Abstract
Adipocytes are important but underappreciated components of bone marrow microenvironment, and their numbers greatly increase with age, obesity, and associated metabolic pathologies. Age and obesity are also significant risk factors for development of metastatic prostate cancer. Adipocytes are metabolically active cells that secrete adipokines, growth factors, and inflammatory mediators; influence behavior and function of neighboring cells; and have a potential to disturb local milleu and dysregulate normal bone homeostasis. Increased marrow adiposity has been linked to bone marrow inflammation and osteoporosis of the bone, but its effects on growth and progression of prostate tumors that have metastasized to the skeleton are currently not known. This review focuses on fat-bone relationship in a context of normal bone homeostasis and metastatic tumor growth in bone. We discuss effects of marrow fat cells on bone metabolism, hematopoiesis, and inflammation. Special attention is given to CCL2- and COX-2-driven pathways and their potential as therapeutic targets for bone metastatic disease.
Keywords: Prostate cancer, Bone metastasis, Adipocytes, Inflammation, COX-2, CCL2
1 Introduction
Bone is a major component of the system that regulates energy metabolism [1, 2]. It is also a major site of metastasis from prostate cancer [3]. Bone metastases occur in 75–80 % of prostate cancer patients and have devastating consequences including bone fractures, pain, hypercalcaemia, and spinal cord compression [4, 5]. Age, obesity, and associated metabolic conditions are considered significant risk factors for aggressive prostate cancer (PCa) [6–15]. Almost 50 % of men with metastatic (M1) PCa are age 75 or older [14]. Independently of age, obesity increases the risk of developing high-grade PCa [16–19], having biochemical recurrence and disease progression after radical prostatectomy [20–23] and radiation treatment [24, 25], as well as increased rate of metastasis and PCa-specific death [26–28]. Notably, risk of developing metastatic disease appears to be 2–3-fold higher in obese and overweight compared to normal-weight men receiving the same treatment [29]. The mechanisms behind obesity-induced changes in the bone microenvironment and their impact on metastatic processes are not well understood.
Adiposity-driven chronic inflammation and oxidative stress are already known risk factors in many cancers including prostate [30–32]. They are also extremely likely to impact both the predisposition and the ability of the tumor to grow and thrive in the skeleton. Increased marrow adiposity is a known culprit in accelerated bone resorption and osteoporosis. Notably, the preferred sites for prostate tumor growth in the bone are the axial skeleton and long bone metaphyses, sites known to be under active remodeling and to have increased marrow cellularity [33]. The increased metastatic potential has been observed in response to experimental treatment with calciotropic hormone, androgen ablation, or cyclophosphamide [34–36]. An adipocyte-rich metabolically active red bone marrow appears to be particularly attractive to metastatic cells colonizing the bone [37, 38]. When enriched in metabolically active fat, the bone marrow niche has a potential to accelerate these events and become even more conducive to tumor growth and survival.
2 Bone-fat relationship: impact of marrow adiposity on bone homeostasis
2.1 Age-induced effects on bone
It is becoming recognized that the relationship between bone and fat formation is reciprocal [39, 40]. Bone marrow adipocytes and osteoblasts share common progenitor cells, known as bone marrow mesenchymal stromal cells (MSCs) [41, 42]. Their lineage commitment appears to be dynamically regulated by the presence of adipogenic (e.g., PPARγ2) vs. osteogenic (e.g., Runx2, Cbfa1) factors in the bone microenvironment [39, 43]. Adipocyte numbers in the bone marrow strongly correlate with age [39, 43–45]. Bone marrow of newborn infants is primarily hematopoietic and contains no fat, whereas 70 % of appendicular skeleton of adults is occupied by adipocytes [43, 45]. Parallel to an increase in fat content, bone acquisition is reduced during growth, and the osteoblast numbers and bone mass decrease [1, 43, 46]. Adipocyte-derived fatty acids accelerate osteoclast differentiation and prolong osteoclast survival [47], while inhibiting differentiation of MSCs to osteoblasts [48]. This is in line with observations from mouse models of aging, where increased adiposity and associated enhancement of PPARγ2 expression, as well as reduced Cbfa1 levels, correlate with decreased bone mass [49, 50]. In addition to changes in MSC fate, aging bone is associated with increases in osteoclast differentiation, process driven by PPARγ and pro-osteoclastic cytokines: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) [43, 44, 51, 52]. Together, these age-related changes in adipo-, osteoblasto-, and osteo-clastogenesis pathways have been linked to low bone mineral density (BMD) and osteoporosis [43, 50].
2.2 Obesity and bone
Effects of age on bone metabolism and function are further complicated by obesity and associated metabolic pathologies. Traditionally, obesity was thought to be protective against dysregulated bone metabolism and osteoporosis, mainly because of positive impact of body weight on bone formation [53, 54]. This has been attributed specifically to mechanical loading-driven proliferation of osteoblasts and osteocytes through Wnt/β-catenin pathway [53, 55]. However, the fact that bone marrow fat localizes to the trabecular area, the site of active bone remodeling, indicates its involvement in bone degradation [1]. Indeed, emerging evidence is increasingly suggesting that expanded adipose tissue mass positively correlates with low BMD [53], although the link between adiposity and increased risk of osteopenia and bone fractures appears to be age-, site-, and sex-specific [53, 56]. In men specifically, the risk of osteoporotic fractures positively correlates with percent body fat, waist circumference, and waist-to-hip ratio [56–58]. Notably, men also have larger amounts of bone marrow fat than age-matched women [1, 59, 60], an observation that further supports the link between marrow adiposity and accelerated bone remodeling. Additional evidence of negative impacts of obesity on bone health comes from diet-induced obesity mouse models. Increased marrow adiposity in obese mice is accompanied by decreases in trabecular bone volume and overall reduced BMD [61–63]. These structural changes are paralleled by increases in osteoclast activity, upregulation of bone-degrading proteases such as cathepsin K, and escalated levels of proinflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor α, (TNF-α) [53, 61–65]. Unfortunately, due to lack of controlled studies on effects of obesity on bone health in humans, the relationship between adiposity and bone turnover remains complex and controversial.
2.3 Marrow adipocytes and bone metabolism: tale of two adipokines
Bone marrow fat, known as yellow adipose tissue (YAT), is proposed to have a mixed white (WAT) and brown fat (BAT) characteristics [39, 66], or potentially even form a distinct class with unique properties [67]. The BAT-like phenotype appears to be involved in providing energy for hematopoietic and mesenchymal compartments [1]. In fact, it has been hypothesized that bone marrow fat can act as a localized energy reservoir and turn on de novo osteogenesis in emergency situations [1, 68]. Its WAT-like functions involve clearing and storing circulating triglycerides and regulating fatty acid metabolism [1, 69, 70]. This suggests that fat cell involvement in regulating events in the bone microenvironment is dynamic and complex.
For a long time, adipocytes have been considered as passive occupants of bone marrow niche or cells filling the spaces after trabecular bone loss [39, 43, 71]. In fact, adipogenesis was suggested to be a default pathway for MSCs that were not able to differentiate into osteoblasts or chondrocytes [43], or a support system in a form of heat for hematopoietic cell development [1, 68]. However, there is a recently growing understanding that bone marrow fat is not inert; it serves as an insulin-sensitive endocrine tissue that affects bone mass, energy expenditure, and insulin metabolism [72, 73]. Marrow adipocytes secrete hormones, cytokines, and fatty acids that have profound effects on metabolism and function of other neighboring cells in the bone microenvironment [43, 45, 61, 63, 64, 74]. Fat cells, including those within bone marrow space, are a significant source of leptin and adiponectin, the adipokines whose receptors are expressed by osteoclasts and osteoblasts [1]. Both of these hormones have been shown to regulate processes in the bone.
Action of leptin on the bone appears to have both positive and negative consequences and is not fully understood [1, 53]. Circulating leptin levels increase in obesity [53, 75], but their correlation with bone mass and fracture risk in humans is not conclusive [1, 76], possibly due to leptin resistance [77]. A positive link has been demonstrated between serum leptin levels and BMD, especially in women, yet a number of other studies suggested no correlation [78]. Several in vitro studies demonstrated positive effects of this peptide hormone on osteoblast proliferation and suppression of osteoblast-dependent osteoclast recruitment [79–81]. In mice, a majority of studies indicated that leptin has a negative influence on bone metabolism and function stemming from its ability to enhance the sympathetic output to bone from the hypothalamus [77]. Yet, a number of other studies reported increased bone formation rate, higher mineral content and mineral density, and reduced number and size of bone marrow adipocytes that appear to be a result of peripheral effects of leptin on bone [1, 77, 82, 83]. In line with these results, ob/ob and db/db mice, both of which are leptin receptor-deficient, exhibit reduced bone mass coupled with significant increase in the number and size of adipocytes in the femoral marrow, suggesting anabolic effects of adipocyte-derived leptin on bone [1,78].
Adiponectin (ACRP30) is a peptide hormone with pivotal roles in glucose metabolism and energy homeostasis [84]. It circulates at much higher concentrations than other adipocyte-derived factors, and its levels are clearly inversely proportional to body mass index (BMI) and visceral adiposity [84, 85]. Its structure is surprisingly similar to that of TNF-α, a cytokine with dynamic roles in regulation of energy metabolism and insulin sensitivity [86]. This similarity may be the potential mechanism behind adiponectin’s ability to mitigate the negative effects of TNF-α on insulin signaling [87]. Despite having clearly defined roles in glucose metabolism, adiponectin’s effects on bone, similarly to those of leptin, are controversial and a subject on ongoing debate [1]. Based on a number of clinical studies, circulating levels of this hormone negatively correlate with BMD, particularly in older adults [1, 88], although a positive association between ACRP30 levels and fracture risk is only apparent in older men, and not older women, indicating potential effects of sex hormones in this process [1, 76]. Adding to the complexity of adiponectin’s effects on bone are the observations from animal studies showing only transient or no effects on bone mass, and the parallel in vitro results demonstrating inhibitory effects of this adipokine on osteoclastogenesis and promoting effects on osteoblastogenesis [1, 86, 88–90]. In line with in vitro findings, the bone phenotype of adiponectin-deficient mice exhibits age-dependent increase in trabecula volume and number, suggesting that adiponectin is indeed a likely contributor to the link between fat and bone mass [88, 90] that calls for further exploration.
2.4 Bone marrow adipocytes and regulation of hematopoietic niche
Numerous studies have linked chronic inflammation with visceral adiposity [91–93]; however, little is known on how bone marrow fat influences inflammatory processes in the skeleton. Bone marrow adipogenesis and osteogenesis are processes tightly linked to hematopoiesis, although their exact effects on hematopoietic stem cell (HSC) niche are not clearly delineated. Human bone marrow adipocytes have been reported to support differentiation of CD34+ of HSCs into myeloid and lymphoid pathways [94]. Accordingly, myelopoiesis was shown to positively correlate with increased adipogenesis and reduced osteoblastogenesis in SAMP6 mouse model of aging [46]. An enhancement in hematopoietic and lymphopoietic bone marrow cell populations was also demonstrated in diet-induced obese mice in correlation with increased marrow adiposity [74]. At the same time, lipid-filled adipocytes in the bone marrow have been linked to repression of growth and differentiation of HSCs [95, 96] and have been considered as the negative regulators of hematopoietic niche [1, 97]. This suppressive activity has been primarily attributed to the reduced production of granulocyte colony-stimulating factor (GM-CSF) and granulocyte stimulating factor (G-CSF) and increased secretion of neuropilin and lipocalin-2 [96, 98, 99]. Interestingly, while inhibiting HSC progenitor cells, adipocytes appear to positively affect the primitive HSCs via secretion of adiponectin and TNF-α [100, 101], a phenomenon proposed to play a role in preserving hematopoietic stem cell pool while preventing progenitor expansion [96]. Indeed, aging in humans and mice, a process associated with increased marrow adiposity [39, 43–45], induces myeloid-biased differentiation in HSCs [102], while promoting overall decrease in marrow cellularity [103]. Collectively, these studies underline the complex nature of bone marrow microenvironment and suggest that the hematopoietic environment in the marrow is governed by the dynamic relationship between adipocyte and osteoblast pathways.
Myeloid cells are the major cell type in undifferentiated bone marrow, which give rise to monocytes, macrophages, and granulocytes [36, 104]. Important contributors to their expansion in the bone marrow are proinflammatory, myelogenic cytokines such as interleukin 6 (IL-6) [36, 105]. Indeed, IL-6 is one of the bone marrow-derived inflammatory genes whose expression is highly upregulated, along with IL-1 and TNF-α in mice fed high-fat diet [63]. All three of these cytokines are highly present in adipose tissue and have been associated with obesity, adipose tissue dysfunction, and metabolic dysregulation [106–108]. They are also known mediators of osteoclastogenesis and bone resorption, predominantly through the regulation of the RANKL/RANK/ osteoprotegrin (OPG) pathway [53, 109]. Blocking TNF-α or IL-1 activity in ovariectomized mice attenuates osteoclast formation and prevents subsequent osteolysis of the bone [110], and neutralizing IL-6 reduces IL-1-driven bone degradation [111]. It has been documented that patients with periodontitis-, pancreatitis-, inflammatory bowel disease-, and rheumatoid arthritis-driven chronic inflammation exhibit accelerated bone resorption and bone loss [53]. Increased circulating levels of IL-6, TNF-α, and C-reactive protein (CRP) have been shown to positively correlate with hip fracture risk in elderly men and women [112], results further underlining the link between proinflammatory events and dysregulated bone remodeling.
2.5 Adiposity and bone marrow inflammation: the role of CCL2/COX-2 axis
One of the key myoelogenic molecules in the bone marrow is a C-motif chemokine ligand 2 (CCL2, MCP1) [36, 105], a low molecular weight monomeric polypeptide known largely for its role in obesity-induced infiltration of adipose tissue by inflammatory cells via its interaction with CCR2 receptor [113–116]. CCL2 overexpression is a major culprit of chronic inflammation in adipose tissue and a contributor to insulin resistance [117, 118]. Accordingly, CCR2 deficiency in a mouse model of diet-induced obesity attenuates the macrophage accumulation and significantly reduces inflammation and systemic insulin resistance [116]. Improved in vivo insulin sensitivity and reduced macrophage content are also observed in mice with preexisting obesity after short-term pharmacologic antagonism of CCR2 [116]. Within bone marrow microenvironment, CCL2 is expressed by several cell types and is an important player in regulating skeletal homeostasis [115]. In fact, CCL2 is one of the most strongly expressed genes in osteoporotic bone [119]. This cytokine significantly increases the number of bone-resorbing osteoclasts when coupled with RANKL [120]. CCL2 is also secreted by osteoblasts that have been stimulated with inflammatory factors [121]. Interestingly, osteoblast-derived CCL2 appears to be involved in monocyte recruitment [115, 122], and in line with these functions, CCL2 deficiency in mice results in higher bone mass [123].
Also important to skeletal metabolism and function is cyclooxygenase-2 (COX-2), an inducible enzyme responsible for the increased prostaglandin levels [124, 125]. COX-2 deficiency does not appear to have significant effects on overall skeletal phenotype, but its activity is essential for fracture healing [126, 127]. Notably, COX-2 overexpression in the bone has been tied to prostaglandin E2 (PGE2)-driven effects on skeletal metabolism [124]. Initially found to stimulate osteoclastogenesis and bone resorption, PGE2 also has also been shown to enhance bone formation [124]. Studies utilizing bone marrow cells from COX-2-deficient mice or treated with selective COX-2 inhibitors demonstrated that COX-2-induced endogenous PGs enhance pro-osteoclastogenic effects of several factors, such as IL-1, IL-6, IL-11, IL-17, TNF-α, PTH, vitamin D3, and BMP-2 [124]. PGE2 appears to have indirect effects on bone resorption either via upregulation of RANKL or by inhibition of its decoy receptor osteoprotegerin (OPG) expression in osteoblastic cells [124]. In general, the PGE2 effects on bone appear to be time and context-dependent: continuous PGE2 administration induces bone loss in rats, whereas an increase is observed with intermittent administration [128].
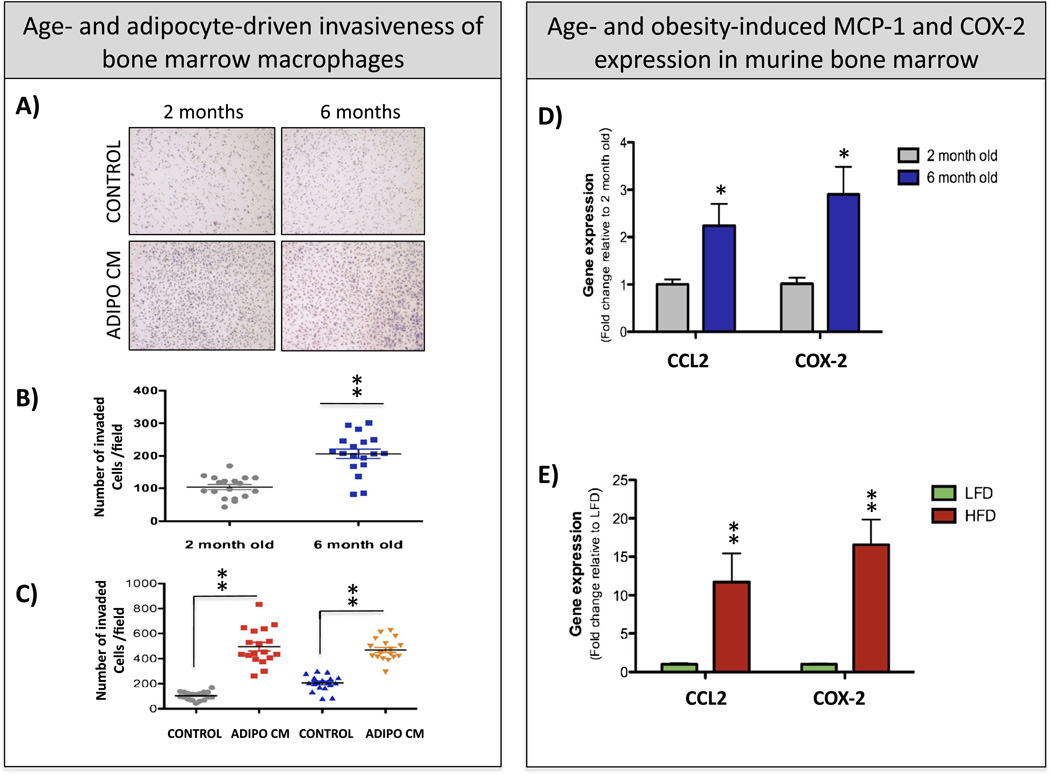
It is noteworthy that during inflammatory events, high expression of COX-2 is often coupled with CCL2 upregulation [129–131]. Specifically, its major metabolite PGE2 is known to induce differentiation of immunoregulatory cells [125, 132], and importantly, COX-2 inhibitors appear to prevent CCL2 production by activated macrophages [133, 134]. Under normal physiological conditions, COX-2 expression in macrophages is low and increases in response to proinflammatory stimuli [135]. In fact, both the COX-2 expression and the PGE2 release by macrophages have been shown to be stimulated by CCL2 and to be important for macrophage migration [136–138]. Interestingly, studies from our laboratory demonstrate that bone marrow macrophage invasiveness through collagen I matrix increases with age (Fig. 1(a, b)) and is highly stimulated by bone marrow adipocyte-derived factors (Fig. 1(a, c)). Notably, age and obesity are also potent inducers of COX-2 and CCL2 levels in murine bone marrow cells (Fig. 1(d, e)), a finding suggesting that the CCL2/COX-2 axis may be playing a functional role in obesity-driven inflammatory events in bone.
Fig. 1.
Age and adiposity increase bone marrow macrophage (BMM) invasiveness and induce COX2 and CCL2 expression in the bone marrow, a Images of invasion filters coated with collagen I matrix. Bone marrow macrophages from 2- and 6-month-old FVBN/N5 mice were plated on top of the filter (120,000 cells/filter) and allowed to invade toward control growth medium (control) or medium conditioned by bone marrow adipocytes (Adipo CM). Cells were allowed to invade for 24 h, and filters were fixed and stained with Diff-Quik dye. b, c Quantitation results showing numbers of invaded cells/filter +/− SD. b Comparative analysis of invasiveness of 2- and 6-month-old BMMs under control conditions; c comparative analysis of 2- and 6-month-old BMMs under control and Adipo CM conditions. Three independent biological replicates with six images/filter each were analyzed, d, e Taqman RT-PCR analysis of CCL2 (Macrophage Chemoattractant Protein; Life Technologies probe ID: Mm00441242_m1 and COX-2 (cyclooxygenase-2; Life Technologies probe ID: Mm00478374_m1) expression in murine bone marrow in a context of age (d) and obesity (e). d Two months old (gray) and 6 months old (blue). Data are shown as fold increases relative to 2-month-old bone marrow, e LFD (green )and HFD (red) mice. Data are shown as fold increases relative to LFD mice. All data are normalized to 18S (Life Technologies probe ID:Mm03928990_m1)
3 Marrow adiposity and bone metastases from prostate cancer
3.1 Effect of adipocytes on prostate cancer cells
Numerous studies have suggested that the biological behavior of tumor cells is affected by adipocyte-supplied factors [139–144]. Adipocytes and associated inflammatory cells secrete adipokines and cytokines, which are known contributors to tumor development and progression [144, 145]. This is particularly true for cancers that grow in adipocyte enriched microenvironments or have a predisposition to metastasize to fat-rich sites (e.g., breast, gastric, and ovarian cancers) [144]. Adipocytes are capable of inducing repolarization of vimentin, downregulating E-cadherin, and promoting tumor cell invasiveness [143]. The presence of adipocytes in the tumor microenvironment leads to an abundance of lipids, which are critical for signaling, cellular trafficking, and migration, particularly when environmental glucose stores are low [146]. Transformed cells have the ability to utilize and store lipids including glycosphingolipids, sphingomyelin, and cholesterol to gain growth advantage in comparison to cells of normal epithelium [147]. Lipids appear to be particularly important to prostatic tumor progression. Prostate tumor cells depend on fatty acid synthesis, have capabilities to upregulate lipogenic enzymes, and utilize both de novo and dietary lipids to sustain growth and proliferation (reviewed in [148, 149]). Interestingly, the favored pathway utilized by prostate cancer cells to generate energy and sustain survival appears to be β-oxidation [144, 150]. Activation of this pathway has been suggested as a mechanism supporting tumor cell viability during conditions of high stress and especially in response to oxidative radical-enriched environments [146, 148].
In line with the evident importance of lipid metabolism to prostate tumor growth, there appears to be a positive association between adipocyte presence, especially within the visceral and periprostatic adipose tissues, and prostate cancer progression and poor prognosis [144]. Adipose tissue adipocytes have been shown to stimulate proliferation, differentiation, and invasive potential in vitro [140, 142]. Clinical studies have linked obesity with prostate cancer aggressiveness, biochemical recurrence, and incidence of metastases [7, 19, 20, 23, 28, 29]. Interestingly, metastases from prostate cancer most commonly occur in adipocyte-rich metabolically active red bone marrow [37, 38]. Tumor cells appear to be attracted to marrow adipocytes, and the interaction between the two cell types results in translocation of adipocyte-stored lipids to the metastatic tumor cells [38, 142], resulting in their increased motility [37]. In addition, data from our laboratory suggest that uptake of bone marrow adipocyte-derived fatty acids and upregulation of lipid chaperone fatty acid-binding protein 4 (FABP4) and IL-1β in tumor cells drives their progression in the bone metastatic site [152]. However, despite increasing evidence linking skeletal metastases and death from prostate cancer with age and obesity, conditions which are implicated in marrow adiposity [8, 14, 19, 21, 29], the mechanisms of marrow fat cell involvement in metastatic growth in bone are still not clearly understood and warrant further studies.
3.2 Regulation of tumor growth in bone by adipocyte-derived leptin and adiponectin
Clinical and epidemiological data provide conflicting reports on the role of leptin in prostate carcinogenesis [152], although positive associations have been found with more advanced, hormone-refractory disease [153]. In vitro studies suggest that this adipokine exerts mitogenic effects in prostate cancer cells via modulation of MAPK pathway [154], and its activity in tumor cells appears to be dependent on hormonal status and to require JNK activation [155]. In the bone microenvironment, effects of adipocyte-derived leptin on tumor growth have been suggested to be indirect and occur via leptin-mediated stimulation of bone resorption [156, 157]. In addition, leptin expression appears to be tied to COX-2/prostaglandin pathways. Specifically, PGE2 has been shown to induce leptin expression and regulate lipolysis in murine and human adipose tissue [158, 159]. It is likely that COX-2 activity will also affect leptin signaling in the bone, with potential downstream effects on metastatic tumor cells.
Although not completely defined, the association of adiponectin with prostate cancer risk and progression appears to be better understood. Circulating levels of adiponectin are inversely correlated with obesity as well as prostate cancer incidence and aggressiveness [160, 161]. Based on limited in vitro studies, adiponectin is a potent inhibitor of prostate cancer cell growth and survival through downregulation of STAT3 signaling [162] and activation of AMPK pathway [161]. Adiponectin receptors are differentially expressed in prostate cancer cell lines and tissues, and their interactions with this adipokine vary depending on the androgen status [163, 164]. In addition, low adiponectin levels in combination with increased leptin secretion have been suggested to regulate prostate cancer progression via modulation of p53 and bcl-2 expression [165]. There have been no studies to date examining the direct role of adiponectin in growth and survival of tumor cells in bone. Similarly to leptin, adiponectin is likely to modulate tumor cell behavior at least in part via its known effects on bone cells and inhibitory effects on adipogenesis [166]. In addition, its reported role(s) in activation of COX-2-prostaglandin pathway and modulation of hematopoiesis [166, 167] suggest involvement in modulation of bone microenvironment with likely implications for affecting tumor growth. Further studies are needed to determine whether a direct interaction of this potent adipokine with receptors on metastatic tumor cells has any impact on their growth and survival in bone.
3.3 COX-2 in bone tumor microenvironment
COX-2 overexpression and aberrant signaling have been implicated in disease progression and decreased survival in several malignancies including breast, prostate, colon, bladder, and lung cancers [168]. COX-2/PGE2 signaling is hijacked in carcinogenesis, predominantly to promote chronic inflammation and immunosuppression associated with tumor evasion of the immune system [169]. Studies have shown that COX-2/PGE2 overexpression in breast tumor cells results in recruitment of regulatory T cells (Tregs) to the primary tumor site and the apoptosis of anti-tumorigenic CD8+ T cells [170]. Eventually, tumor cells progress and metastasize to distant sites, including the bone, and the overutilization of COX-2 by tumor cells may be a selective advantage to metastasis and colonization in the skeletal sites [171]. In the context of bone metastatic disease, COX-2 and PGE2 overexpressions are the major culprits in tumor-associated bone degradation [172, 173]. Increased COX-2 levels in breast cancer cells were shown to promote tumor colonization, osteoclastogenesis, and development of lytic lesions in a mouse model of bone metastasis [174]. In primary osteosarcomas, COX-2 inhibition suppresses proliferation of COX-2-overexpressing UTOS cells and decreased PGE2 levels [175]. In metastatic melanomas, blocking of PGE2 receptor with EP4 antagonist was shown to attenuate osteolysis of the bone [176], a process that was mimicked by use of selective COX-2 inhibitor celecoxib [177]. These effects were mediated by TNF-α, a potent stimulator of tumor proliferation and adhesion, and by COX-2-dependent VEGF overexpression [177].
Several studies have linked COX-2 expression and activity with prostate tumor progression and metastasis. Selective COX-2 inhibition was shown to suppress PC3 tumor growth in vivo via induction of tumor cell apoptosis and decreased angiogenesis due to VEGF downregulation [178]. One of the key factors implicated in COX-2 production by the tumor cells is tumor milieu-derived transforming growth factor β (TGF-β) [179]. PC3 cells treated with exogenous TGF-β exhibit elevated COX-2 expression and increased migration and invasion, events appearing to be linked to the activation of PI3K/Akt/mTor pathway by TGF-β and PGE2 [179]. TGF-β-driven regulation of COX-2 expression and activity is particularly relevant to progression of metastatic tumors in bone. Indeed, TGF-β is known to stimulate bone resorption via production of pro-osteoclastogenic prostaglandins [180].
The role of prostaglandins in tumor-induced osteolysis of the bone is further evident from studies in mice injected intratibially with PC3 cells that have been transfected with a decoy fragment of PGE2 [181]. Suppression of PGE2 pathway resulted in reduced tumor growth, decreased osteolysis, and increased tumor cell death, in parallel with attenuated expression of COX-2 and other pro-inflammatory factors (e.g., IL-1β and IL-6) [181]. Interestingly, in a recent elegant study by Liu et al., COX-2 expression in tumor-associated MSCs was shown to be induced by tumor cell-derived IL-1β, a process that promoted skeletal growth and progression of prostate PC3-ML tumors and was inhibited by IL-1β inhibitor, anakinra [182]. This is in line with previous reports suggesting that in addition to tumor cells themselves, cells of the tumor-associated stroma are key contributors to increased COX-2 expression [183, 184]. In fact, tumors that arise from IL-1β-producing carcinoma cells appear to induce COX-2/mPGES1/PGDH/PGE 2 response in MSCs to support own growth and progression [182, 184].
3.4 CCL2/CCR2 axis and metastatic tumor growth in bone
Growing evidence suggests critical involvement of CCL2/CCR2 axis in modulating the bone microenvironment to support tumor colonization and growth [115]. An important study utilizing bone metastasis tissues from rapid autopsy program at University of Michigan has demonstrated that patients with prostate tumor metastases to the spinal cord have increased levels of CCL2 in the skeletal lesions compared to soft tissue tumors [185]. The same group also revealed that endothelial cells are one of the major sources of bone marrow-derived CCL2 involved in macrophage/monocyte recruitment [185, 186]. In addition to their expression in endothelial cells, CCL2 and its receptor CCR2 have been localized to other cell types in the bone microenvironment, including the metastatic prostate cancer cells [186, 187]. This localization to various components within the marrow space appears to promote an autocrine/paracrine signaling that aids in tumor growth and survival [115]. For instance, recent studies from our laboratory have shown that bone marrow macrophages contribute to increased CCL2 levels in the bone marrow in response to prostate tumor challenge [188]. We have provided evidence for the existence of paracrine signaling between macrophage-and tumor cell-derived CCL2/CCR2 axes, which supports previous reports on prolonged survival and abolished metastasis in response to simultaneous blockade of tumor- and macrophage-derived CCL2 in mice bearing prostate and breast tumors [186, 189, 190].
The pivotal role for CCL2 in bone metastasis has been largely attributed to its effects on osteoclast differentiation and function [115]. Metastatic prostate cancer cells appear to secrete much higher levels of CCL2 compared to primary tumor cells [191]. Tumor cell-derived CCL2 promotes osteoclast differentiation [191, 192] that can be attenuated by CCL2 neutralization [193]. Accordingly, prostate cancer cells that overexpress CCL2 show higher incidence of tumor metastasis and tumor-induced osteolysis of the bone [193]. In line with these findings, targeting CCL2 expression in tumor cells with shRNA leads to reduced bone destruction and osteoclast presence in the tumor [194]. On the other hand, expression of CCL2 by bone-building osteoblasts may also be important to tumor progression in bone, particularly in a context of development of blastic lesions, a common occurrence in prostate cancer [115]. Under normal conditions, CCL2 expression by osteoblasts is low, and its upregulation is stimulated by inflammatory factors and associated with recruitment of monocytes [115]. It is currently not known if osteoblast-derived CCL2 has any direct effects on tumor cells in bone. However, given the fact that the osteoclast-osteoblast pathways are tightly coupled in the bone microenvironment and both are key regulators of bone homeostasis, the activity of CCL2 is likely to be a determinant of how either pathway impacts prostate tumor progression and survival in the marrow niche.
There is no doubt that CCL2 is emerging as a key contributor to site-specific metastasis from prostate and several other cancers [115]; yet the pathways associated with tumor addiction to CCL2 are only beginning to be uncovered. A recent study reported that N-cadherin expression is increased with tumor grade and directly regulates tumor CCL2 production through PI3K/Akt signaling and tumor neovascularization [195]. Additional studies uncovered a possible role for CCL2 in pro-tumorigenic effects of cyclophosphamide, a DNA alkylating chemotherapy drug [36]. Specifically, cyclophosphamide-treated tumor-bearing mice grew larger tumors, displayed significant vascular destruction, and promoted prostate tumor seeding and metastasis in bone, effects that were abrogated with CCL2 inhibition [36]. This suggests that CCL2 signaling may be a critical factor behind chemotherapy-induced bone remodeling and consequent successful tumor adaptation in bone.
3.5 COX-2 and CCL2 pathways: common link between marrow adiposity and bone metastasis?
Clear, positive links have been established between obesity and CCL2 expression [114, 116, 118]. Similar associations have been made between COX-2 and obesity-induced inflammation and insulin resistance [196]. In addition, both factors have been strongly implicated in prostate tumor progression and metastasis [115, 124, 125, 197]. The activities and functions of CCL2 and COX-2 appear to be closely connected, by the virtue of the fact that both factors are often overexpressed together [129–131], and inhibition of one appears to affect expression of the other [133, 134, 198].
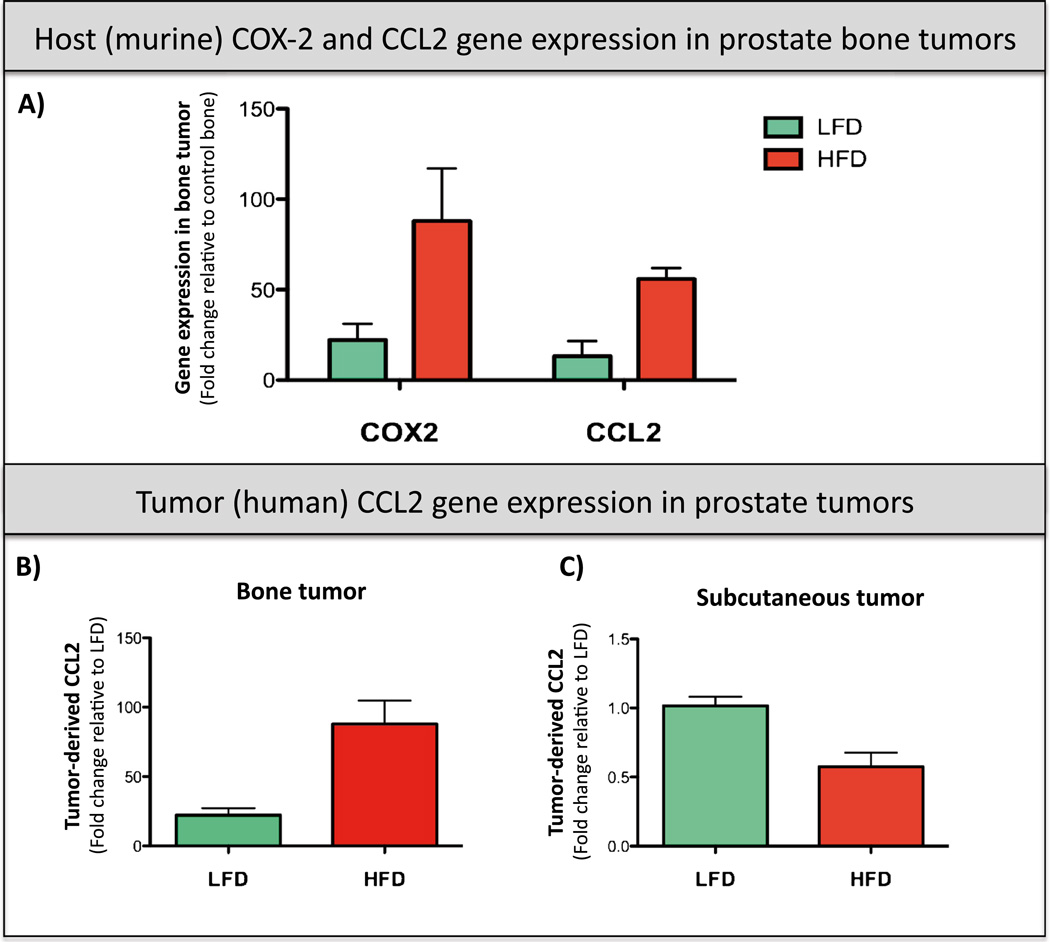
Studies on placing CCL2 and COX pathways as potential links between adiposity and prostate cancer are just beginning to emerge. Increased CCL2 and CCR2 expressions were recently implicated in accelerated tumor progression in prostate tumor-bearing mice on high-fat diet (HFD) [199]. In addition, we have shown recently that inflammatory events during prostate tumor progression in bone involve both the CCL2/CCR2 and COX-2 axes [188]. Interestingly, both the CCL2 and COX-2 pathways appear to be also highly upregulated in prostate bone tumors from mice with diet-induced obesity (Fig. 2). HFD is a known inducer of bone marrow adiposity [61, 63, 64, 152]. We utilized our established xenograft intratibial model of prostate bone tumor growth [188], which we have exposed to low-fat diet (LFD) vs. HFD diets [152] and assessed host (murine)- and tumor (human)-derived CCL2 and COX-2 expression. Our data show that host CCL2 and COX-2 are upregulated in tumor-bearing tibiae of mice receiving normal (LFD) diet (Fig. 2(a)), a result in agreement with our previous findings [188]. Notably, levels of host-derived CCL2 and COX-2 are highly escalated in mice on HFD (Fig. 2(a)) in parallel with accelerated tumor progression in bone [152]. Levels of human tumor-derived CCL2 are also highly increased in bone tumors (Fig. 2(b)) but not subcutaneous tumors (Fig. 2(c)) from HFD mice, suggesting specific functional involvement of CCL2/CCR2 axis in adiposity-driven bone tumor growth. Human tumor-derived COX-2 levels are low under both LFD and HFD conditions (data not shown) suggesting that the majority of COX-2 activity in the bone tumor microenvironment is host-supplied. Collectively, these results underline the involvement of CCL2 and COX-2 pathways in growth and progression of prostate tumors in bone and suggest that these pathways may be functionally tied to adiposity-driven metastatic events in the skeleton.
Fig. 2.
Host (murine) CCL2 and COX-2 and tumor (human) CCL2 are increased with obesity in PC3 prostate bone tumors. FVB/N/N5, Rag-1−/−mice caged in groups of four were started on either a LFD (10 % calories from fat; Research Diets no. D12450Bi) or a HFD (60 % calories from fat; Research Diets no. D12492i) at 5 weeks of age [152]. Mice were maintained on respective diets for 8 weeks prior to and 6 weeks following the PC3 tumor implantation into bone or subcutaneously (total of 14 weeks). a Taqman RT-PCR analysis of murine (host) CCL2 (Macrophage Chemoattractant Protein; Life Technologies probe ID: Mm00441242_m1) and COX-2 (cyclooxygenase 2; Life Technologies probe ID: Mm00478374_m1) in prostate bone tumors from LFD (green bars) and HFD (red bars) mice. Data were normalized to murine HPRT-1 (Life Technologies probe ID: Mm01545399_m1). b, c Taqman RT PCR analysis of human (tumor)-derived CCL2 (Life Technologies probe ID: Hs00234140_m1) in PC3 bone tumors (b) and PC3 subcutaneous tumors (c). Data were normalized to human HPRT1 (Life Technologies probe ID: Hs02800695_m1). At least three biological replicates were analyzed for each group, and data are shown as fold changes +/− SD
3.6 COX-2 and CCL2: therapeutic implications
Bone marrow niche is a complex and dynamic environment where multiple cell types, factors, and events shape the development and progression of metastatic disease. Inflammatory pathways, which under normal physiological conditions are involved in the maintenance and protection of normal bone homeostasis, become “hijacked” by the tumor to promote its growth and survival. COX2 and CCL2 are such examples of inflammatory factors that are important to normal bone function, but when present in overabundance under conditions of high adiposity and inflammation, have a potential to modulate the microenvironment to support aggressive tumor behavior.
Due to strong of evidence of CCL2 involvement in prostate cancer cell proliferation, migration, and invasion, as well as tumor growth in bone [115, 186, 187, 191], this chemokine is becoming a potential target for therapy in men with metastatic disease. Specifically, two humanized monoclonal antibodies have reached phase II clinical trials: Carlumab (CNTO 888), targeting CCL2 and MLN1202 against its receptor CCR2 (Table 1). Carlumab showed promise in preclinical studies both as a single agent slowing prostate tumor growth and reducing macrophage infiltration in mice, and in combination with docetaxel where it induced tumor regression and reduced tumor burden [200]. Unfortunately, a phase II study with Carlumab in metastatic prostate cancer patients with castrate-resistant disease who failed prior docetaxel treatment did not reach clinical objective [200]. Less than sufficient suppression of the target was suggested as a potential reason for the lack of therapeutic benefit. The outcomes of phase II trial with MLN1202 are currently not known.
Table 1.
Clinical development of anti-CCL2/CCR2 and anti-COX-2 therapeutics
| Agent/company | Mechanism of action |
Preclinical findings | Clinical trials in prostate cancer |
|---|---|---|---|
| CCL2 | |||
| Carlumab (CNTO 888) Centocor, Inc. Malvern, PA |
Human monoclonal antibody with high affinity and specificity for CCL2 |
Reduced incidence of tumor metastases, tumor burden, macrophage infiltration, and bone lesions in mice [186, 216] |
|
| MLN1202 SWOG, Portland, OR |
Human monoclonal antibody against CCR2 |
N/A |
|
| COX-2 | |||
| Celebrex® (Celecoxib) Pfizer Inc, Mission, KS |
NSAID that selectively targets COX-2 |
Prostate tumor regression, apoptosis, and inhibition of angiogenesis [201, 204, 220] |
|
| Aspirin | NSAID that targets anti- inflammatory proteins including COX-2 |
Reduces tumor growth [212] and decreased prostate cancer risk [224] |
|
There is also mounting evidence based on preclinical and epidemiological studies that COX-2 inhibition may have therapeutic benefits for prostate cancer patients [201–206]. Several phase II and III clinical trials have been initiated to evaluate the efficacy of Celecoxib in advanced prostate cancer (Table 1). Aspirin, a nonspecific NSAID, is also being examined in combination therapies for localized as well as metastatic disease [207, 208]. NSAIDs have been previously demonstrated to inhibit tumorigenesis through proapoptotic, anti-tumorigenic, and immune-modulating mechanisms [209, 210]. They have been shown to inhibit constitutive and RANKL-induced NFκB activation in osteoclasts [211]. One of the suggested mechanisms of aspirin-mediated effects on prostate tumor cells is modulation of prostaglandin receptor subtype EP3 [212].
It appears that inhibiting CCL2/CCR2 and COX-2 pathways may offer several benefits, particularly in a context of blocking adiposity-induced inflammation and maintaining homeostasis in the bone microenvironment. More studies are needed, however, to fully elucidate the mechanisms and benefits of targeting these axes in bone metastatic disease.
4 Conclusions
At present, metastatic prostate cancer remains an incurable disease, and treatments that are available to patients with skeletal lesions are mainly palliative [213]. Targeting the prostate tumors that have colonized the bone has proven extremely challenging due to complexity and dynamic nature of the bone marrow metastatic niche. The contribution of marrow adipose tissue to metastatic events in the bone is understudied and not well understood. Although considerable research has been dedicated to examining effects of marrow adiposity on bone health, especially in the context of osteoporosis [39, 45, 214, 215], little is known about the role of marrow fat cells in tumor cell adaptation and growth in the skeleton. Clearly, adipocytes have a potential to be active modulators of bone tumor microenvironment via direct interactions with neighboring cells as well as through secretion of adipokines and inflammatory mediators ([141–144] and Fig. 3). Understanding how they influence prostate tumor metabolism, behavior, and mechanisms of adaptation and survival in the bone marrow niche may reveal unique therapeutic targets and treatment opportunities for metastatic disease.
Fig. 3.
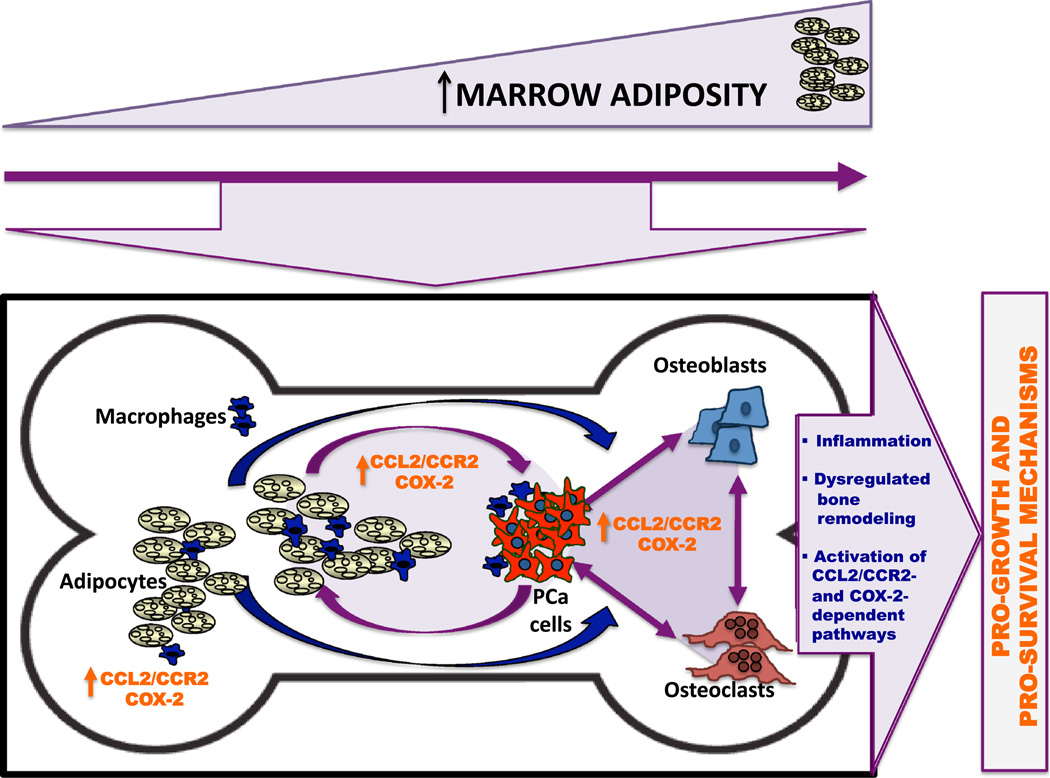
Proposed model of adiposity-induced inflammation and its effects on progression of metastatic tumors in bone. The increased amount of marrow fat content is a consequence of age and/or obesity. Prostate cancer cells that have colonized in adipocyte-rich bone marrow niche thrive and progress via CCL2/CCR2- and COX-2-dependent pathways due to (1) increased inflammation driven by adipocyte-macrophage interactions, (2) dysregulated bone remodeling via CCL2 and COX-2 actions on osteoclasts and osteoblasts, and (3) activation of pathways downstream of CCL2/CCR2 and COX-2 axes to promote tumor growth and survival
Contributor Information
Aimalie L. Hardaway, Department of Pharmacology, Wayne State University School of, Medicine, 540 E. Canfield, Rm 6304, Detroit, MI 48201, USA Karmanos Cancer Institute, Wayne State University School of, Medicine, Detroit, MI 48201, USA.
Mackenzie K. Herroon, Department of Pharmacology, Wayne State University School of, Medicine, 540 E. Canfield, Rm 6304, Detroit, MI 48201, USA
Erandi Rajagurubandara, Department of Pharmacology, Wayne State University School of, Medicine, 540 E. Canfield, Rm 6304, Detroit, MI 48201, USA.
Izabela Podgorski, Email: ipodgors@med.wayne.edu, Department of Pharmacology, Wayne State University School of, Medicine, 540 E. Canfield, Rm 6304, Detroit, MI 48201, USA; Karmanos Cancer Institute, Wayne State University School of, Medicine, Detroit, MI 48201, USA.
References
- 1.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2011;50(2):534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature Reviews. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roodman GD. Mechanisms of bone metastasis. New England Journal of Medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 6.Tuohimaa P, Tenkanen L, Syvala H, Lumme S, Hakulinen T, Dillner J, Hakama M. Interaction of factors related to the metabolic syndrome and vitamin D on risk of prostate cancer. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:302–307. doi: 10.1158/1055-9965.EPI-06-0777. [DOI] [PubMed] [Google Scholar]
- 7.Amling CL. Relationship between obesity and prostate cancer. Current Opinion in Urology. 2005;15:167–171. doi: 10.1097/01.mou.0000165550.94663.fb. [DOI] [PubMed] [Google Scholar]
- 8.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 9.Hsing AW, Chua S, Jr, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. Journal of the National Cancer Institute. 2001;93:783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 10.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. The American Journal of Clinical Nutrition. 2007;86:s843–s857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard JS, Rohrmann S, Landis PK, Metter EJ, Muller DC, Andres R, Carter HB, Platz EA. Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology. 2004;63:253–258. doi: 10.1016/j.urology.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 12.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. British Journal of Cancer. 2006;94:740–742. doi: 10.1038/sj.bjc.6602983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 14.Scosyrev E, Messing EM, Mohile S, Golijanin D, Wu G. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062–3070. doi: 10.1002/cncr.26392. [DOI] [PubMed] [Google Scholar]
- 15.Scosyrev E, Wu G, Mohile S, Messing EM. Prostate-specific antigen screening for prostate cancer and the risk of overt metastatic disease at presentation : Analysis of trends over time. Cancer. 2012;118:5768–5776. doi: 10.1002/cncr.27503. [DOI] [PubMed] [Google Scholar]
- 16.Putnam SD, Cerhan JR, Parker AS, Bianchi GD, Wallace RB, Cantor KP, Lynch CF. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Annals of Epidemiology. 2000;10:361–369. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 17.Dal Maso L, Zucchetto A, La Vecchia C, Montella M, Conti E, Canzonieri V, Talamini R, Tavani A, Negri E, Garbeglio A, Franceschi S. Prostate cancer and body size at different ages: an Italian multicentre case-control study. British Journal of Cancer. 2004;90:2176–2180. doi: 10.1038/sj.bjc.6601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and prostate cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2003;12:1417–1421. [PubMed] [Google Scholar]
- 19.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer and Prostatic Diseases. 2009;72:259–263. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 20.Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG. Pathologic variables and recurrence ates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. Journal of Clinical Oncology. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 21.Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ, Chan JM, Anast JW, Carroll PR, Kane CJ. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–1065. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, Terris MK. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. Journal of Clinical Oncology. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Grubb KA, Yiu SK, Humphreys EB, Nielsen ME, Mangold LA, Isaacs WB, Partin AW. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. The Journal of Urology. 2005;174:919–922. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 24.Palma D, Pickles T, Tyldesley S. Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU International. 2007;100:315–319. doi: 10.1111/j.1464-410X.2007.06897.x. [DOI] [PubMed] [Google Scholar]
- 25.Stroup SP, Cullen J, Auge BK, L’Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007;110:1003–1009. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiology, Biomarkers and Prevention. 2001;10:345–353. [PubMed] [Google Scholar]
- 27.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 28.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 29.Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU International. 2012;110:492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crujeiras AB, Diaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radical Research. 2013;47:243–256. doi: 10.3109/10715762.2013.772604. [DOI] [PubMed] [Google Scholar]
- 31.Gueron G, De Siervi A, Vazquez E. Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer and Prostatic Diseases. 2012;15:213–221. doi: 10.1038/pcan.2011.64. [DOI] [PubMed] [Google Scholar]
- 32.Nelson WG, DeWeese TL, DeMarzo AM. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Reviews. 2002;21:3–16. doi: 10.1023/a:1020110718701. [DOI] [PubMed] [Google Scholar]
- 33.Imbriaco M, Larson SM, Yeung HW, Mawlawi OR, Erdi Y, Venkatraman ES, Scher HI. A new parameter for measuring metastatic bone involvement by prostate cancer: the bone scan index. Clinical Cancer Research. 1998;4:1765–1772. [PubMed] [Google Scholar]
- 34.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 35.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clinical Cancer Research. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 36.Park SI, Liao J, Berry JE, Li X, Koh AJ, Michalski ME, Eber MR, Soki FN, Sadler D, Sud S, Tisdelle S, Daignault SD, Nemeth JA, Snyder LA, Wronski TJ, Pienta KJ, McCauley LK. Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Research. 2012;72:2522–2532. doi: 10.1158/0008-5472.CAN-11-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by omega 6 and its inhibition by omega 3 PUFAS. British Journal of Cancer. 2006;94:842–853. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. Journal of Lipid Research. 2007;48:1846–1856. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23:183–188. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- 40.Rosen C, Bouxsein M. Mechanisms of disease: is osteoporosis the obesity of bone? Nature Clinical Practice Rheumatology. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 41.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Journal of Cell Science. 1992;102(Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 42.Owen M. Marrow stromal stem cells. Journal of Cell Science. 1988;10(Supplement):63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 43.Kawai M, de Paula F, Rosen C. New insights into osteoporosis: the bone-fat connection. Journal of Internal Medicine. 2012;272:317–329. doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle. 2010;9:3648–3654. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Critical Reviews in Eukaryotic Gene Expression. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jilka RL. Osteoblast progenitor fate and age-related bone loss. Journal of Musculoskeletal & Neuronal Interactions. 2002;2:581–583. [PubMed] [Google Scholar]
- 47.Oh S, Sul O, Kim Y, Kim H, Yu R, Suh J, Choi H. Saturated fatty acids enhance osteoclast survival. Journal of Lipid Research. 2010;51:892–899. doi: 10.1194/jlr.M800626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Lazarenko O, Wu X, Tong Y, Blackburn M, Shankar K, Badger T, Ronis M. Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS ONE. 2010;5:el3704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duque G. As a matter of fat: new perspectives on the understanding of age-related bone loss. BoneKEy-Osteovision. 2007;4:129–140. [Google Scholar]
- 51.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nature Medicine. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 52.Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Current Osteoporosis Reports. 2010;8:84–90. doi: 10.1007/s11914-010-0016-1. [DOI] [PubMed] [Google Scholar]
- 53.Cao J. Effects of obesity on bone metabolism. Journal of Orthopaedic Surgery and Research. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villareal DT, Apovian CM, Kushner RF, Klein S American Society for Nutrition, & Naaso TOS. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity Research. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 55.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. Journal of Bone and Mineral Research. 2012;27:1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 57.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. The American Journal of Clinical Nutrition. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 58.Owusu W, Willett W, Ascherio A, Spiegelman D, Rimm E, Feskanich D, Colditz G. Body anthropometry and the risk of hip and wrist fractures in men: results from a prospective study. Obesity Research. 1998;6:12–19. doi: 10.1002/j.1550-8528.1998.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 59.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. Journal of Magnetic Resonance Imaging. 2001;13:263–268. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 60.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Annals of the New York Academy of Sciences. 2010;1192:292–297. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 62.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–1104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Halade G, Rahman M, Williams P, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. Journal of Nutritional Biochemistry. 2010;21:1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halade G, El Jamali A, Williams P, Fajardo R, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental Gerontology. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kyung T, Lee J, Phan T, Yu R, Choi H. Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. Journal of Nutrition. 2009;139:502–506. doi: 10.3945/jn.108.100032. [DOI] [PubMed] [Google Scholar]
- 66.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone-new perspectives. The Journal of Clinical Endocrinology and Metabolism. 2013;98:935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. Journal of Cellular Biochemistry. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 69.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. Journal of Cellular Biochemistry. 1999;74:357–371. [PubMed] [Google Scholar]
- 70.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. Journal of Cellular Biochemistry. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gimble JM. The function of adipocytes in the bone marrow stroma. The New Biologist. 1990;2:304–312. [PubMed] [Google Scholar]
- 72.Paula FJ, Rosen CJ. Obesity, diabetes mellitus and last but not least, osteoporosis. Arquivos Brasileiros de Endocrinologia e Metabologia. 2010;54:150–157. doi: 10.1590/s0004-27302010000200010. [DOI] [PubMed] [Google Scholar]
- 73.Roodman GD. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Review. 2012;31(3–4):569–578. doi: 10.1007/s10555-012-9372-x. [DOI] [PubMed] [Google Scholar]
- 74.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Dielen FM, van’t Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. International Journal of Obesity and Related Metabolic Disorders. 2001;25:1759–1766. doi: 10.1038/sj.ijo.0801825. [DOI] [PubMed] [Google Scholar]
- 76.Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Bauer DC, Cauley JA. Adipokines and the risk of fracture in older adults. Journal of Bone and Mineral Research. 2011;26:1568–1576. doi: 10.1002/jbmr.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Motyl KJ, Rosen CJ. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie. 2012;94:2089–2096. doi: 10.1016/j.biochi.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadie-Van Gijsen H, Crowther NJ, Hough FS, Ferris WF. The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cellular and Molecular Life Sciences. 2012 doi: 10.1007/s00018-012-1211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. Journal of Cellular Biochemistry. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 80.Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells (Dayton, Ohio) 2010;28:1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 82.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. Journal of Bone and Mineral Research. 2004;19:1607–1611. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 83.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. Journal of Bone and Mineral Research. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 84.Berg AH, Combs TP, Scherer PE. ACRP30/ adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endocrinology and Metabolism. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 85.Reid IR. Fat and bone. Archives of Biochemistry and Biophysics. 2010;503:20–27. doi: 10.1016/j.abb.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 86.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, flceda T, Hoshi K, Chung U, Nakamura K, Kawaguchi H. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. Journal of Cellular Biochemistry. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 87.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and Biophysical Research Communications. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 88.Kanazawa I. Adiponectin in metabolic bone disease. Current Medicinal Chemistry. 2012;19:5481–5492. doi: 10.2174/092986712803833146. [DOI] [PubMed] [Google Scholar]
- 89.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochemical and Biophysical Research Communications. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 90.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150:3603–3610. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 91.de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. The Proceedings of the Nutrition Society. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 92.Harte AL, Tripathi G, Piya MK, Barber TM, Clapham JC, Al-Daghri N, Al-Disi D, Kumsaiyai W, Saravanan P, Fowler AE, Oh JP, Kumar S, McTernan PG. Obesity. Silver Spring, MD: 2013. NFkappaB as a potent regulator of inflammation in human adipose tissue, influenced by depot, adiposity, T2DM status, and TNFalpha. [DOI] [PubMed] [Google Scholar]
- 93.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metabolism. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corre J, Planat-Benard V, Corberand JX, Penicaud L, Casteilla L, Laharrague P. Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. British Journal of Haematology. 2004;127:344–347. doi: 10.1111/j.1365-2141.2004.05198.x. [DOI] [PubMed] [Google Scholar]
- 95.Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clinical Biochemistry. 2012;45:874–879. doi: 10.1016/j.clinbiochem.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 98.Belaid-Choucair Z, Lepelletier Y, Poncin G, Thiry A, Humblet C, Maachi M, Beaulieu A, Schneider E, Briquet A, Mineur P, Lambert C, Mendes-Da-Cruz D, Ahui ML, Asnafi V, Dy M, Boniver J, Nusgens BV, Hermine O, Defresne MP. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells (Dayton, Ohio) 2008;26:1556–1564. doi: 10.1634/stemcells.2008-0068. [DOI] [PubMed] [Google Scholar]
- 99.Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. Journal of Cellular Physiology. 2008;215:526–537. doi: 10.1002/jcp.21334. [DOI] [PubMed] [Google Scholar]
- 100.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. The Journal of Immunology. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 101.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 102.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mechanisms of Ageing and Development. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 104.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunology, Immunotherapy. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CDllb+peripheral blood mononuclear cells and induce M2-type macrophage polarization. Journal of Biological Chemistry. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunological Reviews. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stienstra R, Tack CJKT, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metabolism. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological Reviews. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 109.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 110.Kimble RB, Matayoshi AB, Vannice IL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 111.Axmann R. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis and Rheumatism. 2009;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 112.Cauley J, Danielson M, Boudreau R, Forrest K, Zmuda J, Pahor M, Tylavsky K, Cummings S, Harris T, Newman A Health ABC Study. Inflammatory markers and incident fracture risk in older Men and women: the health aging and body composition study. Journal of Bone and Mineral Research. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 113.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? Journal of Clinical Investigation. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Craig MJ, Loberg RD. CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Reviews. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 116.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. Journal of Clinical Investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. Journal of Biological Chemistry. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 118.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. Journal of Clinical Investigation. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Gene expression profile of the bone microenvironment in human fragility fracture bone. Bone. 2009;44:87–101. doi: 10.1016/j.bone.2008.08.120. [DOI] [PubMed] [Google Scholar]
- 120.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. Journal of Biological Chemistry. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 121.Graves DT, Jiang Y, Valente AJ. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Frontiers in Bioscience. 1999;4:D571–D580. doi: 10.2741/graves. [DOI] [PubMed] [Google Scholar]
- 122.Posner LJ, Miligkos T, Gilles JA, Carnes DL, Taddeo DR, Graves DT. Monocyte chemoattractant protein-1 induces monocyte recruitment that is associated with an increase in numbers of osteoblasts. Bone. 1997;21:321–327. doi: 10.1016/s8756-3282(97)00154-3. [DOI] [PubMed] [Google Scholar]
- 123.Sul OJ, Ke K, Kim WK, Kim SH, Lee SC, Kim HJ, Kim SY, Suh JH, Choi HS. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. Journal of Cellular Physiology. 2012;227:1619–1627. doi: 10.1002/jcp.22879. [DOI] [PubMed] [Google Scholar]
- 124.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends in Endocrinology and Metabolism. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang D, Dubois RN. Eicosanoids and cancer. Nature Reviews. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O’Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. Journal of Bone and Mineral Research. 2009;24:251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simon AM, Manigrasso MB, O’Connor JP. Cyclooxygenase 2 function is essential for bone fracture healing. Journal of Bone and Mineral Research. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 128.Tian XY, Zhang Q, Zhao R, Setterberg RB, Zeng QQ, Iturria SJ, Ma YF, Jee WS. Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone. 2008;42:914–920. doi: 10.1016/j.bone.2007.12.228. [DOI] [PubMed] [Google Scholar]
- 129.Chen YC, Sosnoski DM, Gandhi UH, Novinger LJ, Prabhu KS, Mastro AM. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis. 2009;30:1941–1948. doi: 10.1093/carcin/bgp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hsieh PS, Lu KC, Chiang CF, Chen CH. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. European Journal of Clinical Investigation. 2010;40:164–171. doi: 10.1111/j.1365-2362.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 131.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. Journal of Clinical Investigation. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Research. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 133.Muta M, Matsumoto G, Nakashima E, Toi M. Mechanical analysis of tumor growth regression by the cyclooxygenase-2 inhibitor, DFU, in a Walker 256 rat tumor model: importance of monocyte chemoattractant protein-1 modulation. Clinical Cancer Research. 2006;12:264–272. doi: 10.1158/1078-0432.CCR-05-1052. [DOI] [PubMed] [Google Scholar]
- 134.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Research. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO Journal. 2002;27:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. Journal of Clinical Investigation. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, Ozaki H, Hori M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) The Journal of Pharmacology and Experimental Therapeutics. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- 138.Tanaka S, Tatsuguchi A, Futagami S, Gudis K, Wada K, Seo T, Mitsui K, Yonezawa M, Nagata K, Fujimori S, Tsukui T, Kishida T, Sakamoto C. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut. 2006;55:54–61. doi: 10.1136/gut.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology. 2001;280:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 140.Kaneko A, Satoh Y, Tokuda Y, Fujiyama C, Udo K, Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. International Journal of Urology. 2010;17:369–376. doi: 10.1111/j.1442-2042.2010.02472.x. [DOI] [PubMed] [Google Scholar]
- 141.Nieman K, Kenny H, Penicka C, Ladanyi A, Buell-Gutbrod R, Zillhardt M, Romero I, Carey M, Mills G, Hotamisligil G, Yamada S, Peter M, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tokuda Y, Satoh Y, Fujiyama C, Toda S, Sugihara H, Masaki Z. Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU International. 2003;91:716–720. doi: 10.1046/j.1464-410x.2003.04218.x. [DOI] [PubMed] [Google Scholar]
- 143.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang Y, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 144.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et Biophysica Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nature Reviews. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 146.Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochimica et Biophysica Acta. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Llorente A, Skotland T, Sylvänne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochimica et Biophysica Acta. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 149.Suburu J, Chen YQ. Lipids and prostate cancer. Prostaglandins & Other Lipid Mediators. 2012;98:1–10. doi: 10.1016/j.prostaglandins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 151.Herroon M, Rajagurubandara E, Hardaway A, Powell K, Turchick A, Feldmann D, Podgorski I. Bone marrow adipocytes promote tumor growth and survival in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Frontiers in Bioscience. 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 153.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiologic Reviews. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 154.Hoda MR, Theil G, Mohammed N, Fischer K, Fornara P. The adipocyte-derived hormone leptin has proliferative actions on androgen-resistant prostate cancer cells linking obesity to advanced stages of prostate cancer. Journal of Oncology. 2012;2012:280386. doi: 10.1155/2012/280386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. Journal of Biological Chemistry. 2003;278:42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 156.Bussard K, Gay C, Mastro A. The bone microenvi-ronment in metastasis; what is special about bone? Cancer Metastasis Reviews. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 157.Thobe MN, Clark RJ, Bainer RO, Prasad SM, Rinker-Schaeffer CW. From prostate to bone: key players in prostate cancer bone metastasis. Cancers (Basel) 2011;3:478–493. doi: 10.3390/cancers3010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fain JN, Kanu A, Bahouth SW, Cowan GS, Jr, Hiler ML, Leffler CW. Comparison of PGE2, prostacyclin and leptin release by human adipocytes versus explants of adipose tissue in primary culture. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2002;67:467–473. doi: 10.1054/plef.2002.0430. [DOI] [PubMed] [Google Scholar]
- 159.Fain JN, Leffler CW, Bahouth SW. Eicosanoids as endogenous regulators of leptin release and lipolysis by mouse adipose tissue in primary culture. Journal of Lipid Research. 2000;41:1689–1694. [PubMed] [Google Scholar]
- 160.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 161.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. British Journal of Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochemical and Biophysical Research Communications. 2006;340:1158–1166. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 163.Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochemical and Biophysical Research Communications. 2006;348:832–838. doi: 10.1016/j.bbrc.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 164.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. European Urology. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 165.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU International. 2008;101:1317–1322. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 166.Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, Nishida M, Oritani K, Miyagawa J, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. Journal of Clinical Investigation. 2002;109:1303–1310. doi: 10.1172/JCI14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. The Journal of Immunology. 2003;171:5091–5099. doi: 10.4049/jimmunol.171.10.5091. [DOI] [PubMed] [Google Scholar]
- 168.Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? The Lancet Oncology. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- 169.Kalinski P. Regulation of immune responses by prostaglandin E2. The Journal of Immunology. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Karavitis J, Hix L, Shi Y, Schultz R, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS ONE. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lucci A, Krishnamurthy S, Singh B, Bedrosian I, Meric-Bemstam K, Reuben J, Broglio K, Mosalpuria K, Lodhi A, Vincent L, Cristofanilli M. Cyclooxygenase-2 expression in primary breast cancers predicts dissemination of cancer cells to the bone marrow. Breast Cancer Research and Treatment. 2009;117:61–68. doi: 10.1007/s10549-008-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Safina A, Sotomayor P, Limoge M, Morrison C, Bakin A. TAK1-TAB2 signaling contributes to bone destruction by breast carcinoma cells. Molecular Cancer Research. 2011;9:1042–1053. doi: 10.1158/1541-7786.MCR-10-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singh B, Berry J, Shoher A, Ayers G, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 174.Li Z, Schem C, Shi Y, Medina D, Zhang M. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clinical and Experimental Metastasis. 2008;25:389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]
- 175.Lee E, Choi E, Kim S, Park J, Kim H, Ha K, Kim Y, Kim S, Choe M, Kim J, Han J. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Experimental and Molecular Medicine. 2007;39:469–476. doi: 10.1038/emm.2007.51. [DOI] [PubMed] [Google Scholar]
- 176.Takita M, Inada M, Maruyama T, Miyaura C. Prostaglandin E receptor EP4 antagonist suppresses osteolysis due to bone metastasis of mouse malignant melanoma cells. FEBS Letters. 2007;581:565–571. doi: 10.1016/j.febslet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 177.Valcarcel M, Mendoza L, Hernandez JJ, Carrascal T, Salado C, Crende O, Vidal-Vanaclocha F. Vascular endothelial growth factor regulates melanoma cell adhesion and growth in the bone marrow microenvironment via tumor cyclooxygenase-2. Journal of Translational Medicine. 2011;9:142. doi: 10.1186/1479-5876-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. The Journal of Urology. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 179.Vo B, Morton DSK, Millena A, Leath C, Khan S. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 2013;154:1768–1779. doi: 10.1210/en.2012-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hiraga T, Myoui A, Choi ME, Yoshikawa H, Yoneda T. Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor-beta enhances bone metastases in breast cancer. Cancer Research. 2006;66:2067–2073. doi: 10.1158/0008-5472.CAN-05-2012. [DOI] [PubMed] [Google Scholar]
- 181.Takahashi T, Uehara H, Bando Y, Izumi K. Soluble EP2 neutralizes prostaglandin E2-induced cell signaling and inhibits osteolytic tumor growth. Molecular Cancer Therapeutics. 2008;7:2807–2816. doi: 10.1158/1535-7163.MCT-08-0153. [DOI] [PubMed] [Google Scholar]
- 182.Liu Q, Russell MR, Shahriari K, Jernigan DL, Lioni MI, Garcia FU, Fatatis A. Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Research. 2013;73:3297–3305. doi: 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 183.Le Bitoux MA, Stamenkovic I. Tumor-host interactions: the role of inflammation. Histochemistry and Cell Biology. 2008;130:1079–1090. doi: 10.1007/s00418-008-0527-3. [DOI] [PubMed] [Google Scholar]
- 184.Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discovery. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Loberg R, Day L, Harwood J, Ying C, St John , Giles R, Neeley C, Pienta K. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 188.Herroon M, Rajagurubandara EDR, Chalasani A, Hardaway A, Podgorski I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene. 2013;32:1580–1593. doi: 10.1038/onc.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang J, Patel L, Pienta K. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine and Growth Factor Reviews. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lu Y, Cai Z, Xiao G, Keller E, Mizokami A, Yao Z, Roodman G, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Research. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 192.Li X, Loberg R, Liao J, Ying C, Snyder L, Pienta K, McCauley L. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Research. 2009;69:1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mizutani K, Sud S, McGregor N, Martinovski G, Rice B, Craig M, Varsos Z, Roca H, Pienta K. The chemo-kine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A, Zhang J. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clinical and Experimental Metastasis. 2009;26:161–169. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 195.Nalla A, Estes N, Patel J, Rao J. N-cadherin mediates angiogenesis by regulating monocyte chemoattractant protein-1 expression via PI3K/Akt signaling in prostate cancer cells. Experimental Cell Research. 2011;317:2512–2521. doi: 10.1016/j.yexcr.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 196.Hsieh PS, Jin JS, Chiang CF, Chan PC, Chen CH, Shih KC. Obesity. Vol. 17. Silver Spring, MD: 2009. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver; pp. 1150–1157. [DOI] [PubMed] [Google Scholar]
- 197.Bossard MJ, Tomaszek TA, Thompson SK, Amegadzie BY, Hanning CR, Jones C, Kurdyla JT, McNulty DE, Drake FH, Gowen M, Levy MA. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. Journal of Biological Chemistry. 1996;271:12517–12524. doi: 10.1074/jbc.271.21.12517. [DOI] [PubMed] [Google Scholar]
- 198.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Research. 2009;69:7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 199.Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T, Horikawa Y, Tsuchiya N, Habuchi T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate. 2012;72:1779–1788. doi: 10.1002/pros.22531. [DOI] [PubMed] [Google Scholar]
- 200.Pienta K, Machiels J, Schrijvers D, Alekseev B, Shkolnik M, Crabb S, Li S, Seetharam S, Puchalski T, Takimoto C, Elsayed Y, Dawkins K, de Bono J. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investigational New Drugs. 2013;32:760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 201.Abedinpour P, Baron VJW, Borgström P. Regression of prostate tumors upon combination of hormone ablation therapy and celecoxib in vivo. Prostate. 2011;71:813–823. doi: 10.1002/pros.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Antonarakis E, Heath E, Walczak J, Nelson W, Fedor H, De Marzo A, Zahurak M, Piantadosi S, Dannenberg A, Gurganus R, Baker S, Parnes H, DeWeese T, Partin A, Carducci M. Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. Journal of Clinical Oncology. 2009;27:4986–4993. doi: 10.1200/JCO.2009.21.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.James N, Sydes M, Clarke N, Mason M, Dearnaley D, Anderson J, Popert R, Sanders K, Morgan R, Stansfeld J, Dwyer J, Masters J, Parmar M. Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multiarm, multistage randomized controlled trial. BJU. 2009;103:464–469. doi: 10.1111/j.1464-410X.2008.08034.x. [DOI] [PubMed] [Google Scholar]
- 204.Jendrossek V. Targeting apoptosis pathways by Celecoxib in cancer. Cancer Letters. 2013;332:313–324. doi: 10.1016/j.canlet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 205.Pruthi RS, Wallen EM. Cyclooxygenase-2: a therapeutic target for prostate cancer. Clinical Genitourinary Cancer. 2005;4:203–211. doi: 10.3816/CGC.2005.n.034. [DOI] [PubMed] [Google Scholar]
- 206.Sooriakumaran P, Langley SE, Laing RW, Coley HM. COX-2 inhibition: a possible role in the management of prostate cancer? Journal of Chemotherapy. 2007;19:21–32. doi: 10.1179/joc.2007.19.1.21. [DOI] [PubMed] [Google Scholar]
- 207.Effects of ASA on prostate tissue NCT00234299. 2011 www.Clinicaltrials.gov.
- 208.Dexamethasone, aspirin, and diethylstilbestrol in treating patients with locally advanced or metastatic prostate cancer NCT00316927. 2013 www.Clinicaltrials.gov.
- 209.de Groot DJ, de Vries EG, Groen HJ, de Jong S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Critical Reviews in Oncology/ Hematology. 2007;61:52–69. doi: 10.1016/j.critrevonc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 210.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature Reviews. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 211.Robak P, Smolewski P, Robak T. The role of nonsteroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leukemia & Lymphoma. 2008;49:1452–1462. doi: 10.1080/10428190802108854. [DOI] [PubMed] [Google Scholar]
- 212.Kashiwagi E, Shiota M, Yokomizo A, Itsumi M, Inokuchi J, Uchiumi T, Naito S. Prostaglandin receptor EP3 mediates growth inhibitory effect of aspirin through androgen receptor and contributes to castration resistance in prostate cancer cells. Endocrine-Related Cancer. 2013;20:431–441. doi: 10.1530/ERC-12-0344. [DOI] [PubMed] [Google Scholar]
- 213.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nature reviews Urology. 2013;10:522–528. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]
- 214.Duque G. Bone and fat connection in aging bone. Current Opinion in Rheumatology. 2008;20:429–434. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 215.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 216.Loberg R, Ying C, Craig M, Day L, Sargent E, Neeley C, Wojno K, Snyder L, Yan L, Pienta K. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Research. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 217.Sandhu S, Papadopoulos K, Fong P, Patnaik A, Messiou C, Olmos D, Wang G, Tromp B, Puchalski T, Balkwill F, Berns B, Seetharam S, de Bono J, Tolcher A. A first-inhuman, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemotherapy and Pharmacology. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 218.An open-label, multicenter, phase 2 study of single-agent CNTO 888 (an anti-CCL2 monoclonal antibody) for the treatment of subjects with metastatic castrate-resistant prostate cancer. NCT00992186. 2013 www.Clinicaltrials.gov.
- 219.S0916, MLN1202 in treating patients with bone metastases. NCT01015560. 2012 www.Clinicaltrials.gov.
- 220.Zheng X, Cui X, Avila G, Huang M, Liu Y, Patel J, Kong A, Paulino R, Shih W, Lin Y, Rabson A, Reddy B, Conney A. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clinical Cancer Research. 2007;13:5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 221.COX-2 inhibitor reduces serum PSA levels might predict a lower risk of prostatic cancer in men with LUTS/BPH with an elevated PSA level. NCT01678313. 2013 www.Clinicaltrials.gov.
- 222.STAMPEDE: systemic therapy in advancing or metastatic prostate cancer: evaluation of drug efficacy: a multi-stage multi-arm randomised controlled trial. NCT00268476 doi: 10.1016/j.clon.2008.07.002. www.Clinicaltrials.gov. [DOI] [PubMed]
- 223.A trial of COX-2 inhibitors in PSA recurrence after definitive radiation or radical prostatectomy for prostate cancer. NCT00073970. 2013 www.Clinicaltrials.gov.
- 224.Singer E, Palapattu G, van Wijngaarden E. Prostate-specific antigen levels in relation to consumption of nonsteroidal anti-inflammatory drugs and acetaminophen: results from the 2001–2002 National Health and Nutrition Examination Survey. Cancer. 2008;113:2053–2057. doi: 10.1002/cncr.23806. [DOI] [PubMed] [Google Scholar]