Abstract
The role of the gynaecologist in the treatment of female-to-male transsexual patients is largely confined to hysterectomy and vaginectomy. We showed that laparoscopic hysterectomy is feasible and safe in this group. When surgery is not performed completely, follow-up of the remaining organs is necessary.
The major part of this thesis deals with the necessity and acceptability of gynaecological follow-up in male-to-female (MTF) transsexual patients. These patients function well on a physical, emotional, psychological and social level. Sexual function was less satisfactory, especially concerning arousal, lubrication and pain.
Typical gynaecological exams proved to be feasible and well accepted. Transvaginal palpation of the prostate is of poor clinical value, in contrast to transvaginal ultrasound. Mammography was judged almost painless and 98% of transsexual women intend to return for screening. Since there is uncertainty about breast cancer risk in transsexual women, we conclude that breast screening in this population should not differ from that in biological women.
Microflora and cytology of the penile skin-lined neovagina of transsexual women were described for the first time. Vaginal lactobacilli were largely lacking. A mixed microflora of aerobe and anaerobe species, usually found on skin, in bowel or in bacterial vaginosis microflora, was encountered. No high-grade cervical lesions were found, however, one patient displayed a low-grade lesion (positive for HR-HPV with koilocytes).
Finally, low bone mass was highly prevalent in our study group. This finding appeared to be largely determined, in comparison to healthy males, by smaller bone size and a strikingly lower muscle mass.
Keywords: Gender identity, hysterectomy, mammography, osteoporosis, prostate, sexual behaviour, transsexualism, vaginal diseases
Introduction
Gender identity disorder (GID) is a condition in which a person identifies as belonging to the opposite gender as the one he or she was birthed to, termed cross-gender identification, while being persistently and intensely distressed about one’s assigned sex or experiencing a sense of inappropriateness in the gender role of that sex. Transsexualism is considered as the most extreme form of GID (Fisk, 1973) and will most typically require sex reassignment surgery (SRS) (Meyer et al., 2001), which is now generally accepted as an effective means of treating these patients.
As sex reassignment surgery becomes more available and is more commonly performed, health professionals are increasingly likely to see GID patients for pre- and post-transition care. This article gives an overview of the specific role of the gynaecologist in the care of transsexual individuals.
Terminology
An individual with gender dysphoria can be defined as a person whose psychological self-identification is with the other biological sex, and who wishes to alter behaviour and appearance to conform with this internal perception, sometimes with the assistance of hormonal preparations. A transsexual person is a person with GID who wants to undertake hormonal and surgical sex reassignment therapies to conform with the new gender (Witten and Eyler,1999).
Overall, the terminology describing the transgender community is dynamic. Thus a genetic female who considers herself as male and takes medical steps to conform to that perception can be labelled as a female-to-male (FTM) transsexual, while others prefer the term transsexual male, referring to the true gender identity. Equally, a male-to-female (MTF) transsexual is also called a transsexual female.
Diagnosis of GID
Diagnosis of GID is generally made according to the diagnostic criteria of DSM-IV (Table 1). The A-criterion specifies behaviour that signifies cross-gender identification. Criterion B encompasses behaviour that points to discomfort with the own sex or gender role. The diagnosis of GID cannot be given to individuals with disorders of sexual development, although they sometimes can be assigned to the residual diagnosis of ‘GID not otherwise specified’. Furthermore the individual must manifest evidence of distress or impairment as a result of his/her disorder.
Table I. Diagnostic criteria of GID according to DSM-IV.
|
*: new definition is ‘disorders of sex development’, will be implemented in next edition of DSM [Hughes 2008]
It is of utmost importance that any psychological or psychiatric comorbidity is excluded. Therefore the diagnosis of GID has to be made by a mental health professional (preferably a psychiatrist).
The recommended procedure in the Standards of Care of the “Harry Benjamin International Gender Dysphoria Association” (HBIGDA, now called “World Professional Association for Transgender Health” or WPATH) is to arrive at the sex reassignment surgery (SRS) decision in two phases [Meyer et al 2001]. After a first diagnostic phase (based on the DSM-IV criteria) one’s capability to live in the desired role and one’s determination to pursue with SRS is tested in a second diagnostic phase, the so called ‘Real-Life Experience’ or ‘Real-Life Test’ (RLE or RLT). In this period, one has to live permanently in the role of the desired sex, family members must be informed about the impending changes, and a new first name must be chosen. In this phase, considerable variations exist among treatment centres on eligibility of hormone treatment. Some require a period of successful crossgender living without hormone treatment, whereas in others centres hormones are prescribed as soon as cross-gender living has started (Cohen-Kettenis and Gooren, 1999). During the real-life experience, regular contact with a knowledgeable mental health professional is advocated and gender dysphoric patients will only be allowed to undergo definitive SRS when succeeding this RLE.
Prevalence of transsexualism and GID
Prevalence figures of transsexualism show wide variability across studies. Earlier studies have reported prevalences from 1:100.000 to 1:24.000 for MTF and from 1:400.000 to 1:100.000 for FTM) (Pauly, 1968; Walinder, 1971; Hoenig and Kenna, 1974; Ross et al., 1981). Recent studies show prevalences of ~ 1:10.000 for MTF and ~ 1:30.000 for FTM (Tsoi 1988; Bakker et al., 1993; De Cuypere et al., 2007). Figures, however, differ significantly from country to country and from region to region depending on methodology but also on acceptability of GID within a population (Table 2). A recent paper of Vujovic et al. gives a low prevalence of transsexualism in Serbia (< 1:100.000) with a male/female sex ratio of about 1:1 (Vujovic et al., 2008). Prevalence of transsexualism in Belgium is estimated at 1:12.900 for MTF and at 1:33.800 for FTM with a male/female sex ratio of 2.4:1 (De Cuypere et al., 2007).
Table II. Prevalence rates of transsexualism and GID.
| Author | Year | Country | T or GID* | MTF | FTM | MTF/FTM ratio |
|---|---|---|---|---|---|---|
| Tsoi | 1988 | Singapore | T | 1:2900 | 1:8300 | 3:1 |
| Bakker | 1993 | Netherlands | T | 1:11900 | 1:30400 | 2.5:1 |
| Wilson | 1999 | Scotland | GID | 1:7440 | 1:31150 | 4:1 |
| De Cuypere | 2006 | Belgium | T | 1:12900 | 1:33800 | 2.4:1 |
| Vujovic | 2008 | Serbia | T | 1:113636 | 1:105263 | 0.9:1 |
*: transsexualism (T) or gender identity disorders (GID).
Prevalence of GID is even more difficult to estimate since part of these patients do not seek medical help, resulting in underestimates. Recently Olyslager and Conway mathematically estimated the prevalence of GID to be somewhere between 1:500 and 1:2000 (Olyslager and Conway, 2007).
Access to health-care
Even today considerable taboo surrounds people with GID. As a result the threshold for seeking medical help is higher and transgender individuals are more likely to receive suboptimal care or remain silent about important health issues (Dean et al., 2000). A recent survey among transsexual individuals from 13 European countries concluded that, regardless of earnings and social status, healthcare treatment for transsexual people is currently very poor. Indeed, a high majority of transsexuals are not getting any state funding for hormones and/or surgery. Moreover, approximately one third were denied treatment due to non approval of gender reassignment by their primary healthcare practitioner. Apparently many transsexual individuals therefore avoid accessing routine healthcare because they anticipate prejudicial treatment (Whittle et al., 2008).
Providing a supportive environment for individuals with GID is a necessary requirement for effective care (Harrison et al., 2006). Clinicians must address transgender issues in a respectful manner, using appropriate terms and reassuring the patient about confidentiality. However, many clinicians are not acquainted with GID and transsexualism. Therefore gender dysphoric patients are best treated by specialists who are preferably organised in multidisciplinary teams (Sohn and Bosinski, 2007). Within such multidisciplinary teams, surgery is preceded by extensive counselling by the psychiatrist (the diagnostic phase, typically 6-12 months) and by hormonal therapy (hormonal phase, typically 12-18 months).
Role of the gynaecologist
In most multidisciplinary gender teams nowadays a gynaecologist is involved in the surgical treatment (hysterectomy ± vaginectomy ± mastectomy) of FTM transsexual people. Although some gynaecologists are involved in the creation of the neovagina in MTF transsexuals, this is rather uncommon. A potentially more important role for gynaecologists lies in the life-long follow-up of transsexual women. In the next paragraphs the hitherto underestimated and underexplored role of the gynaecologist in the treatment and follow-up of transsexual men and women will be highlighted.
The female-to-male (FTM) transsexual or transsexual man
Screening for gynaecological disorders
Pre-existing hormonal dependent gynaecological disorders (e.g. breast cancer, endometrial hyperplasia) can be worsened by hormonal therapy or can influence the mode of surgery. Therefore a pelvic examination together with a basic hormonal assessment should be performed before initiating any therapy. If the FTM individual has been (hetero-)sexually active a cervical pap-smear should be performed if the last one was performed three or more years before.
A routine gynaecological examination poses a considerable threat for most FTM transsexual individuals. A large proportion of them is still virginal which makes the vaginal access less obvious. A thorough transabdominal ultrasound, with full bladder, is in most cases sufficient to visualise the uterus and the adnexal regions and to exclude significant pathology.
Hormonal treatment in FTM-individuals consists of two phases: in a first phase menstruation is halted by continuous administration of a progestin and/or Gonadotrophin Releasing Hormone (GnRH) analogues for a variable period of time (reversible part). In a second, irreversible phase, testosterone is started to induce male body features: lowering of the voice pitch, augmentation of facial and body hair growth and alteration to a male body hair pattern, hypertrophia of the clitoris and a more masculine body shape. Testosterone administration in transsexual men should in general follow the same guidelines as described by the Endocrine Society for hormone replacement therapy in hypogonadal men: values between 320-1000 ng/dl should be achieved (Bhasin et al., 2006). The recommended endocrine therapy for FTM transsexual individuals is summarized in Table 3.
Table III. Recommended Endocrine Therapy.
| FTM-transsexual individuals | ||
|---|---|---|
| Suppression of original sex hormones | Replacement of the hormones of desired sex* | |
| Oral lynestrenol 5 mg daily (Orgametril®) | Testosterone ester 125-250 mg every two weeks IM (Sustanon®) | |
| Oral medroxyprogesterone acetate 5-10 mg daily | Testosterone Undecanoate 1000 mg every 10-12 weeks | |
| (Provera®, Farlutal®) | IM (Nebido®) | |
| GnRH-analogue: triptoreline 3.75 mg (Decapeptyl®) | Testosterone 100 mg transdermally daily (Androgel®, | |
| IM monthly or goserelin 3.6 mg (Zoladex®) SC monthly | Testim®, Testogel®) | |
| MTF-transsexual individuals | ||
| Suppression of original sex hormones | Replacement of the hormones of desired sex* | |
| Oral cyproterone acetate 50-100 mg daily (Androcur®) | Oral 17β-estradiol valerate 2-4 mg daily (Progynova®) | |
| Transdermal estradiol 1.5-3 mg daily (Estreva®, Oestrogel®) | ||
| Transdermal 17β-estradiol 50-100 µg daily (Climara®, | ||
| Dermestril®, Vivelle Dot®) | ||
Bold: substances preferred by the Ghent Gender Team.
*: continued after SRS.
In most patients the administration of a progestin or GnRH analogue can be ceased after testosterone is started. However, if a transdermal or oral testosterone preparation is used, continuous use of a progestative is sometimes necessary to stop menstruation until surgical castration and hysterectomy are performed.
Administration of testosterone will adversely affect the lipid profile with an increase of triglycerides and a decrease of High Density Lipoprotein (HDL) cholesterol (Giltay et al., 1999; Berra et al., 2006). However, a long-term study by Van Kesteren showed no increase in cardiovascular mortality in transsexual men under testosterone treatment (Van Kesteren et al., 1997).
Administration of suppressive and cross-sex hormones also imply a certain risk of malignant degeneration of the original sex organs. Therefore removal of these genital organs is mandatory. However, if for one reason or another SRS is not (completely) performed, regular follow-up is necessary.
Breast cancer has been reported in residual breast tissue in a FTM transsexual 10 years after bilateral subcutaneous mastectomy (Burcombe et al., 2003). Partial aromatization of androgens to estradiol might have played a role in the occurrence of breast cancer in this patient. There are no reports of incidental breast cancer after mastectomy in FTM transsexual individuals, although it is likely that these cases do occur. In our own experience we encountered an incidental carcinoma in situ in the mastectomy specimen of one patient [not published].
Three cases of ovarian cancer have been described in long-term testosterone treated FTM transsexual individuals (Hage et al., 2000; Dizon et al., 2006). It has been described that ovaries of FTM transsexuals taking androgens show similarities with polycystic ovaries (Spinder et al., 1989; Pache et al., 1991; Baba et al., 2007) and there is still uncertainty about the fact whether polycystic ovaries are more likely to develop malignancies. Therefore, it seems reasonable to remove the ovaries of androgen-treated FTM transsexuals after a successful transition to the male role (Gooren, 2005), even if the absolute risk is probably limited. Moreover, in most countries hysterectomy is a necessary prerogative for the change of birth certificate.
Endometrial hyperplasia is a matter of concern in testosterone-treated FTM transsexual individuals. A high prevalence of endometrial hyperplasia has been noted in a small study of transgender men undergoing hysterectomy (Futterweit and Deligdisch, 1986). As mentioned higher testosterone is partially aromatized into estrogen and as long as the uterus is still in place the endometrium is exposed to this estrogenic action. Consequently, during the period of testosterone administration, periodic uterine sonography, which can be performed through the abdominal wall if technically feasible, is advised if hysterectomy is postponed or not performed (Greenman 2004).
The ultimate goal of SRS in most FTM gender dysphoric patients is the removal of the mammary glands with creation of a male chest, the removal of all female reproductive organs (uterus, cervix, ovaries, tubes and vagina) and the construction of a scrotum and a functional phallus. Needless to say that a supracervical (with preservation of the cervix) hysterectomy is not an option in transsexual men. All of these surgical procedures can be performed separately or combined (Weyers et al., 2006). When complete surgery is performed there is no more need for gynaecological follow-up. In some countries, such as in Belgium, surgery is reimbursed by the social security system and therefore accessible to all transsexual individuals. Worldwide, however, SRS in transsexual people is generally not reimbursed and is therefore in most FTM transsexuals restricted to mastectomy and hysterectomy with bilateral salpingo-oophorectomy. In patients where the vagina is left intact there is no need for regular cytological screening, unless the patient has been treated in the past for cervical cancer or a dysplastic lesion of the cervix with extension into the vagina. However, if these patients have episodes of vaginal blood loss, abnormal discharge or other vaginal complaints a full gynaecological examination with pelvic ultrasound is mandatory. If the FTM patient still has a uterus and/or ovaries, a yearly transvaginal, transrectal or transabdominal sonography is recommended (Mueller et al., 2008).
Technique of hysterectomy
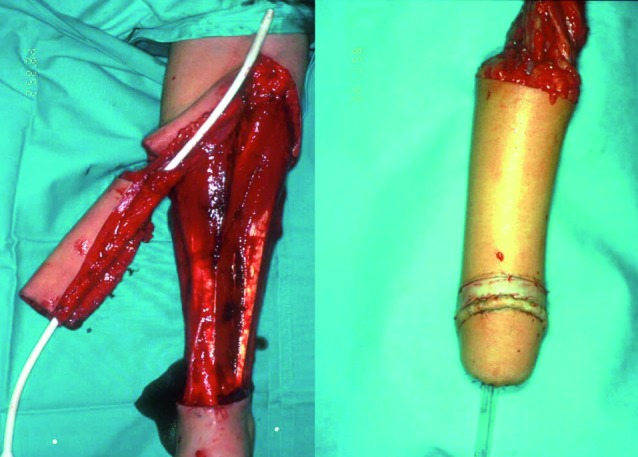
The Ghent gender team initially performed all the surgical procedures in one single operation, which would typically take up to 10 hours and more (Monstrey et al., 2005). In most patients this surgical procedure consisted of the following steps: a subcutaneous mastectomy, a hysterectomy and oophorectomy through a modified Pfannenstiel incision, a vaginectomy via a combined vaginal and abdominal route with reconstruction of the perineal urethra using the vaginal mucosa under the clitoris (Webster et al., 1984), a phalloplasty with a radial fore arm flap (Fig. 1) (Hage et al., 1993; Monstrey et al., 2009) and the creation of a neo-scrotum using the skin of the labia maiora.
Fig. 1. Radial fore arm flap (courtesy of Prof. Dr. S. Monstrey).
From 1993 onwards we performed the subcutaneous mastectomy as a separate first step during the real-life experience. This approach greatly facilitates the adjustment to a male life style. In about one third of these patients hysterectomy had already been performed upon referral, the other two thirds of the patients, however, still had an intact female reproductive tract. When comparing those patients having phalloplasty/vaginectomy/abdominal hysterectomy with those only having phalloplasty/vaginectomy, the latter had significantly less blood loss resulting in less blood transfusions (Weyers et al., 2006). There was also a trend towards a higher major complication rate in those patients having hysterectomy together with phalloplasty/vaginectomy, although the difference was not statistically significant (5.8% vs 0%).
Currently we perform mastectomy together with hysterectomy as a first operative step about 12 months after start of the RLE. About one year later the vaginectomy is combined with the phalloplasty. Performing the SRS in two stages not only smoothens the transition for the patient but equally facilitates the planning for the surgeons. Moreover this change in our protocol made it possible to perform the hysterectomy laparoscopically which prevents a large abdominal scar, shortens hospital stay and quickens the rehabilitation of the patient.
Ideally, hysterectomy is performed using the technique of total laparoscopic hysterectomy (TLH). Indeed, the FTM-transsexual patient is most typically childless and even virginal, which makes the vaginal route more difficult and hazardous. Even a simple procedure such as the closure of the vaginal dome sometimes proves to be quite difficult in these patients. A TLH, with laparoscopic closure of the vaginal dome, necessitates a uterine manipulator with a small vaginal cuff adapted for virginal patients.
Recently O’Hanlan et al described their experience with the technique of TLH in FTM-patients and compared them to TLH-procedures in biological women (O’Hanlan et al., 2007). There was no significant difference in total complication rate (12,2 vs 8,3% respectively) nor in reoperation rate (4,9 vs 4,3% respectively) between transsexual patients and biological women.
We recently reported on 83 laparoscopic hysterectomies in transsexual patients. This is the largest published series of laparoscopic hysterectomies in FTM-individuals. Our major complication rate was 3,6% which is in accordance with the literature. Moreover, our total complication rate (7,2%) and reoperation rate (1,2%) was equally low. These patients form an ideal indication for laparoscopic hysterectomy (Weyers et al., 2008).
Vaginectomy
There remains some debate concerning the need for vaginectomy. Many surgeons choose to leave the vagina unchanged in situ or obliterate the vagina with stitches leaving a small perineal opening (Chapin, 1993; Chesson et al., 1996). However this often gives rise to complaints of discharge and bad smell. It is our experience that vaginectomy is much appreciated by the patients: the vagina has a highly symbolic status in female identity and removal is of great psychological importance.
In the literature little is found about total vaginectomy. Our series of 105 vaginectomies in young patients, performed through a combined vaginal and abdominal approach, is the largest series published so far (Weyers et al., 2006). Our technique of vaginectomy proves to be simple and relatively safe. The risks in vaginectomy are damage to bladder, ureter, sphincter or rectum and bleeding. In the total group of 105 patients, 5,7% needed reoperation for perineal haematoma. There was a tendency for perineal haematomas to occur more frequently in the group of patients not having hysterectomy at the same time, possibly because drainage of blood to the abdominal cavity in these patients was not possible since the peritoneum was not opened.
Currently we perform the vaginectomy through a vaginal approach at the time of phalloplasty. This solely vaginal procedure is easier and leads to less blood loss as compared to our combined vaginal-abdominal approach at the time of the abdominal hysterectomy. We see two explanations for our observation: the upper 1-2 cm of the vagina has been removed at the time of the laparoscopic hysterectomy and the dome of the vagina has not been suspended; moreover, the uterine arteries have been occluded which decreases the blood loss.
In total we performed 320 vaginectomies up till December 2008. In the last 120 procedures, all performed through a sole vaginal approach, an hemostatic matrix (Floseal®, Baxter, Hayward, USA) has been applied to the cavity after vaginectomy. Since then no clinical important hematomas have been observed. For the whole group of 320 vaginectomies we encountered 3 rectal lacerations, one of which could be repaired during the procedure, the 2 others necessitating a temporary colostomy and a re-intervention for the rectal tear. Bladder lesions are more common (10/320 or 3.1%) but are easily recognized during the intervention. Primary closure and urinary derivation by suprapubic catheter always cures the problem [unpublished results].
The male-to-female (MTF) transsexual or transsexual woman
In MTF transsexual individuals endocrinological feminization is achieved by suppression of androgenic effects (reversible part) followed by induction of female physical characteristics (irreversible part). In our centre, suppression of androgenic effects is achieved by the anti-androgen cyproterone acetate, although in other centres spironolactone or GnRH-analogues are being used for this purpose. Estrogen is the principal agent used to induce female characteristics. Oral ethinylestradiol is a potent and inexpensive estrogen, but it is associated with a higher risk on venous thrombosis. Transdermal 17-estradiol or oral 17-estradiol valerate is the treatment of choice (Gooren, 2005). Compared to oral administration, transdermal estradiol administration is the safest route since it is associated with a lower risk of venous thrombosis both in biological and in transsexual women (Van Kesteren et al., 1997; Canonico et al., 2007).
The desired serum level of estradiol is somewhere in between the serum estradiol concentration of the early phollicular phase in premenopausal women and that in postmenopausal women under estrogen replacement therapy. A serum level of 200 ng/ml is usually more than sufficient.
The use of anti-androgens can be halted after surgical castration is performed, the use of estrogens is in general lifelong.
There is no indication that the use of estrogens in transsexual women, when used at appropriate doses, is associated with an increased risk of cardiovascular disease (Van Kesteren et al., 1998). On the contrary, in a large prospective study a favourable change in lipid parameters was seen in transsexual women under estrogen therapy (Elbers et al., 2003). Equally, Van Kesteren showed that cardiovascular mortality was not increased in a large cohort of transsexual women, despite a considerable proportion of smokers (32%) (Van Kesteren et al., 1997). Nevertheless, in transsexual women who smoke the risk of exogenous estrogens should be discussed and cessation of smoking encouraged.
While the correlation between exogenous estrogen use and benign gall bladder disease is well known, there might be a slightly increased risk of induction of gallstones in transsexual women on estrogen therapy (Cirillo et al., 2005; Van Kesteren et al., 1998).
In transsexual women SRS consists of removal of the male reproductive organs (scrotum, testes and penis), creation of a neovagina and -clitoris and, since hormonal breast development is usually insufficient, in about 2/3 of patients implantation of breast prostheses (Monstrey et al., 2001). Transsexual women, although biologically male, might have an increased risk of breast cancer, especially if they have a family history of breast cancer. While the prostate is left intact during SRS, transsexual women remain at a certain risk for developing prostatic disease. Moreover the effect of castration and estrogen replacement therapy on the bone is still a matter of concern (Lapauw et al., 2009).
To study the role of the gynaecologist in the follow-up of MTF-transsexuals a prospective, centre-based observational study was conducted. Since a power calculation was not possible we assumed that a sample of 50 patients could give us a reliable descriptive picture. After informed consent was obtained, all women completed the study proctocol between March and June 2007. Table 4 gives an overview of the main patient characteristics of this study.
Table IV. Characteristics for severe PPGP.
| Age – years (Mean ± SD) | 43.06 ± 10.42 |
| Interval since vaginoplasty – months (Mean ± SD) | 75.46 ± 77.16 |
| Body Mass Index (BMI) – kg/m2(Mean ± SD) | 25.30 ± 5.37 |
| Smoking years (Mean ± SD) | 17.40 ± 11.48 |
| Smoking currently | 18 (36%) |
| Ever smoked | 31 (62%) |
| Regular sport | 20 (40%) |
| Chronic disease | 13(26%) |
| Family history of thrombosis | 11(22%) |
| Family history of breast cancer | 6 (12%) |
| Estradiol – pg/dl (Median, IQ range) | 49.13 (28.60-96.17) |
| Testosterone – ng/dl (Median, IQ range) | 29.57 (21.45-38.24) |
| Sex Hormone Binding Globulin (SHBG) – mmol/l (Median, IQ range) | 66.09 (47.76-107.36) |
| Breast augmentation | 48 (96%) |
| Vocal cord surgery | 20 (40%) |
| Facial feminising surgery | 18 (36%) |
| Cricoid reduction performed | 15 (30%) |
| History of thrombosis | 4 (8%) |
| Use of estrogen therapy | 47 (94%) |
| Use of anti-androgens | 2 (4%) |
| Engaged in a relationship | 27 (54%) |
| Quality of this relationship (Median, IQ range) | 9 (8-10) |
| Heterosexual orientation (= attracted to men) | 22 (44%) |
| Homosexual orientation (= attracted to women) | 11 (22%) |
| Bisexual orientation | 14 (28%) |
| Not sexually interested | 3 (6%) |
| Importance of sex in a relationship – 0 to 10 score (Median, IQ range) | 6 (5-9) |
| Has a general practitioner | 47 (94%) |
| Has no problem with consulting this GP with urogyn problems | 41 (87%) |
| Would prefer consulting gynaecologist with urogyn problems | 37 (74%) |
| Would prefer consulting gynaecologist specialised in gender disorders | 23 (46%) |
| Worries about their newly created genital organs | 29 (58%) |
| Worries about continuous use of estrogens | 21 (45%) |
| Has ever consulted a gynaecologist | 2 (4%) |
| Thinks a regular gynaecological check-up is necessary | 46 (92%) |
| Thinks a regular gynaecological exam is a confirmation of femininity | 33 (66%) |
Unless otherwise specified results are shown as n (%)
In the following paragraphs the conclusions of this study will be discussed and the main areas of interest in the gynaecological follow-up of transsexual women highlighted.
Health-seeking behaviour
Nearly all MTF-individuals in our study indicated to have a general practitioner (GP) (92%). Noteworthily, most subjects revealed to have no problems in consulting their GP with ‘women’s problems’ or urogynaecological complaints (87%) (Weyers et al., 2009). While the medical profession is generally deemed to be rather unfamiliar with transsexualism, it is certainly reassuring that transsexual women were actually found to have confided in a family physician or a gynaecologist even in the case of more delicate, gender-related problems. Three out of four would prefer consulting a gynaecologist in the case of a urogynaecological problem, about half would even prefer a gynaecologist who is an expert in gender identity disorders.
More than half of transsexual women admitted to have some worries about their newly created genital organs and about half do worry about the use of estrogen on their health. Only 4% had ever consulted a gynaecologist whereas nearly all thought they should have a regular gynaecological check-up. Two out of three felt that a regular gynaecological check-up would represent a confirmation of their femininity (Table 4).
Sexual functioning
Little attention has been given to this issue, and nearly all research has been based on self-reports. As expected, there seems to be a correlation between sexual function and the quality of the neovagina (Green, 1998). It was shown earlier that sexual satisfaction was rather high despite inadequate sexual functioning, however, at that time construction of a neoclitoris was not yet part of SRS (Lief and Hubschman, 1993; Green, 1998). Even more adequate genital sensitivity and reassuring sexual satisfaction can be expected with techniques where part of the glans penis with its neurovascular pedicle is preserved for the construction of the neoclitoris (Selvaggi et al., 2007; Soli et al., 2008).
In an earlier study from our group, the sexual health of 32 MTF-individuals and 28 FTM-transsexuals was evaluated through the ‘Biographical Questionnaire for Transsexuals and Travestites’ (Verschoor and Poortinga, 1988) and through a self-developed questionnaire. A large proportion of MTF- (75.8%) and FTM-patients (75.0%) reported an improvement of their sexual life after SRS. There was a trend towards higher sexual satisfaction, more sexual excitement and more easily reaching orgasm in the FTM-group (De Cuypere et al., 2005).
As there is growing evidence that androgen levels are in fact important for female libido and sexual enjoyment, the pharmacologically induced androgen depletion of MTF transsexuals might play an important role. The circulating androgen levels in these women are in fact lower than in genetic women (Gooren, 2005). Elaut et al recently showed that MTF transsexuals have significantly lower serum levels of total and calculated free testosterone compared to ovulating women. Nevertheless there was no difference in the level of sexual desire or in the occurrence of hypo-active sexual desire disorder (HSDD) between both groups (Elaut et al., 2008).
In our group of 50 MTF-individuals, female sexual functioning, as assessed with the Female Sexual Functioning Index (FSFI), was less optimal than might be expected (Weyers et al., 2008). Overall FSFI scores were actually found to approximate those obtained in non-transsexual women eliciting sexual complaints (Ter Kuile et al., 2006). Sexual functioning and satisfaction clearly differed with respect to sexual orientation: women with a homosexual preference presented with markedly lower sexual functioning scores as compared to women with heterosexual or bisexual orientation. Furthermore, lesbian transsexual women were found to have significantly worse sexual functioning indices as compared to a historical cohort of non-transsexual lesbian women (Tracy and Junginger, 2007). It may be added that transsexual lesbian women also attributed the lowest importance to sex as compared to the remainder of transsexual women in this series (Weyers et al., 2008). Transsexual women in a heterosexual partnership primarily reported problems with arousal, lubrication and pain, while data on desire, the attainment of orgasm, and most importantly sexual satisfaction were in line with those obtained from non-transsexual heterosexual women without sexual complaints. Elaut et al. previously observed the absence of a difference in sexual desire between transsexual women and biological women, but found a more pronounced sexual dissatisfaction in transsexual women, possibly resulting from the use of different measurement instruments (Elaut et al., 2008).
Interestingly, post-transitional alteration of sexual orientation was observed in one out of every four MTF-individual in our study. A post-transitional change in the choice of the sexual partner was however not related to sexual functioning or satisfaction scores. Also noteworthy is that transsexual women who indicate a higher degree of satisfaction with their appearance also report better sexual functioning. The same was found in an American study: in particular, both men and women who were more dissatisfied with their body appearance were also less content with their sex lives (Hoyt and Kogan, 2001).
The vagina
Since the first use of amniotic membrane for reconstructing the aplastic vagina by Brindeau in 1934 several procedural developments for reconstructing tissue to create a neovagina, such as the use of the split-thickness and full-thickness skin graft, perineal pedicled local flap, peritoneum, rectosigmoid, penile skin flap and combined penile and scrotal skin flap, have been adopted (Fang et al., 2003).
This last technique is nowadays the standard technique for the creation of the neovagina in transsexual patients (Sohn and Bosinski, 2007). Ideally, permanent depilation of the scrotum and penile shaft is pre-operatively perfomed to minimize hair growth in the neovagina. Pedicled intestinal transplants, mostly rectosigmoid segments, have also been used in transsexual patients, however this approach requires additional transabdominal surgery with all its possible inherent complications. Moreover other problems such as introital stenosis, persistent odor and diversion colitis make this technique less attractive (Hage et al., 1995). Whatever the technique, the construction of the neovagina results in abnormal exposure of the original tissue, and little or nothing is known about the long-term effects of this exposure.
Genital HPV-infection is estimated to occur in about 70% of all people. Of the more than 100 different types of HPV about 40 are specific for the anogenital region. Some of these anogenital HPV-types are classified as ‘high-risk’ and are involved in nearly all cases of cervical, vaginal and vulvar cancer (Munoz et al., 2006). The ‘low-risk’ types are responsible for the condylomata and for a proportion of low-grade dysplastic lesions of the genital tract.
The prevalence of HPV-infection (detected by HPV-DNA in the cervix) in biological women is age- and population-dependent and varies somewhere between 14-90% with an overall prevalence of 20.8% in US adolescents (Revzina and DiClemente, 2005) A recent systematic review of the prevalence of HPV-infection in men showed the same wide variations: prevalence varies somewhere between 1.3-72.9% depending on the population tested, the genital site (glans penis, corona penis, penile shaft, scrotum, semen, urine, …), the sampling method and the sensitivity of the assay for the detection of HPV DNA. Population studies show prevalences below 15%. Prevalences prove to be consistently higher in male partners of women with cervical dysplasia or cancer or in high-risk populations (military, men attending STD-clinics, …). The anogenital HPV-types detected in men varied by study but were more or less similar to those detected in women, with type 16 consistently among the most common. The prevalence of HPV-detection on the penile shaft and the scrotum is between 5.6-51.5% and 7.1-46.2% respectively (Dunne et al., 2006).
While the skin of the penile shaft is used for the creation of the neo-vagina and the scrotum is used for the creation of the labia this ample prevalence of HPV has to be taken into account and occurrence of HPV-related lesions in these newly constructed organs is not unlikely.
Up till now there is little information regarding the cytology of the neovagina of transsexual women treated with the technique of the inverted penile skin. Yet knowledge of this cytology in transsexual women can be considered essential for their state-of-the-art follow-up. Neoplasia has been documented several times (at least 16 case reports) in the neovagina of biological women but only twice in a transsexual woman (Lawrence, 2001; Harder et al., 2002). Condylomata have been described in the neovagina of a transsexual woman (Liguori et al., 2004) and we recently treated condylomata in three transsexual women with laser ablation (not published).
Recently, vaccines against the most frequent causal agents of genital dysplasia and condylomatosis have been commercialised and are reimbursed for young girls (11-16/18 years of age) in most European countries. Vaccination of older girls, women, boys and men is still under debate (Newall et al., 2007). In addition, vaccination of MTF-transsexuals should be considered, especially if they have male partners.
While the composition of the normal vaginal microflora (VMF) has been extensively studied by conventional culture techniques and molecular methods (Frederics et al., 2005; Verhelst et al., 2004), there is no information regarding the vaginal microflora in transsexual women treated with the technique of the inverted penile skin. Under normal conditions, the lower genital tract of the biological female harbors a commensal microflora that primarily consists of lactobacilli which confer antimicrobial protection to the vagina. In addition, given adequate vaginal estrogen levels, the vaginal epithelium and its associated mucous layers helps to regulate and support the intrinsic bacterial and mucosal defense system (Marrazzo, 2004). However, in case the vaginal hydrogen peroxide producing lactobacilli fail to thrive, an overgrowth by other commensal bacterial vaginosis-associated micro-organisms is observed (Sobel, 2000). These commensals include Gardnerella vaginalis, A topobium vaginae, Prevotella spp., anaerobic Gram-positive cocci, Mobiluncus spp. and Mycoplasma hominis.
Vaginal complaints are in fact one of the most common reasons for gynaecological consultation. Frequent episodes of troublesome vaginal discharge is mentioned by some 20% (14.5-57% depending on the population studied) of biological women (Goldacre et al., 1979; Koenig et al., 1998; Patel et al., 2005). Figures about the background incidence of vulvo-vaginal irritation in biological women are difficult to find: one study from 1979 gives a figure of 9.1% (Goldacre et al., 1979). Nevertheless, most biological women sooner or later will be confronted with one or more episodes of vaginitis. Bacterial vaginosis (BV) and vaginal candidiasis are the two most frequent diagnoses (Anderson et al., 2004).
The only report on the microflora of the neovagina concerned 15 patients who were treated with pedicled sigmoid transplants (Toolenaar et al., 1993). Yet knowledge of the VMF in transsexual women is essential in their proper follow-up, e.g. in case these women present with vulvar or vaginal complaints (pain, odour, itch, etc) or in case of overt genital inflammation and/or infection. Gonococcal infection of the skin-lined neovagina of a MTF-patient has twice been published (Bodsworth et al., 1994, Haustein, 1995).
Feasibility and acceptability of vaginal exams
In our study only 4% of women ever had a gynaecological exam, nevertheless 84% thought that a regular vaginal exam should be part of their follow-up.
A normal-size speculum (2.5 cm wide and 10 cm long Collins speculum) could be used in 74% of women, a smaller type (2 cm wide) had to be used in the others.
A normal digital vaginal exam (with two fingers inserted) was possible in nearly half (44%) of transsexual women, while in the remainder of women only one finger could readily be inserted. The mean mobility of the vagina, as subjectively rated by the clinician on a scale from 0 to 3 was 1.70 (Standard Deviation (SD) = 0.71), i.e. rather mobile on average. The mean vaginal length as measured by the physician was 6.99 cm (SD = 1.81) with a median of 7.50 cm (Inter quartile (IQ) range 6.00-8.12).
From 28 patients we recovered the mean vaginal length, as self-measured by the patients at home after dilation for at least 5 minutes and use of lubricant: this was 9.85 cm (SD = 2.77). This highly significant difference in the measuring of the vaginal length (p = 0.008) was however not surprising: besides the ‘observer-bias’ there is also an important influence of the circumstances and environment. Indeed, when measured by the clinician, patients had been instructed to refrain from any vaginal manipulations (dilatation, coitus, vaginal rinsing,…) in the three days before, thus probably negatively influencing the length measured at the time of consultation. Moreover, since most of them never had a gynaecological exam, they were without any doubt anxious which also could have influenced the ease and depth of speculum insertion. The correlation between dilatation habits and the vaginal length as measured by the clinician just marginally missed significance (P = 0.053). On the one hand this could be due to the small numbers but it could also be an indication that regular dilatation is not a necessary prerogative for maintaining the vaginal length.
Both speculum and digital exams were very well tolerated by the participants: when asked for by an independent study nurse the mean pain score for the speculum exam was rated 2.10 (SD = 2.37) and for the vaginal digital exam 1.74 (SD = 2.32) on a Visual Analogue Scale (VAS) from 0 to 10.
The vagina from a transsexual woman’s perspective
The importance MTF-individuals attributed to the aspect of their vulva/vagina was very high (median of 9 on a scale from 0 to 10). The satisfaction with their vulva/vagina was equally high with a median of 8. This is in accordance with earlier studies: Lawrence showed that the ‘overall happiness with the postoperative result of SRS’ was high (Lawrence, 2003) whereas transsexual women in the study of De Cuypere indicated satisfaction with vaginoplasty in 86.2% (De Cuypere et al., 2005).
Sixty-eight percent of women indicated to dilate regularly using the prosthesis or a vibrator. Three patients indicated that there was no need to dilate because they had regular intercourse, which resulted in a total of 77% of patients who ‘dilated’ on a regular basis. The other 23% indicated not to dilate because of pain. In fact 41% experienced some degree of pain during dilatation, with a mean score for pain experienced of 3.92 (SD = 1.89).
More than half of women (55.9%) reported the regular (once a week or more) use of products for vaginal hygiene. More than half of them were using a iodine solution (Isobetadine Gynaecological solution, Meda Pharma, Brussels, Belgium), about one in three used a solution with low pH containing lactic acid and milk serum (different manufacturors), the others were using a body douche gel or plain water.
About one in four (23.5%) had frequent (once a month or more) episodes of bad-smelling vaginal discharge. The mean vaginal pH was 5.88 (SD = 0.49, range 5.0-7.0), which is considerably higher than in biological women (reference range 4.0-4.5). This of course is not surprising since these vaginas are made from penile skin. There was no correlation between vaginal rinsing habits on the one hand and the vaginal pH, malodorous vaginal discharge or pain at dilatation on the other.
Likewise there was no correlation between the vaginal pH and complaints of irritation, dysuria or smelly discharge.
Vaginal cytology
In our population cytological abnormalities were found in 5 patients (10%): in one patient low-grade dysplasia was present (this patient proved high-risk HPV positive) and four patients showed cells compatible with Atypical Squamous Cells of Uncertain Significance (ASCUS), however without HPV infection. Inflammation was present in 22% of the population. Concerning the quality of the specimens, more than a quarter (28%) merely contained non vital cells. In the penile skin-lined neovagina there is always a considerable amount of sebaceous material and cellular debris. This is probably the reason why in more than a quarter of the patients the smear of the vaginal vault only contained avital material. In only 4% of the specimens our findings correlated with normal ectocervical cytology, where superficial, intermediate and parabasal cells as well as Döderlein bacilli are present.
Vaginal Microflora (VMF)
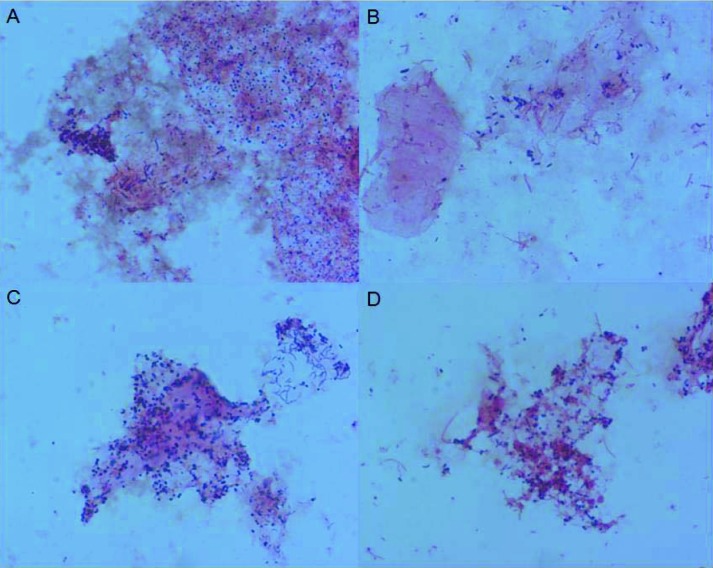
The fifty neovaginal swabs in our study were all Gram stained. For six smears, one of which contained pus cells, only few bacteria were found. Fourty-four smears revealed a mixed microflora that was more complex than bacterial vaginosis microflora and contained mostly filamentous and fusiform shaped cells and Mobiluncus and Spirochaetes cell types (Fig. 2). Candida hyphens or spores were not present in any of the smears, the presence of Lactobacilli was confirmed in only one patient.
Fig. 2. Microscopic image (1000x) of Gram-stained neovaginal smears illustrating the observed diversity: various amounts of cocci (A), polymorphous Gram negative and Gram positive rods, often with fusiform (B) and comma-shaped rods (C), and sometimes even with spirochetes (D).
A total of 79 different species could be cultured. Eighty-four percent of these species demonstrated more than 98% similarity with previously known bacterial species belonging to 32 different genera. No specimen was sterile. On average we identified 8.6 species per woman (range 4-14). The species most often found were Bacteroides ureolyticus (n = 10), Corynebacterium sp. (n = 12), Enterococcus faecalis (n = 13), Mobiluncus curtisii (n = 10), Staphylococcus epidermidis (n = 19) and Streptococcus anginosus group spp. (n = 16). DNA extracts of 50 neovaginal samples were amplified with 16S rRNA gene based primers specific for A. vaginae, G. vaginalis and Mobiluncus curtisii. Remarkably, more than 80% (41 of 50) of neovaginal specimens showed an amplicon after amplification with M. curtisii primers.
There was no correlation between dilatation habits, having coitus, rinsing habits and malodorous vaginal discharge on the one hand and the presence of a particular species on the other. There was, however, a highly significant correlation between the presence of Enteroccus Faecalis and sexual orientation: in heterosexual transsexual women (i.e. attracted to male partners) Enterococcus Faecalis was present in 78.6% while it was only present in 14.2% of homosexual transsexual women and in 12.5% of bisexual transsexual women (p = 0.003). Similarly, in heterosexual transsexual women there was a significant correlation between Enterococcus Faecalis and the occurrence of regular coitus with a male partner: in those having regular coitus Enterococcus Faecalis was present in 75% while in only 25% of those not having coitus (p = 0.027) (Weyers et al., 2009).
In transsexual women with a penile-skin lined neovagina a complex mixture of aerobe and (facultative) anaerobe species usually encountered either on the skin, in the intestinal microflora or in a bacterial vaginosis microflora was found. While Toolenaar isolated lactobacilli from 10 of 15 women who were treated with pedicled intestinal (sigmoid) transplants, only one of our women with a penile skin-lined neovagina was colonized by lactobacilli (Toolenaar et al., 1993). As expected, the environment of the penile skin-lined neovagina does not support the growth of lactobacilli due to the absence of glycogen rich epithelial cells. Therefore transsexual women are possibly more vulnerable to infections and information on intimate hygiene and prevention of infections should be part of the follow-up of these women.
If patients report symptoms of sexually transmitted infection, they must be screened and treated as per local guidelines.
The breasts
One of the desired effects of estrogen therapy is gradual growth of breast tissue. This effect, however, is highly variable: some patients will hardly develop some breast buds even after years of estrogen therapy whereas others have full breast development after 1-2 years. This variability is likely based on estrogen sensitivity. Eventually a large proportion of transsexual women need breast prostheses to achieve a satisfactory female chest contour.
Data on the necessity of performing screening mammographies in transsexual women are lacking. In one publication, mammography is recommended after 10 years of hormonal therapy for women older than 40 years of age (Oriel, 2000). Another report advises screening mammography from the age of 50 in the presence of additional risk factors [Feldman and Goldberg 2006], but evidence from prospective studies is lacking.
The Women’s Health Initiative study showed that the breast cancer risk in postmenopausal women taking conjugated equine estrogen without the addition of progesterone was not increased (Anderson et al., 2004). In a recent French study (NH3-EPIC) however there proved to be an unequal risk for breast cancer according to the type of hormone replacement therapy. More specifically there was a significant increase in breast cancer risk for users of estrogen-alone while for combined use of estrogen and progestins it depended on the type of progestin used (Fournier et al., 2008).
Breast cancer is uncommon in men, accounting for <1% of all male malignancies. Unlike female breast cancer, for which incidence rates are rising throughout the world, the comparative incidence of male breast cancer has remained relatively stable in most countries (Ravandi-Kashani and Hayes,1998). It is not unlikely, however, that in transsexual women under estrogen therapy the risk of developing breast cancer will prove to be higher than in males. So far, reports of transsexual women developing breast cancer are scarce (Symmers, 1968; Pritchard et al., 1988; Ganley and Taylor, 1995).
Biological women with breast implants are not at a higher risk of developing breast cancer (Brinton et al., 2000). Similarly, women with breast implants are not diagnosed at a later stage, do not have more recurrences and have no shorter survival (Hoshaw et al., 2001). However, it has been shown that the sensitivity of screening mammography in detecting breast cancer is lower in biological women with breast implants although the false-positive rate is not augmented (Miglioretti et al., 2004; Mc Intosh and Horgan, 2008). Miglioretti et al. in turn showed that invasive breast tumors in women with implants are prognostically equal to similar tumors in patients without implants (Miglioretti et al., 2004]).
Feasibility and acceptability of breast exams
In our study 80% of women thought that a regular breast check-up is necessary and 90% would come if sollicited for mammographic screening (Weyers et al., 2009).
In Belgium, all women between the ages of 50 and 69 are invited for a free screening mammography on a two-yearly basis. Ten transsexual women already had a mammography performed, three of which were ≥ 50 years of age and mammography took place within the framework of the regional screening programme. The reasons for mammography in the 7 others were diverse.
Eight percent of transsexual women in our study had at least one first grade relative with breast cancer.
Pain during mammography can discourage women to attend a screening mammography. Moreover, if the first mammography is painful this is cited as the main reason for not re-attending the next screening visit (Andrews, 2001). Compared to women without implants, those biological women with breast implants do not expect mammography to be more painful neither do they experience more pain during the mammography (Brown et al., 2004). In our study in MTF-transsexuals both expected pain (4.37) and experienced pain (2.00) were judged fairly low. The experienced pain was marginally inversely correlated with the volume of the prostheses (r² = -0.319, p=0.048) but not with their location nor with mammographic density. There was no significant difference in expected pain between those who already had mammography and those who did not. There was, however, a significantly positive correlation between expected and experienced pain. This confirms findings in several other studies: expected discomfort, whether or not from own experience, is an important risk factor associated with pain during mammography (Bruyninckx et al., 1999; Andrews, 2001).
In 60% of our patients the breasts were judged “very dense” to “dense” on mammography. There was no correlation between the density of the breast tissue and estrogen levels. About one third of the images, both on cranio-caudal incidence as on oblique incidence, were rated suboptimal (‘over-‘ or ‘underexposed’). Performing mammographies in patients with breast-implants always poses a diagnostic challenge.
In two patients an abnormality was detected on mammography: one patient had empty prostheses (patient was acquainted with it) and in the other a fibro-adenoma was suspected.
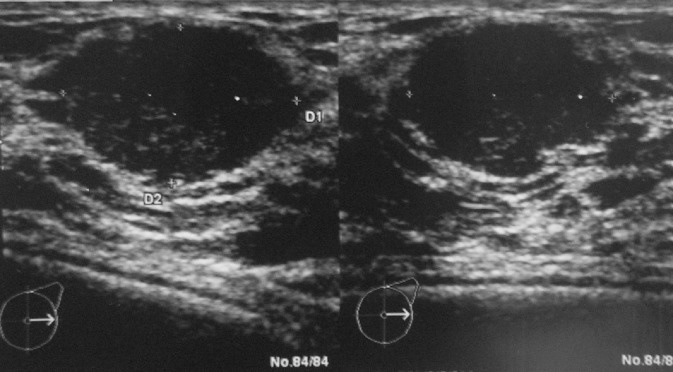
Sonographic density was equally scored by the radiologist. In only one patient the breasts were very echodense, 36% were judged “dense” and 36% “fatty” whereas the remaining 26% were “slightly dense”. There was a significant correlation between the density on mammography and on sonography (0.770, P < 0.001). In five patients abnormalities (other than small cysts) were visualized on sonography: one patient had a fibro-adenoma (Fig. 3), two had a lipoma, in one patient both prostheses were empty while in another rupture of one of the prostheses was suspected. A fibro-adenoma has only been described twice in transsexual women and is extremely rare in the male breast (Kanhai et al., 1999; Lemmo et al., 2003). Since in our patient the fibroadenoma was small (1.3 cm) and had perfectly benign features on echography we decided not to perform a biopsy.
Fig. 3. Sonography of a fibroadenoma in a transsexual woman.
The breasts from a transsexual woman’s perspective
The importance transsexual women attributed to the aspect of their breasts was very high (median of 9 on a scale from 0 to 10). Luckily, the results met their expectations: the satisfaction with their breasts was equally high with a median of 9. This confirms findings from an earlier study from our group: transsexual women in the study of De Cuypere indicated satisfaction with breast augmentation in 95.2% (De Cuypere et al., 2005).
Fourty-two percent of women experience episodes of breast tenderness, most of them once a month or more. The severity of this breast tenderness had a mean score of 3.90 (± 1.97) as selfscored by the patient on a visual analogue scale. A striking finding in our recent study was that about one in four patients is not aware of the type of breast prosthesis that was used and even four out of five do not know the position of the prostheses in relation to the pectoral muscles (retro- or prepectoral).
From our study we concluded that mammography as well as breast sonography are technically feasible and well accepted in transsexual women. Since both exams were judged as nearly painless, after our study exams 98% of transsexual women agreed to come back if invited. As a result of these findings and since there is uncertainty about the real breast cancer risk of transsexual women, we suggest that breast screening habits in this population should not differ from those of biological women (Weyers et al., 2008). Whenever an abnormality is suspected (clinically or on imaging) Magnectic Resonance Imaging must be considered as this exam has a considerably higher sensitivity in women with breast protheses.
The prostate
During vaginoplasty the prostate and the seminal vesicles are left in place to avoid the considerable short- and long-term morbidity associated with radical prostatectomy. Therefore follow-up of the prostate status could be warranted as part of the post-transition care for these patients. Prostatic disease, including benign prostatic hyperplasia (BPH) (Goodwin and Cummings, 1984; Yokoyama et al., 1998; Brown and Wilson, 1997; Casella and Bubendorf, 2005) and prostate carcinoma (Markland, 1975; Thurston, 1994; Van Haarst et al., 1998; Miksad et al., 2006; Dorff et al., 2007) has been reported among transsexual women. All transsexual women diagnosed with prostate carcinoma were ≥ 50 years of age when they had started cross-sex hormone treatment and it is not clear whether these cancers were estrogen-sensitive or whether they were present before estrogen administration started and then progressed to become androgen-independent (Gooren, 2005). Anyhow, it might be expected that these new clinical entities will become more prevalent as more transsexual patients receive SRS.
In the total group of transsexual women that were operated by our gender team (n = 320) we have observed 2 cases of prostatitis and one case of prostatic abscess after SRS. The clinical presentation did not differ from the clinical presentation in males with fever, dysuria and elevated Prostatic Specific Antigen (PSA). The prostatic abscess was drained transvaginally without any further problems while the patients with prostatitis were treated with antibiotic treatment. These patients had no other urological problems which could be at the base of this prostatitis (unpublished results).
Feasibility and acceptability of transvaginal palpation and transvaginal ultrasound of the prostate
In our own study population only one transsexual woman already had a vaginal ultrasound examination, while none ever had a vaginal digital examination, even if most women considered a regular vaginal examination (92%) and prostate examination (80%) necessary for a good follow-up. Mean ‘anticipated pain’ scores for digital vaginal examination and vaginal ultrasound, on a 0 to 10 VAS, was 5.20 (SD = 2.91) and 3.67 (SD = 2.72), respectively. Median serum levels for testosterone (ng/dl) and Prostatic Specific Antigen (PSA, ng/ml) were 29.57 (IQ range 21.45-38.24) and 0.0300 (IQ range 0.0300-0.0815) respectively. Estradiol levels were not reported due to the diversity of used formulations.
Vaginal palpation of the prostate was possible in merely half of the transsexual women (48%). This was not explained by prostate size, which proved to be consistently small (< 35 mL on ultrasound) and within or below the normal range for young men (normal range 14-44.8 mL in men 30-50 years of age) (Roehrborn, 1999). The latter had been previously documented in two historical case series of eunuchs (Wilson and Roehrborn, 1999; Van Kesteren et al., 1996). Rather, palpability of the prostate was to a considerable extent explained by the length (0.332, P = 0.018) and the tissue rigidity of the neovagina (0.396, P = 0.004). There was no correlation between palpability of the prostate and patient age.
In our study it proved possible in all patients to visualize the prostate using the endocavitary probe. Introduction of the probe was judged ‘easy’ in 74%. Transvaginal scanning was feasible among 94% while in three patients the vagina was too short to allow introduction of the tip of the probe but the prostate could adequately be visualized transperineally. Proper imaging of the seminal vesicles through transvaginal ultrasound was obtained in 80% of the patients.
Jin et al. showed that in 14 transsexual women, all taking estrogens but 10 of them not yet surgically castrated, the mean prostatic volume, as measured transrectally, was significantly lower than among age-matched controls (19.3 vs. 28.2 mL, p < 0.001) (Jin et al., 1996). Thereby they documented the net effect of estrogen substitution on prostate volume. The mean volume as measured transvaginally in our population was 14.19 cm3 (range 5-35, SD 5,95). There was a statistically significant positive correlation between the volume of the prostate on the one hand and serum value of PSA (0.362, p = 0.012) on the other hand. However, there was no relation between the volume of the prostate and the serum levels of total testosterone, as was observed by Jin et al. Similarly there was no correlation between the volume of the prostate on ultrasound and the age of the patient. We observed no significant influence of the duration of estrogen intake or the interval since surgical castration on the volume of the prostate.
No gross anomalies of the prostate were observed in our case series. When asked for by the study nurse, the painfulness of the vaginal ultrasound was very low (1.10, SD 1.66).
Clearly, prostate volume and PSA levels are considerably lower than in biological men of corresponding age and hence also well below the diagnostic cut-off values for prostatic disease. This finding was not quite unexpected as all patients had been surgically castrated and presented with low testosterone levels, and moreover nearly all of them were on estrogen replacement therapy (Weyers et al., 2009).
Therefore it may be concluded that surgical castration but even more so estrogen replacement therapy suppresses prostatic growth, also with increasing age. Hence, MTF-individuals, especially when operated at a young age, may be assumed to have a considerably lower risk of developing benign prostatic hyperplasia and prostatic cancer than biological males. Nevertheless, prostatic disease has been reported in transsexual women and therefore regular screening through PSA-dosage from the age of 50 on could theoretically be considered, assuming that such screening would be found to be cost-effective. It must be stressed, however, that screening for prostate cancer even for biological men is under much debate (Ilic et al., 2007), and therefore some restraint in applying screening exams is appropriate in transsexual women. If prostatic enlargement is suspected transvaginal ultrasound is probably the first imaging exam indicated since it is perfectly feasible and well tolerated by the patients.
Endocrine treatment and the bone
Endocrine treatment regimens in MTF-individuals show wide variation among treatment centers. This is particularly apparent with regard to estrogen dose in older patients and the addition of a progestin and/or antiandrogen to the treatment regimen. Results from a survey of MTF transsexual people demonstrated markedly elevated hormone doses and even greater complexity in their treatment regimens. Estrogen doses were often at alarming levels, and multiple formulations were used (estradiol up to 100 mg IM every two weeks, ethinyl estradiol up to 100 µg/d PO, conjugated equine estrogen up to 5 g/d PO, transdermal estradiol benzoate up to 25 mg every week, transdermal 17-β estradiol up to 8 mg/d) (Moore et al., 2003).
The effects of feminizing hormones on bone density in transsexual women remain controversial. Long-term estrogen exposure in non-castrated MTF-individuals (with or without treatment with gonadotropin releasing hormone agonist) results in an increase in bone mineral density (Reutrakul et al., 1998; Mueller et al., 2005). Concerning the testosterone deprived biological male the majority of available literature suggests that estrogen replacement therapy, with or without the addition of anti-androgen therapy, does in fact not result in significant bone loss (Lips et al., 1989; Van Kesteren et al., 1996; Schlatterer et al., 1998; Ruetsche et al., 2005; Haraldsen et al., 2007). Other studies indicate that there is in fact a risk of bone loss in post-transitional MTF transsexual individuals (Van Kesteren et al., 1998; Lapauw et al., 2008). Loss of density, however, is more likely in those patients who are less compliant in taking their estrogen therapy.
Data on the degree by which bone loss correlates with the risk of fractures, a relation which has clearly been established in biological women and men (De Laet et al., 1997), is lacking in transsexual women. There is no consensus on the minimal dose of estrogen needed to preserve the bone mineral density after surgical castration, although some studies in postmenopausal women suggest that very low doses of estrogen may be sufficient (Doeren et al., 2000; Evans and Davie, 1996). Whether these conclusions in postmenopausal women are also valid in transsexual women remains unclear.
Generally the hormone treatment for MTF transsexual persons is guided by the induction and maintenance of a feminizing physical state which is acceptable for the woman herself, at the same time avoiding both short- and long-term adverse effects (T’Sjoen et al., 2009). In most centres, anti-androgen therapy precedes or is given in conjunction with the estrogen therapy, at least during the first years of hormone treatment and before surgical castration is performed. After the SRS, estrogen therapy alone is usually sufficient to maintain feminisation. There is growing evidence that, as in postmenopausal women, the use of transdermal preparations is the first-choice estrogen treatment for transsexual women of all age groups (Van Kesteren et al., 1997; Greenman, 2004]. The recommended endocrine therapy MTF transsexual individuals is summarized in Table 3.
In our centre, hormonal sex reassignment is initiated using anti-androgen therapy (cyproterone acetate 50-100 mg/day) alone up to a maximum of 1 year, followed by addition of exogenous estrogen administration (De Cuypere et al., 2005). Current estrogen treatment is not completely standardized. In a recent study we conducted 50% of patients were on transdermal estrogen treatment (estradiol gel 1.5 mg, n = 22; estradiol patch 50 µg/24 h., n = 3), whereas 44% were on oral estrogen tablets and 6% were not taking any estrogen therapy due to a previous thromboembolism (T’Sjoen et al., 2009). Choice of estrogen treatment was mainly determined by patient preference. In this study age was significantly different between patients on transdermal or oral estrogen treatment, with patients on oral formulations generally being younger.
Depending on the site measured, 2-26% (hip vs distal radius) of these transsexual women were diagnosed with low bone mass. This finding is indeed in contrast with the majority of the literature on bone health in this specific transsexual patient group. Differences in bone quality in transsexual women may be dependent on time of follow up, but may also be based on centre-specific timing, dosing and choice of sex steroid hormones. As we use a strong anti-androgen as sole therapy during the first months of treatment this could lead to loss of muscle, gain of fat mass and decrease in BMD, analogous with effects as described in men treated for prostate carcinoma (Diamond et al., 2004) or following surgical castration (Stepan et al., 1999). Furthermore, once estrogen therapy is initiated, we cautiously advise moderately dosed estrogens in an attempt to avoid potential adverse effects/complications.
In this same study, serum levels of gonadotropins were rather high (mean ± SD for LH = 28 ± 17 U/l; median with IQ range for FSH = 43, 26-60 U/l). No differences in steroid hormone levels between oral and transdermal administration were observed. However LH had a tendency to be lower in patients using transdermal estrogens (p = 0.061). In contrast to Van Kesteren et al we saw no correlation between LH-levels and BMD (Van Kesteren et al., 1998). The rather high serum levels of gonadotropins might be considered as a marker for suboptimal estrogen supplementation, however, SHBG-levels were equally high suggesting sufficient estrogen doses.
No significant difference in bone size or density was observed between patients on transdermal or oral estrogens, though muscle size was higher in patients on transdermal estrogens. No difference in whole body or regional fat mass was observed between subjects using transdermal estrogens or oral treatment. Interestingly, transdermal estrogens not only seem to suppress LH better than oral estrogen compounds, but are also associated with higher muscle mass and this despite the significant higher age of transdermal estrogen users. The etiology for this remains unknown. No relationship between the duration of estrogen therapy and bone density or size was found. The relation between low bone mass and fracture risk has so far not been established in transsexual women.
Based on our pQCT-data we demonstrated that low cortical bone mass was highly dependent on low bone size, more than on low cortical bone density, confirming data from earlier work from our group (Lapauw et al., 2008). The most plausible explanation for these findings are lower strains on the bones due to lower muscular loading. On the other hand, an additional explanation for the low cortical bone size could be the testosterone deficiency in these patients, since there is general acceptance of androgen action on periosteal bone formation (Vanderschueren et al., 2006). No relation of testosterone or DHEAS with bone size or cortical thickness was observed in our cohort. In general androgen concentrations are low in these patients and could not be sufficient to promote periosteal apposition.
Calcium (1200 mg daily) and Vitamin D (600 IU daily) supplementation together with weight bearing exercise are indicated for all transsexual women. In our own study, however, not more than 40% of transsexual women gave an account of regular sport’s activities (at least twice a week for 30 minutes or more) and a mere 24% reported the regular use of Calcium/Vitamin D supplements.
Based on our results we would advocate to perform bone density measurement before onset of hormonal therapy and thereafter every 5 years, even in young transsexual women. Whenever low bone mass is detected frequency of bone density measurement should be increased. The effect of bisphosphonates has not been established in transsexual patients, however it seems likely that the effect proven in biological men and women can also be expected in transsexual women (Watts, 2001).
In our opinion endocrine treatment in transsexual patients should be initiated by an endocrinologist or a gynaecologist specialised in transgender endocrine therapy. As gynaecologists are well aware of the desired and side effects of different regimens of estrogens, they might constitute a valuable partner in the endocrine follow-up of transsexual women.
Conclusions
For the gynaecologist there is a substantial role in transsexual health care, not only concerning the treatment of FTM-patients but also in the follow-up of MTF-individuals. The gynaecologist has ample experience in all diagnostic examinations concerning female sexual health. Moreover MTF-patients appreciate follow-up of their newly created and their remaining original sex organs. The key points of the role of the gynaecologist in the treatment and follow-up of transsexual men and women are summarised in Table 5.
Table V. Role of the gynaecologist in the treatment and follow-up of transsexual individuals.
| FTM-transsexual individuals |
|---|
| Treatment |
|
| Follow-up |
|
| MTF-transsexual individuals |
| Treatment |
|
| Follow-up |
|
Acknowledgments
Petra De Sutter is holder of a fundamental clinical research mandate by the Flemish Foundation for Scientific Research (FWO-Vlaanderen).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (3rd ed.) Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed.) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women’s health initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Andrews JA. Pain during mammography: implications for breast screening programmes. Australas Radiol. 2001;45:113–117. doi: 10.1046/j.1440-1673.2001.00889.x. [DOI] [PubMed] [Google Scholar]
- Baba T, Endo T, Honnma H. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007;22:1011–1016. doi: 10.1093/humrep/del474. [DOI] [PubMed] [Google Scholar]
- Bakker A, van Kesteren P, Gooren LJG, et al. The prevalence of transsexualism in the Netherlands. Acta Psychiatry Scand. 1993;87:237–238. doi: 10.1111/j.1600-0447.1993.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Berra M, Armillotta F, Emidio L. Testosterone decreases adiponectin levels in female-to-male transsexuals. Asian J Androl. 2006;8:725–729. doi: 10.1111/j.1745-7262.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- Bodsworth N, Price R, Davies S. Gonococcal infection of the neovagina in a male-to-female transsexual. Sex Transm Dis. 1994;21:211–212. doi: 10.1097/00007435-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Brown JA, Wilson TM. Benign prostatic hyperplasia requiring transurethral resection of the prostate in a 60-year-old male-to-female transsexual. BJU Int. 1997;80:956–957. doi: 10.1046/j.1464-410x.1997.00342.x. [DOI] [PubMed] [Google Scholar]
- Brown L, Todd JF, Do Luu H-M. Breast implant adverse events during mammography: reports to the food and drug administration. J Women’s Health. 2004;13:371–378. doi: 10.1089/154099904323087042. [DOI] [PubMed] [Google Scholar]
- Bruyninckx E, Mortelmans D, Van Goethem M, et al. Risk factors of pain in mammographic screening. Soc Science Med. 1999;49:933–941. doi: 10.1016/s0277-9536(99)00181-1. [DOI] [PubMed] [Google Scholar]
- Burcombe RJ, Makris A, Pittam M, et al. Breast cancer after bilateral subcutaneous mastectomy in a female-to-male trans-sexual. Breast. 2003;12:290–293. doi: 10.1016/s0960-9776(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Canonico M, Oger E, Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women. Impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- Casella R, BubendorfL , Schaefer DJ, et al. Does the prostate really need androgens to grow? Transurethral resection of the prostate in a male-to-female transsexual 25 years after sex-changing operation. Urol Int. 2005;75:288–290. doi: 10.1159/000087811. [DOI] [PubMed] [Google Scholar]
- Chapin DS. Laparoscopically assisted vaginal hysterectomy in female-to-male transsexuals. Plast Reconstr Surg. 1993;91:962. doi: 10.1097/00006534-199304001-00045. [DOI] [PubMed] [Google Scholar]
- Chesson RR, Gilbert DA, Jordan GH, et al. The role of colpocleisis with urethral lengthening in transsexual phalloplasty. Am J Obstet Gynecol. 1996;175:1443–1450. doi: 10.1016/s0002-9378(96)70088-1. [DOI] [PubMed] [Google Scholar]
- Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- Cohen-Kettenis PT, Gooren LJG. Transsexualism: a review of etiology, diagnosis and treatment. J Psychosom. 1999;46:315–333. doi: 10.1016/s0022-3999(98)00085-3. [DOI] [PubMed] [Google Scholar]
- De Cuypere G, Sjoen G, Beerten R, et al. Sexual and physical health after sex reassignment surgery. Arch Sex Behav. 2005;34:679–690. doi: 10.1007/s10508-005-7926-5. [DOI] [PubMed] [Google Scholar]
- De Cuypere G, Van Hemelrijck M, Michel A, et al. Prevalence and demography of transsexualism in Belgium. Eur Psychiatry. 2007;22:137–141. doi: 10.1016/j.eurpsy.2006.10.002. [DOI] [PubMed] [Google Scholar]
- De Laet CE, Van Hout BA, Burger H, et al. Bone density and risk of hip fracture in men and women: cross sectional analysis. BMJ. 1997;315:221–225. doi: 10.1136/bmj.315.7102.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sutter P, Kira K, Verschoor A, et al. The desire to have children and preservation of fertility in transsexual women: a survey. Int J Transgenderism. 2002;6 [Google Scholar]
- Dean L, Meyer IH, Robinson K, et al. Lesbian, Gay, Bisexual, and Transgender Health: Findings and Concerns. J Gay Lesbian Med Ass. 2000;4:102–151. [Google Scholar]
- Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–899. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- Dizon DS, Tejada-Berges T, Koelliker S, et al. Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol Obstet Invest. 2006;62:226–228. doi: 10.1159/000094097. [DOI] [PubMed] [Google Scholar]
- Doeren M, Samsioe G. Prevention of postmenopausal osteoporosis with estrogen replacement therapy and associated compounds: update on clinical trials since 1995. Hum Reprod Update. 2000;6:419–426. doi: 10.1093/humupd/6.5.419. [DOI] [PubMed] [Google Scholar]
- Dorff TB, Shazer RL, Nepomuceno EM, et al. Successful treatment of metastatic androgen-independent prostate carcinoma in a transsexual patient. Clin Genitourin Cancer. 2007;5:344–346. doi: 10.3816/CGC.2007.n.016. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Nielson CM, Stone KM, et al. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- Elaut E, De Cuypere G, De Sutter P, Gijs L, et al. Hypoactive sexual desire in transsexual women: prevalence and association with testosterone levels. Eur J Endocrin. 2008;158:393–399. doi: 10.1530/EJE-07-0511. [DOI] [PubMed] [Google Scholar]
- Elbers JM, Giltay EJ, Teerlinck T, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrin. 2003;58:562–571. doi: 10.1046/j.1365-2265.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- Evans SF, Davie MW. Low and conventional dose transdermal estradiol are equally effective at preventing bone loss in spine and femur at all postmenopausal ages. Clin Endocrin. 1996;44:79–84. doi: 10.1046/j.1365-2265.1996.637459.x. [DOI] [PubMed] [Google Scholar]
- Fang RH, Chen TJ, Chen TH. Anatomic study of vaginal width in male-to-female transsexual surgery. Plast Reconst Surg. 2003;112:511–514. doi: 10.1097/01.PRS.0000070946.57378.BB. [DOI] [PubMed] [Google Scholar]
- Feldman J, Goldberg J. Transgender Primary Medical Care: Suggested Guidelines for Clinicians in British Columbia. http://www.vch.ca/transhealth/resources/library/tcpdocs/guidelines-primcare.pdf
- Fisk N. Laub D, Gandy P, editors. Proceedings of the second interdisciplinary symposium on gender dysphoria syndrome. Palo Alto, California: Stanford University Press; 1973. Gender dysphoria syndrome (the how, what and why of the disease) pp. 7–14. [Google Scholar]
- Fournier A, Berrino F, Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- Futterweit W, Deligdisch L. Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J Clin Endo Metab. 1986;62:16–21. doi: 10.1210/jcem-62-1-16. [DOI] [PubMed] [Google Scholar]
- Ganley I, Taylor E. Breast cancer in a transsexual man recieving hormone replacement therapy. Br J Surg. 1995;82:341. doi: 10.1002/bjs.1800820319. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Lambert J, Gooren LJ, et al. Sex steroids, insulin and arterial stiffness in women and men. Hypertension. 1999;34:590–597. doi: 10.1161/01.hyp.34.4.590. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Watt B, Loudon N, et al. Vaginal microbial flora in normal young women. BMJ. 1979;1:1450–1453. doi: 10.1136/bmj.1.6176.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin WE, Cummings RH. Squamous metaplasia of the verumontanum with obstruction due to hypertrophia: long-term effects of estrogen on the prostate in an aging male-to-female transsexual. J Urol. 1984;131:553–554. doi: 10.1016/s0022-5347(17)50493-0. [DOI] [PubMed] [Google Scholar]
- Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(Suppl 2):31–36. doi: 10.1159/000087751. [DOI] [PubMed] [Google Scholar]
- Green R. Sexual functioning in post-operative transsexuals: male-to-female and female-tomale. Int J Impot Res. 1998;10(suppl 1):S22–S24. [PubMed] [Google Scholar]
- Greenman Letter to the editor: The endocrine care of transsexual people. J Clin Endocrin Metabol. 2004;89:1014. doi: 10.1210/jc.2003-031620. [DOI] [PubMed] [Google Scholar]
- Hage JJ, Bouman F, de Graaf F, et al. Construction of the neophallus in female-to-male transsexuals: the Amsterdam experience. J Urol. 1993;149:1463–1468. doi: 10.1016/s0022-5347(17)36416-9. [DOI] [PubMed] [Google Scholar]
- Hage JJ, Karim R, Aschemann H, et al. Unfavorable long term results of rectosigmoid neocolpopoiesis. Plast Reconst Surg. 1995;95:842–849. [PubMed] [Google Scholar]
- Hage JJ, Dekker JJ, Karim RB, et al. Ovarian cancer in female-to-male transsexuals: report of two case. Gyn Oncol. 2000;76:413–415. doi: 10.1006/gyno.1999.5720. [DOI] [PubMed] [Google Scholar]
- Haraldsen IR, Haug E, Falch J, et al. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav. 2007;52:334–343. doi: 10.1016/j.yhbeh.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Harder Y, Erni D, Banic A. Squamous cell carcinoma of the penile skin in a neo-vagina 20 years after male-to-female reassignment. Br J Plast Surg. 2002;55:449–451. doi: 10.1054/bjps.2002.3868. [DOI] [PubMed] [Google Scholar]
- Harrison AE, Silenzio VM. Lesbian, bisexual and transgender health care. Glass’s office gynecology. Lippincott, Williams and Wilkins (LWW), Wolters Kluwer; 2005. [Google Scholar]
- Haustein U. Pruritus of the artificial vagina of a transsexual patient caused by gonococcal infection. Hautartz. 1995;46:858–859. doi: 10.1007/s001050050354. [DOI] [PubMed] [Google Scholar]
- Hoenig J, Kenna JC. The prevalence of transsexualism in England and Wales. Br J Psychiatry. 1974;124:181–190. doi: 10.1192/bjp.124.2.181. [DOI] [PubMed] [Google Scholar]
- Hoshaw SJ, Klein PJ, Clark BD, et al. Breast implants and cancer: causation, delayed detection, and survival. Plast Reconstr Surg. 2001;107:1393–1407. doi: 10.1097/00006534-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Hoyt D, Kogan LR. Satisfaction with body image and peer relationships for males and females in a college environment. Sex Roles. 2001;45:199–215. [Google Scholar]
- Hughes IA. Disorders of sex development: a new definition and classification. Best Pract Res Clin Endocrinol Metab. 2008;22:119–134. doi: 10.1016/j.beem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Ilic D, Connor D, Green S, et al. Screening for prostate cancer: A Cochrane systematic review. Cancer causes control. 2007;18:279–285. doi: 10.1007/s10552-006-0087-6. [DOI] [PubMed] [Google Scholar]
- Jin B, Turner L, Walters WAW, et al. Androgen or Estrogen Effect on Human Prostate. J Clin Endocrin Metabol. 1996;81:4290–4295. doi: 10.1210/jcem.81.12.8954029. [DOI] [PubMed] [Google Scholar]
- Kanhai RC, Hage JJ, Bloemena E, et al. Mammary fibroadenoma in male-to-female transsexual. Histopathology. 1999;35:183–184. doi: 10.1046/j.1365-2559.1999.0744c.x. [DOI] [PubMed] [Google Scholar]
- Koenig M, Jejeebhoy S, Singh S, et al. Investigating women’s gynaecological morbidity in India: not just another KAP survey. Reprod Health Matters. 1998;6:1–13. [Google Scholar]
- Lapauw B, Taes Y, Simoens S, et al. Bone composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone. 2009;43:1016–1021. doi: 10.1016/j.bone.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lawrence A. Vaginal Neoplasia in a Male-to-Female Transsexual: Case Report, Review of the Literature, and Recommendations for Cytological Screening. http://www.symposium.com/ijt/ijtvo05no01_01.htm The Int J Transgenderism. 2001;5 [Google Scholar]
- Lawrence AA. Factors associated with satisfaction or regret following male-to-female sex reassignment surgery. Arch Sex Behav. 2003;32:299–315. doi: 10.1023/a:1024086814364. [DOI] [PubMed] [Google Scholar]
- Lemmo G, Garcea N, Corsello S, et al. Breast fibroadenoma in a male-to-female transsexual patient after hormonal treatment. Eur J Surg Suppl. 2003;588:69–71. [PubMed] [Google Scholar]
- Lief HI, Hubschman L. Orgasm in the postoperative transsexual. Arch Sex Behav. 1993;22:145–155. doi: 10.1007/BF01542363. [DOI] [PubMed] [Google Scholar]
- Liguori G, Trombetta C, Bucci S S, et al. Condylomata acuminata of the neovagina in a HIV-seropositive Male-To-Female Transsexual. Urol Int. 2004;73:87–88. doi: 10.1159/000078811. [DOI] [PubMed] [Google Scholar]
- Lips P, Asscheman H, Uitewaal P, et al. The effect of cross-gender hormonal treatment on bone metabolism in male-to-female transsexuals. J Bone Miner Res. 1989;4:657–662. doi: 10.1002/jbmr.5650040503. [DOI] [PubMed] [Google Scholar]
- Markland C. Transsexual Surgery. Ann Obstet Gynecol. 1975;4:209–220. [PubMed] [Google Scholar]
- Mc Intosh SA, Horgan K. Augmentation mammoplasty: effect on diagnosis of breast cancer. J Plast Reconstr Aesthet Surg. 2008;61:124–129. doi: 10.1016/j.bjps.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Marrazzo JM. Evolving issues in understanding and treating bacterial vaginosis. Expert Rev Anti Infect Ther. 2004;2:913–922. doi: 10.1586/14789072.2.6.913. [DOI] [PubMed] [Google Scholar]
- Meyer W III, Bockting W, Kettenis P, et al. The Standards of Care for Gender Identity Disorders – Sixth Version. http://www.symposion.com/ijt/soc_2001/index.htm Int J Transgenderism. 2001;5 [Google Scholar]
- Miglioretti DL, Rutter CM, Geller BM, et al. Effect of breast augmentation on the accuracy of mammography and cancer chracteristics. JAMA. 2004;291:442–450. doi: 10.1001/jama.291.4.442. [DOI] [PubMed] [Google Scholar]
- Miksad A, Bubley G, Church P, et al. Prostate Cancer in a Transgender Woman 41 Years After Initiation of Feminization: Research Letter. JAMA. 2006;296:2316–2317. doi: 10.1001/jama.296.19.2316. [DOI] [PubMed] [Google Scholar]
- Monstrey S, Hoebeke P, Dhont M, et al. Surgical therapy in transsexual patients: a multi-disciplinary approach. Acta chir Belg. 2001;101:200–209. [PubMed] [Google Scholar]
- Monstrey S, Hoebeke P, Dhont M, et al. Radial forearm phalloplasty: a review of 81 cases. Eur J Plast Surg. 2005;28:206–212. [Google Scholar]
- Monstrey S, Hoebeke P, Selvaggi G, et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plastic and Reconstructive Surgery. 2009;124:510–518. doi: 10.1097/PRS.0b013e3181aeeb06. [DOI] [PubMed] [Google Scholar]
- Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrin Metabol. 2003;88:3467–3473. doi: 10.1210/jc.2002-021967. [DOI] [PubMed] [Google Scholar]
- Mueller A, Dittrich R, Binder H, et al. High dose estrogen treatment increases bone mineral density in male-to-female transsexuals receiving gonadotropin-releasing hormone agonist in the absence of testosterone. Eur J Endocrinol. 2005;153:107–113. doi: 10.1530/eje.1.01943. [DOI] [PubMed] [Google Scholar]
- Mueller A, Gooren L. Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2008;159:197–202. doi: 10.1530/EJE-08-0289. [DOI] [PubMed] [Google Scholar]
- Munoz N, Castellsague X, Berrington de Gonzalez A, et al. HPV in the etiology of human cancer. Vaccine. 2006;24(S3):S3/1–S3/10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- Newall AT, Beutels P, Wood JG, et al. Cost-effectiveness analyses of human papillomavirus vaccination. Lancet Inf Dis. 2007;7:289–296. doi: 10.1016/S1473-3099(07)70083-X. [DOI] [PubMed] [Google Scholar]
- Hanlan KA, Dibble SL, Spint M. Total laparoscopic hysterectomy for female-to-male transsexuals. Obstet Gynecol. 2007;110:1096–1101. doi: 10.1097/01.AOG.0000286778.44943.5a. [DOI] [PubMed] [Google Scholar]
- Olyslager F, Conway L. On the calculation of the prevalence of transsexualism. Abstract Book of the WPATH 20th Biennial Symposium, 5/9/2007-8/9/2007, Chicago, USA. 2007 [Google Scholar]
- Oriel KA. Medical care of transsexual patients. J Gay Lesbian Med Assoc. 2000;4:185–194. [Google Scholar]
- Pache TD, Chadha S, Gooren LJ, et al. Ovarian morphology in long-term androgen-treated female-to-male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19:445–452. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Patel V, Pednekar S, Weiss >H, et al. Why do women complain of vaginal discharge? A population survey of infectious and pyschosocial risk factors in a South Asian community. Int J Epidemiol. 2005;34:853–862. doi: 10.1093/ije/dyi072. [DOI] [PubMed] [Google Scholar]
- Pauly I. The current status of the change of sex operation. J Nerv Ment Dis. 1968;147:460–471. doi: 10.1097/00005053-196811000-00003. [DOI] [PubMed] [Google Scholar]
- Pritchard T, Pankowsky A, Crowe J, et al. Breast cancer in a male-to-female transsexual. A case report. JAMA. 1988;259 [PubMed] [Google Scholar]
- Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34:1341–1347. doi: 10.1016/s0959-8049(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Reutrakul S, Ongphiphadhanakul B, Piaseu N, et al. The effects of estrogen exposure on bone mass in male to female transsexuals. Clin Endocrin. 1998;49:811–814. doi: 10.1046/j.1365-2265.1998.00614.x. [DOI] [PubMed] [Google Scholar]
- Revzina NV, DiClemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD & AIDS. 2005;16:528–537. doi: 10.1258/0956462054679214. [DOI] [PubMed] [Google Scholar]
- Roehrborn C, Boyle P, Gould AL, et al. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–589. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- Ross MW, Walinder J, Lundström B, et al. Cross-cultural approaches to transsexualism. Acta Psychiatrica Scand. 1981;63:75–82. doi: 10.1111/j.1600-0447.1981.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Ruetsche AG, Kneubuehl R, Birkhaeuser MH, et al. Cortical and trabecular bone mineral density in transsexuals after long-term cross-sex hormonal treatment: a cross-sectional study. Osteoporos Int. 2005;16:791–798. doi: 10.1007/s00198-004-1754-7. [DOI] [PubMed] [Google Scholar]
- Schachter MB. The natural way to a healthy prostate. New Canaan, CT: Keats Publishing Inc; 1995. [Google Scholar]
- Schlatterer K, Auer DP, Yassourdis A, et al. Transsexualism and osteoporosis. Exp Clin Endocrinol Diabetes. 1998;106:365–368. doi: 10.1055/s-0029-1211999. [DOI] [PubMed] [Google Scholar]
- Selvaggi G, Monstrey S, Ceulemans P, et al. Genital Sensitivity After Sex Reassignment Surgery in Transsexual Patients. Annals of Plastic Surgery. 2007;58:427–433. doi: 10.1097/01.sap.0000238428.91834.be. [DOI] [PubMed] [Google Scholar]
- Sobel JD. Bacterial vaginosis. Annu Rev Med. 2000;51:349–356. doi: 10.1146/annurev.med.51.1.349. [DOI] [PubMed] [Google Scholar]
- Sohn M, Bosinski HA. Gender Identity Disorders: Diagnostic and surgical aspects. J Sex Med. 2007;4:1193–1208. doi: 10.1111/j.1743-6109.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- Soli M, Brunocilla E, Bertaccini A, et al. Male to female gender reassignment: modified surgical technique for creating the neoclitoris and mons veneris. J Sex Med. 2008;5:210–216. doi: 10.1111/j.1743-6109.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- Spinder T, Spijkstra JJ, van den Tweel JG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metabol. 1989;69:151–157. doi: 10.1210/jcem-69-1-151. [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Lachman M, Zverina J, et al. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1999;69:523–527. doi: 10.1210/jcem-69-3-523. [DOI] [PubMed] [Google Scholar]
- Symmers W. Carcinoma of breast in transsexual individuals after surgical and hormonal interference with the primary and secondary sex characteristics. BMJ. 1968;2:83–85. doi: 10.1136/bmj.2.5597.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T'Sjoen G, Weyers S, Taes Y, et al. Prevalence of low bone mass in relation to estrogen treatment and body composition in male-to-female transsexual persons. J Clin Densitom. 2009;12:306–313. doi: 10.1016/j.jocd.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Ter Kuile M, Brauer M, Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS): Psychometric Properties within a Dutch Population. J Sex Marital Therapy. 2006;32:289–304. doi: 10.1080/00926230600666261. [DOI] [PubMed] [Google Scholar]
- Thurston Carcinoma of the prostate in a transsexual. BJU. 1994;73:217. doi: 10.1111/j.1464-410x.1994.tb07503.x. [DOI] [PubMed] [Google Scholar]
- Toolenaar TA, Freundt I, Wagenvoort JH, et al. Bacterial flora of the sigmoid neovagina. J Clin Microbiol. 1993;31:3314–3316. doi: 10.1128/jcm.31.12.3314-3316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JK, Junginger J. Correlates of Lesbian Sexual Functioning. J Women’s Health. 2007;16:499–509. doi: 10.1089/jwh.2006.0308. [DOI] [PubMed] [Google Scholar]
- Tsoi WF. The prevalence of transsexualism in Singapore. Acta Psychiatrica Scand. 1988;78:501–504. doi: 10.1111/j.1600-0447.1988.tb06373.x. [DOI] [PubMed] [Google Scholar]
- Van Haarst E, Newling D, Gooren L, et al. Metastatic prostatic carcinoma in a male-to-female transsexual. Br J Urol. 1998;81:776. doi: 10.1046/j.1464-410x.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- Van Kesteren P, Lips P, Deville W. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81:2227–2232. doi: 10.1210/jcem.81.6.8964856. [DOI] [PubMed] [Google Scholar]
- Van Kesteren P, Meinhardt W, van der Valk P, et al. Effects of Estrogens Only on the Prostates of Aging Men. J Urol. 1996;156:1349–1353. doi: 10.1097/00005392-199610000-00026. [DOI] [PubMed] [Google Scholar]
- Van Kesteren P, Megens JAJ, Asscheman H, et al. Side effects of cross-sex hormone administration in transsexuals. Clin Endocrinol. 1997;47:337–342. doi: 10.1046/j.1365-2265.1997.2601068.x. [DOI] [PubMed] [Google Scholar]
- Van Kesteren P, Lips JM, Gooren L, et al. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol. 1998;48:347–354. doi: 10.1046/j.1365-2265.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Venken K, Ophoff J, et al. Sex Steroids and the Periosteum – Reconsidering the Roles of Androgens and Estrogens in Periosteal Expansion. J Clin Endocrinol Metab. 2006;91:378–382. doi: 10.1210/jc.2005-1766. [DOI] [PubMed] [Google Scholar]
- Verhelst R, Verstraelen H, Claeys G, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor AM, Poortinga J. Psychosocial differences between Dutch male and female transsexuals. Arch Sex Behav. 1988;17:173–178. doi: 10.1007/BF01542666. [DOI] [PubMed] [Google Scholar]
- Vujovic S, Popovic S, Milosevic G, et al. Transsexualism in Serbia: A twenty-year follow-up study. J Sex Med. 2008;6:1018–1023. doi: 10.1111/j.1743-6109.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Walinder J. Incidence and sex ratio of transsexualism in Sweden. Br J Psychiatry. 1971;119:195–196. doi: 10.1192/bjp.119.549.195. [DOI] [PubMed] [Google Scholar]
- Watts NB. Treatment of osteoporosis with biphosphonates. Rheum Dis Clin North Am. 2001;27:197–214. doi: 10.1016/s0889-857x(05)70194-0. [DOI] [PubMed] [Google Scholar]
- Webster G, Sihelnik S, Stone A. Urethrovaginal fistula: a review of the surgical management. J Urol. 1984;132:460–462. doi: 10.1016/s0022-5347(17)49691-1. [DOI] [PubMed] [Google Scholar]
- Weyers S, Selvaggi G, Monstrey S, et al. Two-stage versus one-stage sex reassignment surgery in female-to-male transsexual individuals. Gynecol Surg. 2006;3:190–194. [Google Scholar]
- Weyers S, Monstrey S, Hoebeke P, et al. Laparoscopic hysterectomy as the method of choice for hysterectomy in female-to-male gender dysphoric individuals. Gynecol Surg. 2008;5:269–273. [Google Scholar]
- Weyers S, Decaestecker K, Verstraelen H, et al. Clinical and transvaginal sonographic evaluation of the prostate in transsexual women. Urology. 2009;74:191–196. doi: 10.1016/j.urology.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Weyers S, Elaut E, De Sutter P P, et al. Long-term assessment of the physical, mental, and sexual health among transsexual women. J Sex Med. 2009;6:752–760. doi: 10.1111/j.1743-6109.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- Weyers S, Verstraelen H, Gerris J J, et al. Microflora of the penile skin-lined neovagina of transsexual women. BMC Microbiology. 2009;9:102. doi: 10.1186/1471-2180-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers S, Villeirs G, Van Herreweghe E E, et al. Mammography and breast sonography in transsexual women. European Journal of Radiology. 2009 doi: 10.1016/j.ejrad.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Whittle S, Turner L, Combs R R, et al. Transgender Eurostudy: legal survey and focus on the transgender experience of health care. European region of the International Lesbian and Gay Association (ILGA Europe) ; 2008. [Google Scholar]
- Wilson JD, Roehrborn C. Long-term consequences of castration in men: lessons from the Skoptzy and the eunuchs of the Chinese and Ottoman courts. J Clin Endocrinol Metab. 1999;84:4324–4331. doi: 10.1210/jcem.84.12.6206. [DOI] [PubMed] [Google Scholar]
- Wilson P, Sharp C, Carr S. The prevalence of gender dysphoria in Scotland: a primary care study. Br J Gen Pract. 1999;49:991–992. [PMC free article] [PubMed] [Google Scholar]
- Witten T, Eyler E. Hate crimes and violence against the transgendered. Peace Review. 1999;11:461–468. [Google Scholar]
- Yokoyama M, Seki N, Tamai M, et al. Benign prostatic hyperplasia in a patient castrated in his youth. J Urol. 1989;142:134–135. doi: 10.1016/s0022-5347(17)38685-8. [DOI] [PubMed] [Google Scholar]