Abstract
Metal chelators masked with protecting groups for targeted release have the potential to conditionally modulate cellular metals. We report a new route to prepare cis-cinnamate protecting groups that enabled development of a prochelator with chemical stimulus response, fluorescent reporting and active compound release in a single structure.
Misregulation of biological metal ions has been implicated as a contributor to many diseases. Altering the concentration and distribution of select metal ions can therefore be a powerful pharmacological strategy for fighting disease. For example, β-thalassemia patients are often treated with the chelating agent desferrioxamine to avoid organ damage from iron overload.1 In Wilson’s disease, chelation therapy can ameliorate neurological and hepatological symptoms associated with malfunctioning copper transport.2 However, administering therapeutic chelators carries unintended risks of systemic metal depletion and associated toxic effects.1, 3 Our laboratory and others therefore seek to develop molecules, termed ‘prochelators’, that bind metals only under disease conditions. We recently reported that a non-toxic prochelator named QBP converts to 8-hydroxyquinoline (8HQ) in response to the oxidative burst of activated macrophages, an event that releases reactive oxygen and nitrogen species that deprotect the boronate masking group.4 The released 8HQ elicits copper-dependent killing of a fungal pathogen in vitro and in mouse pulmonary infection.4 A multifunctional agent that provides a fluorescent signal upon activation would be useful to probe the localization and timing of such agents in order to understand their mechanism of action and improve disease targeting.
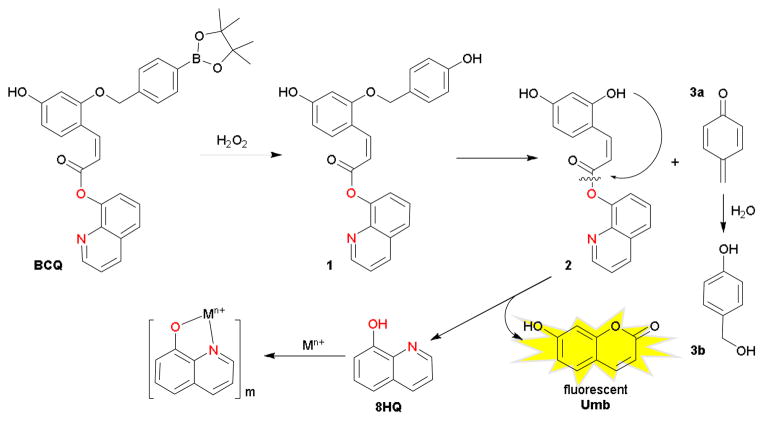
The present work describes the preparation of such a multifunctional prochelator, named BCQ. The design takes advantage of the intramolecular acyl substitution of cis-(ortho-hydroxy)cinnamic acid derivatives to form coumarin chromophores. The rapid lactonization reaction was previously utilized as a deprotection strategy for esterase-sensitive prodrugs.5 The concept was also notably extended to benzylidenemalonates for triggerable luminescence sensing.6 As shown in Scheme 1, BCQ relies on oxidation of an aryl boronic ester to yield an unstable phenol that undergoes spontaneous 1,6 benzyl elimination to release quinone methide (a reactive species that is ultimately quenched in water to 4-hydroxybenzyl alcohol, 3b) and a cis-(o-hydroxy)cinnamic acid ester. The exposed hydroxyl group is then expected to attack intramolecularly the electrophilic carboxylate carbon, with collapse of the tetrahedral intermediate leading to ejection of the 8HQ metal-binding unit7 simultaneously with 7-hydroxycoumarin (umbelliferone, Umb). Umbelliferone is a well-known fluorophore, absorbing near-UV and emitting in the blue region of the visible spectrum. By releasing equimolar quantities of chelator and fluorophore, real-time monitoring of prochelator activation may be possible.
Scheme 1.
Activation of the fluorogenic prochelator BCQ with hydrogen peroxide
Reported syntheses of compounds with cis-coumaric acid masking groups have primarily relied on reduction of the coumarin acid moiety or photochemical isomerization of the trans-coumarate.8 Neither of these routes yielded sufficient quantities of the cis configuration for our purposes. Other reported reactions utilize conditions that would degrade the bromomethyl arylboronic ester reagent required for BCQ and were thus not suitable.9 Instead, we developed an alternative synthetic route for opening coumarin rings and installing a trigger that renders the compound sensitive to H2O2.
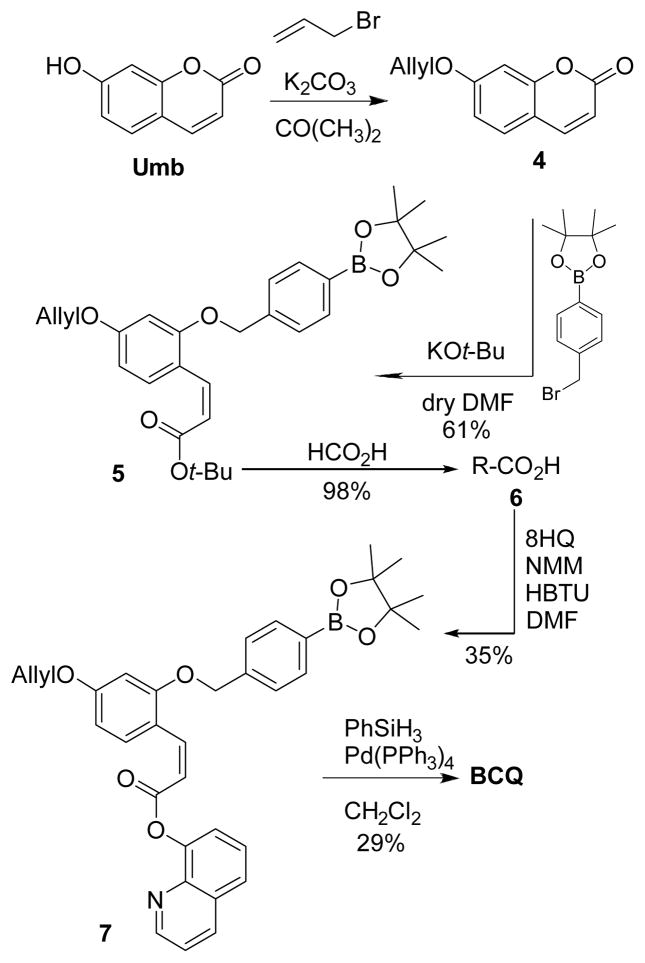
BCQ was prepared in five steps starting with the deconstruction of commercially available Umb (Scheme 2). Because this structure will ultimately be reconstructed upon peroxide activation, the scheme allows the flexibility to incorporate alternative coumarin derivatives that could be desirable for their different biological or physicalproperties. Allyl protection of the coumarin is suitable to block its phenol during subsequent esterification with 8HQ and is stable to both acidic and basic conditions used in the synthesis. The opening of the lactone ring in 4 under strongly basic conditions enables alkylation of the exposed phenolate with the bromomethyl arylboronic ester, thereby preventing ring closure upon quenching. Coupling with 8HQ, final deprotection, and purification by column chromatography yields the product as one isomer, which by 1H NMR spectroscopy reveals a 3J H-H coupling constant of 13 Hz that is characteristic for cis olefins. BCQ is obtained as a white solid dissolvable in DMSO in high concentrations useful as stock solutions.
Scheme 2.
Synthesis of BCQ
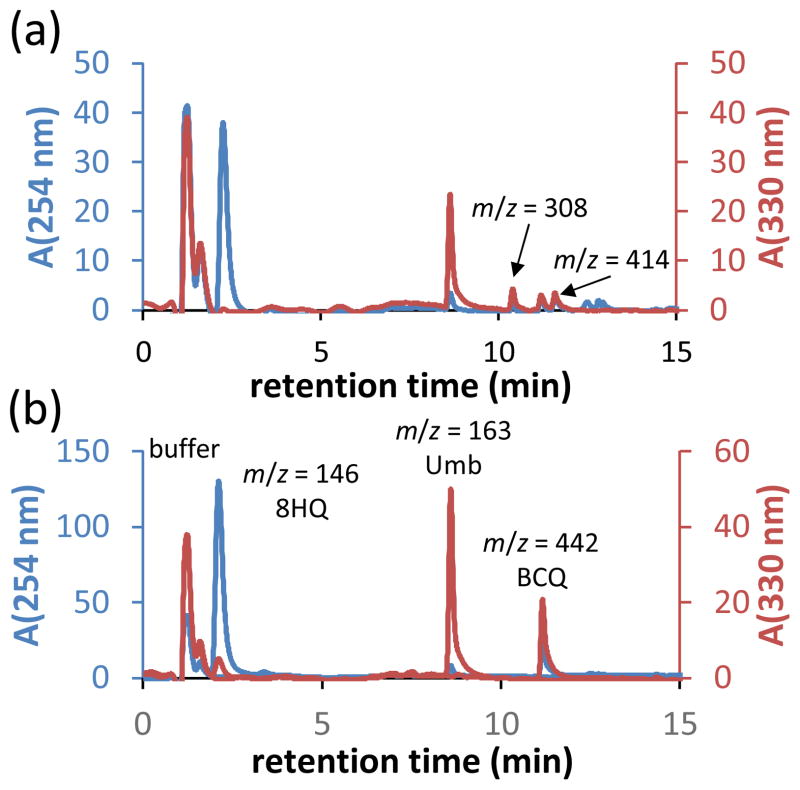
The reaction of BCQ with H2O2 was monitored by liquid chromatography-mass spectrometry (LC-MS) (Figure 1). Prior to peroxide addition, BCQ elutes as the boronic acid species at 11 min (m/z = 442). The lability of the pinacol ester in HPLC conditions has been previously reported.11 After 10 min of reaction, very little BCQ remains, but new peaks appear at 2.0 and 8.6 min with respective mass-to-charge (m/z) signals of 146 and 163 and absorption profiles best visualized at 254 and 330 nm, respectively (Figure 1a). These data match authentic 8HQ and Umb samples run under identical conditions (Figure 1b). In addition to these prominent peaks, two smaller peaks elute at 10.5 and at 11.5 min with corresponding m/z values of 308 and 414, respectively. The 414 species, which is consistent with intermediate 1 in Scheme 1, is not observed in chromatograms collected 1 h after peroxide addition when the starting material has been ~99% consumed (data not shown). This result fits a deprotection mechanism wherein rapid oxidation of the boronate moiety precedes rate-limiting 1,6 benzyl elimination. The 4-hydroxybenzyl alcohol was not observed by LC-MS, but its presence as a reaction product was verified by NMR (δ (ppm): 7.28 (d, J = 6 Hz, 2H); 6.79 (d, J = 6 Hz, 2H)).10 These results support the proposed activity of BCQ as a peroxide-dependent source of 8HQ and Umb.
Figure 1.
(a) A BCQ solution monitored by LC-MS 10 min following addition of 5 mM H2O2. UV detection at 254 nm (blue trace) and 330 nm (red trace) reveals only a trace signal of BCQ eluting ~12 min, with new species consistent with products 8HQ and Umb, as well as an intermediate (m/z = 414) and a side-product (m/z = 308). (b) Reference chromatograms of unreacted BCQ and authentic 8HQ and Umb samples (all 50 μM in 1:1 PBS:MeOH + 2% DMSO).
The small signal with m/z = 308 observed after 10 min of reaction persists with the same relative abundance even 12 h after peroxide addition (data not shown). This mass fits that expected for a species with molecular formula C18H14NO4 ([M+H]+ ion), consistent with intermediate 2 in Scheme 1.
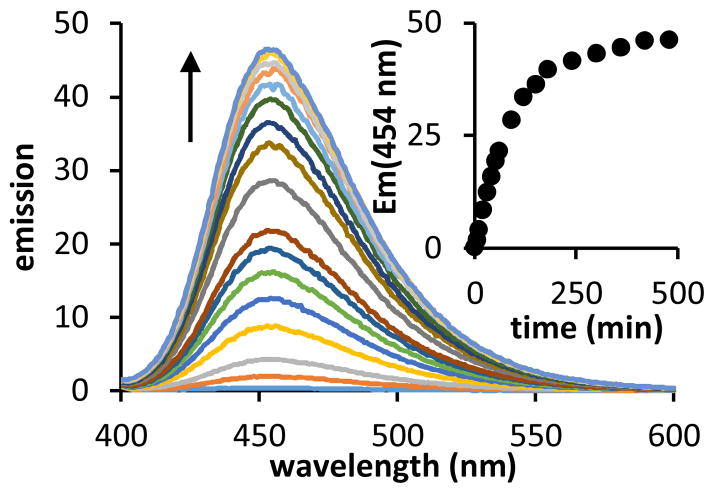
The proposed BCQ activation scheme is further supported by spectroscopic evidence. The fluorescence emission of a BCQ solution was monitored following treatment with a 100-fold molar excess of H2O2 (Figure 2). Upon mixing, the solution displayed a time-dependent growth of emission intensity. The resulting spectra (λex = 350 nm, λem,max = 454 nm) are consistent with Umb fluorescence. After reaction completion, the integrated emission intensity is 90-fold greater than that of the starting solution. The emission data fit a first-order reaction model with an observed pseudo-first order rate constant kobs = 0.010 s−1 (Figure 2 inset).
Figure 2.
A 1-μM solution of BCQ in PBS (0.1% DMSO) is nonfluorescent. Addition of 100 μM H2O2 results in growth of emission over time (λex=350 nm).
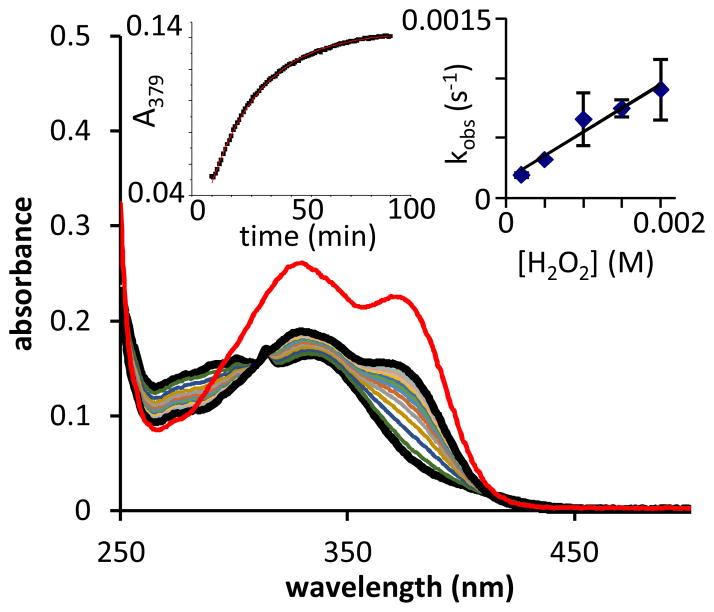
The absorption spectrum of BCQ in 50:50 PBS:methanol shows three peaks at 301, 314, and 335 nm (Figure 3). Due to the cinnamate chromophore, BCQ has significant absorption extending to ~425 nm. As the reaction with peroxide proceeds, the spectra shift with formation of isosbestic points at 305 and 410 nm. The spectrum obtained at 90 min (after no further spectral change is observed) bears the same features as a solution containing equimolar 8HQ and Umb.
Figure 3.
Absorption spectra show the change in spectral features as BCQ (dashed line, 20 μM in 1:1 PBS:MeOH) converts to products over the course of 90 min following addition of 1 mM H2O2 (t = 90 min, black, bold line). Intermediate spectra shown were collected at 5 min intervals. A solution of 20 μM 8HQ + 20 μM Umb in 1:1 MeOH:PBS (red, bold line) shown for comparison. Left inset: Absorbance vs. time data fit a pseudo-first order model for product formation during the reaction (red line). Right inset: A linear fit of kobs vs. [H2O2] gives the second-order rate constant for product formation.
Kinetic analysis of the UV-visible data collected under pseudo-first order conditions gives a second order rate constant of 0.54 M−1 s−1 for the appearance of product. This rate constant is consistent with those observed for other compounds protected with benzyl ether-linked boronic esters.10–11 We conclude from this observation that the inclusion of the pro-coumarin cinnamate moiety does not alter 8HQ release kinetics in this system.
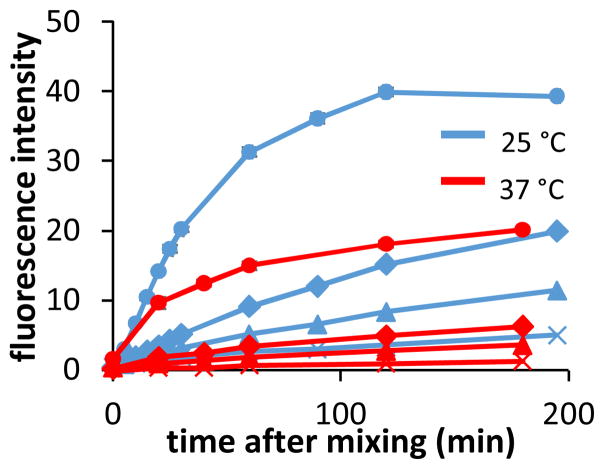
To gauge its feasibility for use in biological assays, BCQ (2 μM in PBS buffer) was mixed in microplate wells with H2O2 ranging in concentration from 156 nM to 200 μM. Umbelliferone emission was then monitored over several hours. The experiment was performed both at 25 °C and 37 °C (Figure 4). Wells containing as little as 2.5 μM H2O2 elicited discernible increases in fluorescence compared to negative control wells. However, the observed emission from activated BCQ wells did not match the intensities observed in wells loaded with Umb at the same concentration.
Figure 4.
Treatment of BCQ with 200 (●), 25 (◆), 10 (▲), or 0 μM H2O2 on a microplate results in dose-dependent fluorescence increases. Samples were incubated at 25 °C (blue) or 37 °C (red). Error bars for 200 μM samples represent the standard deviation from triplicate samples and are representative for the entire set. BCQ solutions were diluted to 2 μM in PBS from 1 mM stock in DMSO.
While spectroscopic and chromatographic data provide evidence of 8HQ and Umb release, results from these experiments consistently indicate that less product is generated than would be predicted based on the initial concentration of BCQ. A possible explanation for this deficit is a thermal isomerization of the cis configuration of intermediate 2 to the trans configuration. Such a change would reposition the ester and thereby disfavor lactonization. Only one unexpected species was observed in the final H2O2 reaction mixture: a compound that elutes at 10.5 min with an m/z value of 308 in the LC-MS (Figure 1a), which is consistent with either a cis or trans species 2. The cis-(o-hydroxy)cinnamate should undergo rapid cyclization to produce the product coumarin. This process clearly occurs on a rapid timescale given the appearance of coumarin in the chromatograms. The persistence of the m/z 308 species hours after reaction completion is consistent with the presence of a deactivated intermediate 2.
The temperature-dependent differences in emission intensity observed in the microwell plate assay also support this hypothesis (Figure 4). Wells incubated at 37 °C during activation exhibited substantially lower emission than those at room temperature; the increase in thermal energy would speed up not only peroxide activation but also the undesired isomerization reaction. Based on these combined data, we attribute the unexpected mass and the temperature dependence of emission to a byproduct trans-2.
BCQ exhibits many desirable attributes for a chemical tool to modify local metal coordination environments in response to an oxidative stimulus. Fluorescence detection could be used in microplate assays or fluorescence microscopy to learn more about the interaction of prochelators with oxidatively stressed cells. However, there are several characteristics of BCQ that warrant further development. BCQ exhibits low solubility in aqueous solutions without organic co-solvent, limiting its use to low concentrations (< 5 μM) or organic/aqueous solvent mixtures. Although Umb fluorescence is detectable at these low concentrations, studies from our laboratory suggest that higher prochelator concentrations are required to alter the fate of stressed cells.4,10 Consequently, improvements are desirable for future applications in living cells.
The turn-on response of BCQ could be enhanced by minimizing the thermal deactivation of the cis isomer. Depletion of the active form by isomerization lowers the yield of released chelator and fluorophore, so efforts to inhibit this process would benefit both activity and sensitivity. The reactivity of BCQ was explored here with H2O2, though it is important to keep in consideration that aryl boronates also undergo facile deprotection by peroxynitrite (ONOO−).12
In conclusion, a new synthetic route to transform coumarin fluorophores into stable cis-cinnamates enabled the development of a stimulus-responsive prochelator with fluorescent reporting. The synthesized compound, BCQ, demonstrates reactivity to hydrogen peroxide that results in oxidation of a boronic ester, followed by spontaneous 1,6-benzyl elimination and intramolecular nucleophilic substitution to release a desired metal chelator and an emissive fluorophore. The unactivated prochelator is nonfluorescent and exhibits a 90-fold increase in emission intensity upon exposure to hydrogen peroxide. BCQ signifies a proof of principle and first generation attempt to incorporate stimulus response, visual readout, and active compound release in a single molecule.
Supplementary Material
Acknowledgments
We thank Dr. George Dubay for assistance with LC–MS, the NIH for funding (GM084176), and the Burroughs Wellcome Fund administered by Duke Chemistry for fellowship support to ATF.
Footnotes
Electronic Supplementary Information (ESI) available: synthetic protocol, compound characterization data. See DOI: 10.1039/c000000x/
References
- 1.Faa G, Crisponi G. Coord Chem Rev. 1999;184:291. [Google Scholar]
- 2.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Lancet. 2007;369:397. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kell DB. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, Burt AD, Fleming KA. New Engl J Med. 1998;339:417. doi: 10.1056/NEJM199808133390701. [DOI] [PubMed] [Google Scholar]; (c) Bendova P, Mackova E, Haskova P, Vavrova A, Jirkovsky E, Sterba M, Popelova O, Kalinowski DS, Kovarikova P, Vavrova K, Richardson DR, Simunek T. Chem Res Toxicol. 2010;23:1105. doi: 10.1021/tx100125t. [DOI] [PubMed] [Google Scholar]
- 4.Festa RAH, Helse ME, Fran KJ, Thiele DJ. 2014 in press http://dx.doi.org/10.1016/j.chembiol.2014.06.009.
- 5.(a) Wang B, Huijuan Z, Wei W. Bioorg Med Chem Lett. 1996;6:945. [Google Scholar]; (b) Wang BH, Zhang HJ, Zheng AL, Wang W. Biorg Med Chem. 1998;6:417. doi: 10.1016/s0968-0896(98)00014-5. [DOI] [PubMed] [Google Scholar]; (c) Wang W, Jiang J, Ballard CE, Wang B. Curr Pharm Design. 1999;5:265. [PubMed] [Google Scholar]; (d) Hershfield R, Schmir GL. J Am Chem Soc. 1973;95:7359. doi: 10.1021/ja00803a025. [DOI] [PubMed] [Google Scholar]
- 6.Pershagen E, Nordholm J, Borbas KE. J Am Chem Soc. 2012;134:9832. doi: 10.1021/ja3004045. [DOI] [PubMed] [Google Scholar]
- 7.Dickens MG, Franz KJ. ChemBioChem. 2010;11:59. doi: 10.1002/cbic.200900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Zheng AL, Wang W, Zhang HJ, Wang B. Tetrahedron. 1999;55:4237. [Google Scholar]; (b) Xie Q, Wang XL, Wang XH, Jiang ZQ, Qiu ZB. Bioorg Med Chem Lett. 2005;15:4953. doi: 10.1016/j.bmcl.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.(a) Cairns N, Harwood LM, Astles DP, Orr A. J Chem Soc, Chem Commun. 1986:182. [Google Scholar]; (b) Grimm EL, Levac S, Trimble LA. Tet Lett. 1994;35:6847. [Google Scholar]
- 10.Kielar F, Helsel ME, Wang Q, Franz KJ. Metallomics. 2012;4:899. doi: 10.1039/c2mt20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Jourden JLM, Daniel KB, Cohen SM. Chem Commun. 2011;47:7968. doi: 10.1039/c1cc12526e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jourden JLM, Cohen SM. Angew Chem Int Ed. 2010;49:6795. doi: 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Chem Res Toxicol. 2012;25:1793. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.