Abstract
Three transgenic Anopheles stephensi lines were established that strongly inhibit transmission of the mouse malaria parasite Plasmodium berghei. Fitness of the transgenic mosquitoes was assessed based on life table analysis and competition experiments between transgenic and wild-type mosquitoes. Life table analysis indicated low fitness load for the 2 single-insertion transgenic mosquito lines VD35 and VD26 and no load for the double-insertion transgenic mosquito line VD9. However, in cage experiments, where each of the 3 homozygous transgenic mosquitoes was mixed with nontransgenic mosquitoes, transgene frequency of all 3 lines decreased with time. Further experiments suggested that reduction of transgene frequency is a consequence of reduced mating success, reduced reproductive capacity, and/or insertional mutagenesis, rather than expression of the transgene itself. Thus, for transgenic mosquitoes released in the field to be effective in reducing malaria transmission, a driving mechanism will be required.
Over 3 billion people live under the threat of malaria worldwide. Malaria causes a mortality of over 1 million people each year (World Malaria Report 2005). This underscores the urgent need to develop new tools to fight the disease. The mosquito is the obligatory vector of Plasmodium, the causative agent of malaria. After the mosquito ingests an infected blood meal, the parasites undergo complex sporogonic development that includes invasion of 2 epithelia (midgut and salivary gland). Transmission occurs when the mosquito bites another person and inoculates sporozoites stored in the salivary gland. To control malaria transmission, early efforts have been focusing on the genetics of naturally resistant mosquitoes. The advent of mosquito germ line transformation made the creation of transgenic mosquitoes impaired in malaria transmission a reality (Ito et al. 2002; Moreira et al. 2002). The next major challenge is to devise means to introduce and spread the transgenes that confer refractoriness into wild mosquito populations. To assess the feasibility of this strategy, it will be crucial to determine whether the transgene imposes a fitness load on the mosquito and use this information to design gene-driving strategies (Boete and Koella 2002; Scott et al. 2002; Sinkins and Gould 2006).
It has been proposed that transgenic organisms are less fit because they are evolutionary novelties with reduced viability (Tiedje et al. 1989). Catteruccia et al. (2003) have shown that homozygous transgenic Anopheles stephensi have lower fitness than wild type. Similar conclusions have been reached for transgenic Aedes aegypti (Irvin et al. 2004). Possible causes for reduced fitness observed in the transgenic population include the presence of recessive genes near the transgene insertion point that confers reduced fitness when homozygous (hitchhiking effect), overexpression of a foreign protein (e.g., green fluorescent protein [GFP]) in somatic tissues, and insertional mutagenesis (Marrelli et al. 2006). Recently, we compared the fitness of hemizygous transgenic mosquitoes that secrete into the mosquito midgut either of 2 effector proteins under the control of the blood-inducible carboxypeptidase promoter (Moreira et al. 2004). One effector protein is SM1, a dodecapeptide that binds to the lumenal side of the midgut epithelium and to the surface of the salivary glands while interfering with Plasmodium invasion (Ghosh et al. 2001). The other is phospholipase A2 (PLA2), a protein that also inhibits Plasmodium midgut invasion (Zieler et al. 2001). Heterozygous SM1-expressing transgenic mosquitoes exhibited no detectable fitness load, whereas PLA2-expessing mosquitoes showed reduced fitness (Moreira et al. 2004) and midgut damage (Abraham et al. 2005), presumably as a result of phospholipase enzymatic activity. In another recent study, we found that when fed on mice infected with Plasmodium berghei, the SM1 transgenic mosquitoes exhibited a fitness advantage over sibling nontransgenic mosquitoes, as evidenced by higher fecundity and lower mortality in the transgenic population (Marrelli et al. 2007). All previous fitness studies have neither examined transgenic mosquitoes that carry antiparasite effector genes nor compared the fitness traits of homozygous transgenic mosquitoes carrying effector genes with their nontransgenic counterparts.
Here we report on experiments that measure the fitness of transgenic mosquitoes that express SM1 under the control of the blood-inducible vitellogenin promoter and secrete the protein into the hemocoel. Plasmodium berghei sporozoite invasion of salivary glands is strongly inhibited in these transgenic mosquitoes (85% average, 68–98% range; Li C, Jacobs-Lorena M, unpublished data). We find that the fitness load of the 3 independently derived homozygous transgenic mosquitoes can be attributed to lower mating success possibly derived from hitchhiking effect or insertional mutagenesis and not from a change of intrinsic rate of increase or to a physiological effect of the transgenic protein.
Materials and Methods
Mosquito Strain
The An. stephensi mosquitoes used in the present study probably originated from India, and they have been maintained in laboratory conditions since 1970. Mosquitoes were reared in an insectary regulated at 27°C ± 0.2°C and 80 ± 2% humidity with photoperiod 14:10 h (light/dark). Larvae were reared in sparse density (~250 larvae per 30 × 34 cm tray) with daily supplement of cat food. Adults were reared in metal cage (20 × 20 × 20 cm3) supplemented with 5-ml 1-day-old tap water and 10% honey-saturated cotton on the top of the cage. Tap water was replaced everyday, whereas honey-saturated cotton was covered with a plastic cup to avoid quick evaporation and replaced every 4 days.
Germ Line Transformation of Anopheles stephensi
Germ line transformation used SM1 effector gene and the vitellogenin promoter and followed the methods previously described (Catteruccia et al. 2000; Ito et al. 2002). A mixture of pBac[3×P3-eGFPafm]-AeVg-SM14 (0.5 mg/ml) and the piggyBac helper plasmid (0.3 mg/ml) (Handler and Harrell 1999) was injected into embryos. The surviving adults were pooled into families as follows: each adult transgenic male was crossed with 5–10 nontransgenic virgin females. Each virgin transgenic female was mated with 3–5 nontransgenic males. After mating, females were blood fed and eggs were collected and reared as previously described (Moreira et al. 2002). Transformants were selected by screening cold-immobilized larvae for GFP expression using fluorescence microscopy. Transgenic mosquitoes express GFP resulting from the eye-specific 3×P3 promoter, and a tetramer of the Plasmodium invasion inhibiting SM1 peptide (SM14) (Ito et al. 2002) from the fat body-specific vitellogenin promoter (Kokoza et al. 2001). Three transgenic lines were produced: 1 with a double transgene insertion (VD9) and 2 with a single transgene insertion (VD35 and VD26) after conformation by Southern blots (Li C, Jacobs-Lorena M, unpublished data). The produced transgenic stocks had been kept in hemizygous conditions by crossing transgenic males with virgin wild-type females from laboratory population cages for at least 25 generations. The repeated backcrossing procedure assures that the transgenic populations used for subsequent fitness comparisons had same genetic background as their sibling nontransgenic counterparts. Therefore, despite that the nontransgenic mosquitoes have been colonized in the laboratory for over 3 decades, the fitness comparisons between the transgenic mosquitoes and nontransgenic counterparts were valid because they shared same genetic background.
Selection of Homozygous Transgenic Mosquitoes
To select homozygous lines, 60 hemizygous transgenic males and 40 hemizygous transgenic virgin females from the same line were crossed in a small cage (12 × 12 × 12 cm3). After 24 h, mosquitoes were blood fed and F0 females were individually placed in single cups. Two days later, filter papers were placed in each cup to collect eggs. Eggs were hatched and larvae reared under the conditions described above. The fourth-instar F1 larvae were placed individually in a single cup. After eclosion, 40 mating pairs (1 transgenic F1 virgin female and 1 transgenic F1 male) were set up. Progeny from individual F1 pairs were reared as one population to the F2 generation. Individual F2 adults were examined under a fluorescent microscope to ensure all mosquitoes were transgenic that carried GFP. To confirm that the mosquitoes were homozygous, a bioassay was conducted by crossing between transgenic males with virgin nontransgenic females. Because GFP marker is dominant, all individuals in the offspring population from homozygous transgenic lines should carry GFP. Three homozygous lines (VD9, VD35, and VD26) were obtained in this manner. Subsequent fitness comparisons used these 3 homozygous transgenic lines and the nontransgenic population from which the transgenic mosquitoes were derived.
Egg Hatchability, Larval-to-Pupal Viability, and Adult Survivorship
Twenty newly eclosed homozygous transgenic males and females were selected and used to set up pairwise mating for each of the 4 experimental lines (nontransgenic, VD9, VD26, and VD35). Female mosquitoes were then exposed to anesthetized mouse for blood feeding. Eggs were collected in oviposition cups 2 days after blood feeding. To assess egg viability, about 100 newly laid eggs were collected on a wet filter paper and kept in a wet Petri dish. After 2 days at 27°C, the number of larvae and nonhatched eggs was counted with the aid of a dissecting microscope. To estimate larval-to-pupal viability, 70 newly hatched larvae from each line were reared under the same conditions, dead larvae were removed daily till all developed into pupae. To assess adult sex ratio, 200 pupae of each line were randomly selected and placed into different cages (20 × 20 × 20 cm3). Eclosed adults were assessed to be female or male with the aid of a microscope. To estimate adult survivorship, 200 adults were pooled in a cage, the total number of surviving adults in the cage was recorded, and dead adults were removed daily with a hand vacuum from the cage till all died. Two replicates for each transgenic and nontransgenic line were performed for the above experiments. Care was taken that the rearing conditions (larval density, food, and temperature) were optimal, and mosquitoes from all lines were reared at the same time and in the same room. Therefore, differences in the fitness traits among the transgenic lines are likely due to their genetic differences or gene and environmental interactions but not due to environment itself.
Measurement of Amount of Blood Ingested
Mosquitoes were exposed to a noninfected mouse for 45 min. The guts of 10 nontransgenic and 10 transgenic mosquitoes were dissected, and the blood contents were collected in 200 µl 1 × phosphate-buffered saline (PBS). The gut sheets were further rinsed in 800 µl 1 × PBS and then discarded. The hemoglobin content in the collected blood was determined as previously described (Suzuki 1998).
Life Table Analysis
This experiment was to determine the overall net reproductive rate of the transgenic lines in comparison to the nontransgenic counterpart. Approximately 12 h after eggs were laid, 217–253 first-instar larvae from each homozygous transgenic line or from nontransgenic mosquitoes were counted and transferred to a plastic rearing container (50 × 35 × 30 cm3). The number of live larvae was counted twice a day, and the developmental stage of all larvae was recorded based on the size of the head capsule, until they pupated. Pupae were then transferred to a mosquito cage (25 × 25 × 25 cm3), and the mosquitoes that emerged were provided a 10% sugar solution daily. The number and sex of the newly emerged mosquitoes were determined every 12 h. Live males and females were recorded every day. Mosquitoes were blood fed every 4 days after emergence of the first female. Eggs from blood-fed females were collected and counted, and the percentage of eggs that successfully hatched was counted. This experiment lasted till no live mosquitoes were observed.
Male Mating Success
Homozygous transgenic and nontransgenic male mosquitoes (5 each) were placed within 12 h of eclosion in a cage (25 × 25 × 25 cm3) with a single virgin nontransgenic female for 2 days. After providing a blood meal, eggs were collected from each female and reared to larvae as described above. Fourth-instar larvae were immobilized in ice-cold water and observed for GFP expression with the aid of a fluorescent microscope. Ten replicates were performed for each line. Male mating success was determined by progeny phenotype and measured as the portion of females being sired by the genotype. The progeny from transgenic and nontransgenic males could be readily distinguished by the presence or absence, respectively, of the green florescent marker.
Transgene Frequency Changes in Cage Populations
A total of 200 mosquitoes (50 in each of the following 4 genotypes: nontransgenic and transgenic homozygous males and wild type and homozygous transgenic virgin females) were placed in a cage and provided with 10% sugar solution. The mosquitoes were blood fed, and their eggs were used to start the next generation (F1). After the adults eclosed, 100 male and 100 female mosquitoes were picked at random to start the following generation (F2). The populations were maintained for a total of 6 generations for each of the 3 transgenic lines (VD9, VD35, and VD26). Transgenic frequency was quantified at each generation by examining randomly selected adult females for green eye fluorescence. Presence or absence of fluorescence and the intensity of fluorescence allowed the identification of the mosquitoes as nontransgenic, hemizygous transgenic, and homozygous transgenic (Catteruccia et al. 2003). There were 2 replicates for each mixture between a transgenic line and its nontransgenic counterpart.
Data Analysis
Mann–Whitney test was applied to compare nontransgenic and transgenic mosquitoes for egg hatchability (proportion of eggs that hatched into larvae), larval viability (proportion of first-instar surviving to pupal stage), larval-to-adult developmental time (for both male and female), lifetime fecundity (number of eggs laid) per female, mean reproductive longevity, and 3 life table indices (see below). The duration of the adult reproductive life was calculated as the number of days elapsed between the laying of the first and the last batch of eggs. A female was considered infertile when no more eggs were laid after 2 consecutive blood meals. Adult survivorship was analyzed using the Kaplan– Meier survival analysis. Chi-square test was applied to test mating success between nontransgenic and transgenic males.
The 3 life table indices, including net reproductive rate (R0), cohort generation time (Tc), and intrinsic rate of population growth (rm), were estimated from data obtained from life table analysis. Net reproductive rate is defined as the total number of offspring that an average individual will produce during its lifetime. Generation time is defined as the mean period of time elapsing between the birth of an individual and the birth of its offspring. Intrinsic rate of population growth is the instantaneous per-capita rate of increase. R0 is calculated as ∑lxmx, where lx is the proportion of the cohort surviving to age x and mx is the average fecundity at age x. Tc is calculated as ∑xlxmx/∑lx mx, where x is mosquito age. This rm is calculated as ln(R0/Tc). Transgene frequency was calculated using the standard population genetic method, and the statistical difference between the observed transgene frequency and the expected frequency in the cage studies were conducted using the chi-square test.
Results
Anopheles stephensi Transformation
Of 286 embryos injected with the AeVg-SM14 construct, 49 adults were obtained, from which 3 independent transgenic lines were established. Three independent homozygous transgenic lines were subsequently established from these lines, and they were labeled VD9 (from the VD9 line, double insertion), VD35 and VD26 (from the VD3 and VD13 lines, respectively, single insertions). We have not observed nontransgenic mosquitoes from any of the 3 homozygous lines during the subsequent 2 years (observation of more than 20 generations with more than 500 adult mosquitoes per generation). This observation confirmed that the mosquitoes were homozygous for the insertion and that the transgene is stably integrated.
Egg Hatching, Larval-to-Pupae Viability, and Adult Survivorship
No differences between transgenic and nontransgenic lines were detected in egg hatchability, larval-to-pupae viability, or adult survivorship based on Kaplan–Meier survival analysis (Table 1). There was also no difference in sex ratio when adults eclosed (results not shown).
Table 1.
Comparison of egg hatchability, larval-to-pupal viability, and daily adult survivorship between homozygous transgenic and nontransgenic Anopheles stephensi mosquitoes
| Mosquito line | Replicate | Egg hatchability (%)a |
Larval-to-pupal viabilityb |
Adult survivorshipb,c |
|---|---|---|---|---|
| Nontransgenic | 1 | 76.9 (n = 104) | 0.97 (n = 70) | 0.93 (n = 200) |
| 2 | 79.1 (n = 91) | 0.98 (n = 70) | 0.93 (n = 200) | |
| Transgenic VD9 | 1 | 62.1 (n = 103) | 0.97 (n = 70) | 0.92 (n = 200) |
| 2 | 81.7 (n = 71) | 0.99 (n = 70) | 0.92 (n = 200) | |
| Transgenic VD35 | 1 | 80.3 (n = 76) | 0.96 (n = 70) | 0.88 (n = 200) |
| 2 | 74.5 (n = 94) | 0.99 (n = 70) | 0.89 (n = 200) | |
| Transgenic VD26 | 1 | 75.7 (n = 74) | 0.99 (n = 70) | 0.89 (n = 200) |
| 2 | 70.9 (n = 86) | 0.99 (n = 70) | 0.91 (n = 200) |
The number of total eggs for each experiment is given in parenthesis. No significant difference was detected for egg hatchability (P > 0.05 between nontransgenic and all transgenic mosquitoes).
The number of individuals is given in parenthesis. No significant difference was detected for larval-to-pupal viability (P > 0.05 between nontransgenic and transgenic mosquitoes).
Developmental Time, Female Fecundity, and Reproductive Longevity
Small but significant differences were observed in the duration of both male and female larval development. Compared with wild-type mosquitoes, all 3 transgenic mosquito lines require a significantly longer time to reach the adult stage, whereas no significant differences were detected among the 3 transgenic lines (Table 2).
Table 2.
Comparison of larval-to-adult development time, female lifetime fecundity, and mean reproductive longevity among homozygous transgenic and nontransgenic Anopheles stephensi mosquitoes
| Mosquito line | Larval-to-adult development time in days (female) |
Larval-to-adult development time in days (male) |
Lifetime fecundity per female* |
Mean reproductive longevity** |
|---|---|---|---|---|
| Nontransgenic | 10.7 ± 0.5 (n = 90)a | 10.4 ± 0.5 (n = 119)a | 190.9 ± 15.4a | 17.2 ± 1.4a |
| Transgenic VD9 | 11.0 ± 0.2 (n = 101)b | 10.9 ± 0.3 (n = 112)b | 183.1 ± 17.1a | 16.3 ± 1.4b |
| Transgenic VD35 | 11.1 ± 0.3 (n = 78)b | 10.8 ± 0.4 (n = 88)b | 104.0 ± 7.8b | 12.0 ± 1.9c |
| Transgenic VD26 | 11.0 ± 0.3 (n = 93)b | 10.8 ± 0.4 (n = 103)b | 114.9 ± 10.6c | 11.4 ± 2.0c |
Values are means ± standard deviation (SD). Letters following the numerical values in a column indicate the results of multiple comparison tests; the values with the same letter were not statistically significant.
Lifetime fecundity is the number of eggs laid per female during her entire reproductive life. Number of females assayed was 40.
Mean reproductive longevity is the average number of days elapsed between the first and the last batch of eggs laid. Number of females assayed was 40.
Nontransgenic females laid significantly more eggs than VD35 and VD26 but not VD9 transgenic females during their lifetime (Table 2). We also observed significant differences in fecundity among the 3 transgenic mosquitoes (Table 2), even though the amount of blood ingested by females from these lines was similar. Significant differences in reproductive longevity were observed between non-transgenic and transgenic mosquitoes and between double-insertion transgenic VD9 and single-insertion transgenic VD35 or VD26 (Table 2).
Net Reproductive Rate, Generation Time, and Intrinsic Rate of Population Growth
The net reproductive rate of single-insertion transgenic VD35 and VD26 populations was consistently lower than nontransgenic population and double-insertion VD9 population, but they were not statistically significant (Table 3). Similar results were found for generation time and intrinsic rate of growth.
Table 3.
Life table parameters of transgenic and nontransgenic Anopheles stephensi
| Mosquito line | Net reproductive rate (R0) |
Generation time (Tc) |
Intrinsic rate of growth (rm) |
|---|---|---|---|
| Nontransgenic | 58.52 ± 13.02 | 24.38 ± 1.28 | 0.1655 ± 0.0005 |
| VD9 | 51.23 ± 5.04 | 23.72 ± 1.09 | 0.1655 ± 0.0015 |
| VD35 | 32.58 ± 4.58 | 21.18 ± 1.16 | 0.1644 ± 0.0008 |
| VD26 | 36.36 ± 0.74 | 21.80 ± 0.16 | 0.1648 ± 0.0021 |
Data are mean ± SD. No significant differences were observed by the Mann–Whitney test for the 3 parameters between nontransgenic and the homozygous transgenic lines.
Mating Success
Mating success was evaluated using a competition assay in which an equal number of transgenic and nontransgenic males were placed in a cage with a single virgin non-transgenic female, followed by assessment of mating success via progeny phenotype (GFP fluorescence). We found that males from the 3 transgenic lines exhibited lower but statistically insignificant mating success rate than the nontransgenic counterparts (Table 4).
Table 4.
Male mating success of homozygous transgenic and nontransgenic Anopheles stephensi mosquitoes
| Male genotype | Percentage of progeny produced by transgenic males (%) |
Relative mating success of transgenic line to nontransgenic line* |
|---|---|---|
| Transgenic VD9 | 46 ± 5.2 (n = 100) | 0.85 |
| Transgenic VD35 | 44 ± 5.2 (n = 100) | 0.79 |
| Transgenic VD26 | 42 ± 4.2 (n = 100) | 0.72 |
Data are mean ± SD. No significant differences were observed between nontransgenic and transgenic mosquitoes for any of the 3 transgenic lines.
Relative mating success is calculated as the ratio of percentage of progeny from transgenic males to wild-type males. For example, VD9 relative mating success is calculated as: 46/(100 – 46).
Transgene Population Dynamics in Cage Experiments
The observation that homozygous transgenic lines showed a significant reduction in fecundity prompted us to conduct cage population experiments to assess the competitiveness of the transgenic mosquitoes. The experiments started by placing in a cage an equal number of homozygous transgenic mosquitoes of one sex and wild-type mosquitoes of the opposite sex. These mosquitoes were maintained without selection and at each generation, transgene frequency was estimated by determining the phenotype (homozygous transgenic, hemizygous transgenic, or non-transgenic) of a randomly selected sample of 200 mosquitoes. If the mixed populations were in Hardy–Weinberg equilibrium, one would expect transgene frequency to be 0.5 and the frequency of homozygous mosquitoes to remain at 0.25 and of hemizygous at 0.5.
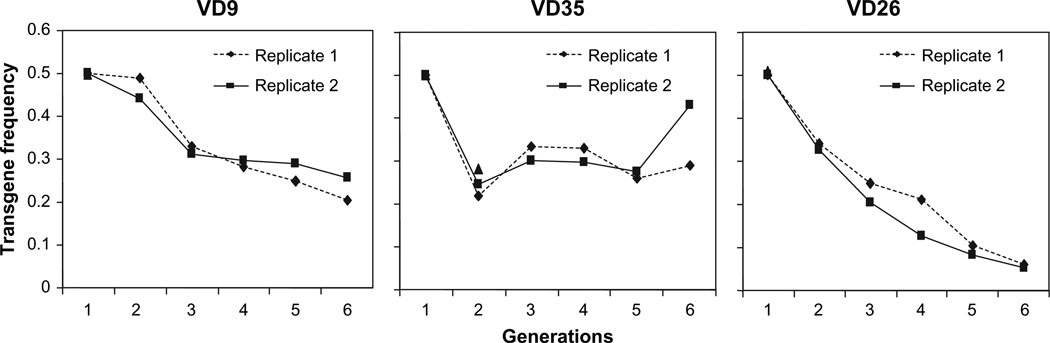
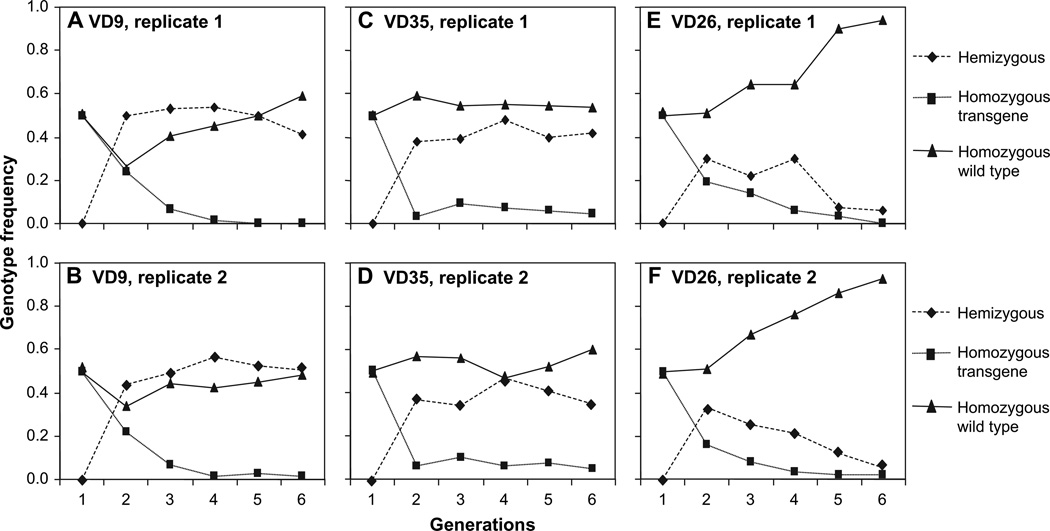
We found that by generation 6, transgene frequencies were significantly lower than the expected frequency of 0.5 (Figure 1; For VD9, χ2 = 2418, degree of freedom [df] = 1, P < 0.0001; for VD35, χ2 = 33 356, df = 1, P < 0.0001; and for VD26, χ2 = 1108, df = 1, P < 0.0001). Further, examination of the genotype frequency changes revealed that the frequency of hemizygotes remained at around 0.5 for VD9 and VD35 lines (Figure 2). The frequency of homozygous transgenic genotypes was reduced rapidly below the 0.25 expected value for all 3 transgenic lines. For VD26 that exhibited the lowest fecundity and male mating success rate, the wild-type frequency gradually increased and reached to ~0.9 at generation 6, significantly higher than the expected 0.5 value (P < 0.0001) (Figure 2).
Figure 1.
Changes of transgene frequency as a function of generation number in population cage experiments. An equal number (50 virgin males and 50 virgin females) of transgenic and nontransgenic mosquitoes were placed in a cage to initiate the experiment. At each generation, transgene frequency was assessed by measuring the presence or absence of the transgene marker protein (GFP) with a fluorescence microscope. The experiment had 2 replicates.
Figure 2.
Genotype frequency changes in the population cage studies as a function of generation number.
Discussion
The feasibility of genetic manipulation of mosquito vectorial capacity has been demonstrated (Ito et al. 2002; Kim et al. 2004), and several methods to drive refractory genes into natural populations have been proposed (Durvasula et al. 1997; Curtis and Sinkins 1998; Turelli and Hoffmann 1999; Sinkins and Gould 2006; Chen et al. 2007; Huang et al. 2007). However, before contemplating the implementation of any of these methods in the field, it is important to evaluate the parameters affecting their success, such as fitness and effectiveness of the effector proteins (Boete and Koella 2003), in addition to the social and ethical issues with the field release of genetically modified mosquitoes (Touré and Manga 2006).
In this study, we generated 3 independent stable homozygous transgenic lines that secrete the SM1 peptide into the hemocoel and inhibit sporozoite invasion of the salivary gland. Advantages of using homozygous lines for future genetic approach to control vector-borne diseases include: 1) effector gene expression in homozygous mosquitoes is expected to be stronger and more effective than in hemizygous mosquitoes, 2) mass rearing of homozygous mosquitoes for an eventual field release is much easier than rearing of hemizygous mosquitoes, 3) introgression of transgenes in the field should be more efficient with homozygous than with hemizygous mosquitoes, and 4) the presence of hitchhiking effects of potential recessive deleterious mutations near the site of transgene insertion may only be detected in homozygous mosquitoes and can be avoided by utilizing a more favorable insertional event. That we succeeded in isolating only 3 homozygous lines from 120 hemizygous mating pairs, a much lower number than predicted by Mendelian assortment (13 expected), raises the possibility that lethal recessive genes or genes imposing a substantial fitness load may reside in the general vicinity of the transgenes.
Fitness load has been detected in homozygous transgenic mosquitoes (Catteruccia et al. 2003; Irvin et al. 2004) but not in hemizygous mosquitoes (Moreira et al. 2004). Several events could be associated with homozygosity, such as founder effects and inbreeding depression. Thus, fitness tests with homozygous transgenic mosquitoes may reveal fitness load not directly derived from expression of the transgene itself. Moreover, other factors, such as abundant accumulation of a foreign protein (GFP) in the cytoplasm of a wide variety of cell types (Catteruccia et al. 2003; Irvin et al. 2004), could contribute to decreased fitness (Liu et al. 1999).
When transgenic and nontransgenic mosquitoes were reared together in cage experiments, we found that hemizygous VD9 and VD35 were rather stable whereas that of VD26 was reduced gradually (Figure 1). This is consistent with tested fitness parameters in which fecundity, male mating success rate and net reproductive rates of VD26 were substantially lower than the nontransgenic counterparts and the VD9 and VD35 lines. The reduction of transgene frequency is not due to transgene instability because for over 2 years, we have never observed a nontransgenic mosquito among the homozygous mosquito populations. Rather, the following factors may account for the frequency decrease. First, homozygous transgenic males compete less effectively for females than wild-type males (Table 4) resulting in fewer transgenic progeny. Second, slower development of homozygous larvae delays attainment of sexual maturity (Table 2); thus, wild-type males may be more competitive to mate than transgenic males due to earlier eclosion. Third, lower fecundity of some of the homozygous transgenic mosquito lines (Table 2) may reduce representation of transgenic mosquitoes in the population. However, the adverse effects associated with homozygous mosquitoes may not pertain to their hemizygous counterparts. For example, in Drosophila melanogaster the effect of P element insertion on viability was completely recessive (Mackay et al. 1992; Lyman et al. 1996).
The observation that our homozygous transgenic mosquitoes were quickly replaced by wild-type mosquitoes, whereas in 2 out of 3 tested mosquito lines heterozygotes persisted with nontransgenic mosquitoes (Figure 2), supports the idea that recessive adverse traits are associated with each of the transgenic lines. That in previous experiments, a fitness load was not observed in hemizygous mosquitoes (Moreira et al. 2004) is consistent with this interpretation. Moreover, the difference in kinetics of the 3 hemizygous lines strongly suggests that genetic elements residing near the transgene insertion point, and not expression of the transgenes themselves, affect the fitness of the transgenic mosquitoes. An important conclusion from our experiments is that for eventual field applications, multiple lines should be produced and the one with the lowest fitness cost selected because each line exhibits a different fitness load. In addition, our experiments highlight the need to use effector genes that minimize mosquito fitness costs and to select lines with integration events that do not reside near loci that confer high fitness costs when homozygous.
Acknowledgments
Funding
National Institutes of Health (AI031478).
Excellent technical support by Neil Cheddie and Jessica Brown is gratefully acknowledged. We thank Dr. Daniel Tisch at Center for Global Health and Diseases for help with Statistical Analysis and 2 anonymous reviewers for valuable comments on the manuscript.
References
- Abraham EG, Donnelly-Doman M, Fujioka H, Ghosh A, Moreira L, Jacobs-Lorena M. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol Biol. 2005;14:271–279. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Boete C, Koella JC. A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J. 2002;1:3. doi: 10.1186/1475-2875-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boete C, Koella JC. Evolutionary ideas about genetically manipulated mosquitoes and malaria control. Trends Parasitol. 2003;19:32–38. doi: 10.1016/s1471-4922(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Catteruccia F, Godfray HC, Crisanti A. Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science. 2003;299:1225–1227. doi: 10.1126/science.1081453. [DOI] [PubMed] [Google Scholar]
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- Curtis CF, Sinkins SP. Wolbachia as a possible means of driving genes into populations. Parasitol. 1998;116(Suppl):S111–S115. doi: 10.1017/s0031182000084997. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, Richards FF, Beard CB. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc Natl Acad Sci USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM, Harrell RA2nd. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Magori K, Lloyd AL, Gould F. Introducing desirable transgenes into insect populations using Y-linked meiotic drive—a theoretical assessment. Evolution Int J Org Evolution. 2007;61:717–726. doi: 10.1111/j.1558-5646.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- Irvin N, Hoddle MS, O’Brochta DA, Carey B, Atkinson PW. Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. Proc Natl Acad Sci USA. 2004;101:891–896. doi: 10.1073/pnas.0305511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Kim W, Koo H, Richman AM, Seeley D, Vizioli J, Klocko AD, O’Brochta DA. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J Med Entomol. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ. Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Lyman RF, Lawrence F, Nuzhdin SV, Mackay TF. Effects of single P-element insertions on bristle number and viability in Drosophila melanogaster. Genetics. 1996;143:277–292. doi: 10.1093/genetics/143.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF, Lyman RF, Jackson MS. Effects of P element insertions on quantitative traits in Drosophila melanogaster. Genetics. 1992;130:315–332. doi: 10.1093/genetics/130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci USA. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli MT, Moreira CK, Kelly D, Alphey L, Jacobs-Lorena M. Mosquito transgenesis: what is the fitness cost? Trends Parasitol. 2006;22:197–202. doi: 10.1016/j.pt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Wang J, Collins FH, Jacobs-Lorena M. Fitness of anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics. 2004;166:1337–1341. doi: 10.1534/genetics.166.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW, Takken W, Knols BG, Boete C. The ecology of genetically modified mosquitoes. Science. 2002;298:117–119. doi: 10.1126/science.298.5591.117. [DOI] [PubMed] [Google Scholar]
- Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat Rev Genet. 2006;7:427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Determination of human hemoglobin in blood based on its spectral change due to the solvent effect of ethanol. Anal Sci. 1998;14:1013–1016. [Google Scholar]
- Tiedje JM, Colwell RK, Grossman YL, Hodson RE, Lenski RE, Mack RN, Regal PJ. The planned introduction of genetically engineered organisms: ecological considerations and recommendations. Ecology. 1989;70:298–315. [Google Scholar]
- Touré YT, Manga L. Ethical, legal and social issues in the use of genetically modified vectors for disease control. In: Knols BGJ, Louis C, editors. Bridging laboratory and field research for genetic control of disease vectors. Dordrecht (The Netherlands): Springer; 2006. pp. 221–225. [Google Scholar]
- Turelli M, Hoffmann AA. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- Zieler H, Keister DB, Dvorak JA, Ribeiro JM. A snake venom phospholipase A (2) blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J Exp Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]