SUMMARY
Quantitative assays for human DNA and mRNA were used to examine the paradox that intravenously (i.v.) infused human multipotent stromal cells (hMSCs) can enhance tissue repair without significant engraftment. After 2 × 106 hMSCs were i.v. infused into mice, most of the cells were trapped as emboli in lung. The cells in lung disappeared with a half-life of about 24 hr, but <1000 cells appeared in six other tissues. The hMSCs in lung upregulated expression of multiple genes, with a large increase in the anti-inflammatory protein TSG-6. After myocardial infarction, i.v. hMSCs, but not hMSCs transduced with TSG-6 siRNA, decreased inflammatory responses, reduced infarct size, and improved cardiac function. I.v. administration of recombinant TSG-6 also reduced inflammatory responses and reduced infarct size. The results suggest that improvements in animal models and patients after i.v. infusions of MSCs are at least in part explained by activation of MSCs to secrete TSG-6.
INTRODUCTION
Currently there is interest in developing new therapies with cells that can repair nonhematopoietic tissues (Caplan, 2007; Giordano et al., 2007; Prockop and Olson, 2007). Among the cells being tested are the adult stem/progenitor cells from bone marrow initially referred to as fibroblastic colony-forming units, then as marrow stromal cells, subsequently as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells or MSCs (Dominici et al., 2006). The first clinical trials with MSCs were in osteogenesis imperfecta (Horwitz et al., 1999), then in lysosomal storage diseases (Koc et al., 1999), and subsequently in graft versus host disease (Le Blanc and Ringden, 2006). Currently MSCs are being employed in clinical trials in heart disease, Crohn’s disease, cartilage repair, stroke, spinal cord injury, and several other diseases (Prockop and Olson, 2007; Caplan, 2007; Giordano et al., 2007). In many of the experimental animal models and in clinical trials, the cells were intravenously (i.v.) infused (Pereira et al., 1998; Horwitz et al., 1999; Akiyama et al., 2002; Koc et al., 2002; Nomura et al., 2005; Le Blanc et al., 2007; Wu et al., 2008). The functional improvements were surprising, since MSCs infused i.v. into mice or rats were rapidly trapped in lung (Gao et al., 2001; Schrepfer et al., 2007) as microemboli that are potentially lethal (Lee et al., 2009). Trapping of MSCs in the lung is not in itself unexpected, since the cells tightly adhere to culture plasticware and to each other as they are expanded in culture (Colter et al., 2001; Owen and Friedenstein, 1988). Also, trapping of cells in lungs occurs with metastatic tumors (Khanna et al., 2004) and probably hematopoietic stem cells (Dooner et al., 2004). However, it was not clear how MSCs trapped in the lung can enhance repair of the heart, brain, and other tissues.
Here we first developed assays to provide quantitative data on the fate of human cells infused into mice. We then demonstrated that i.v.-infused human MSCs (hMSCs) produced functional improvement in mice with myocardial infarction (MI) at least in part because the cells trapped as emboli in lung are activated to express the anti-inflammatory factor TNF-α-induced protein 6 (TNAIP6 or TSG-6).
RESULTS
Clearance from Blood and Trapping of Systemically Infused hMSCs
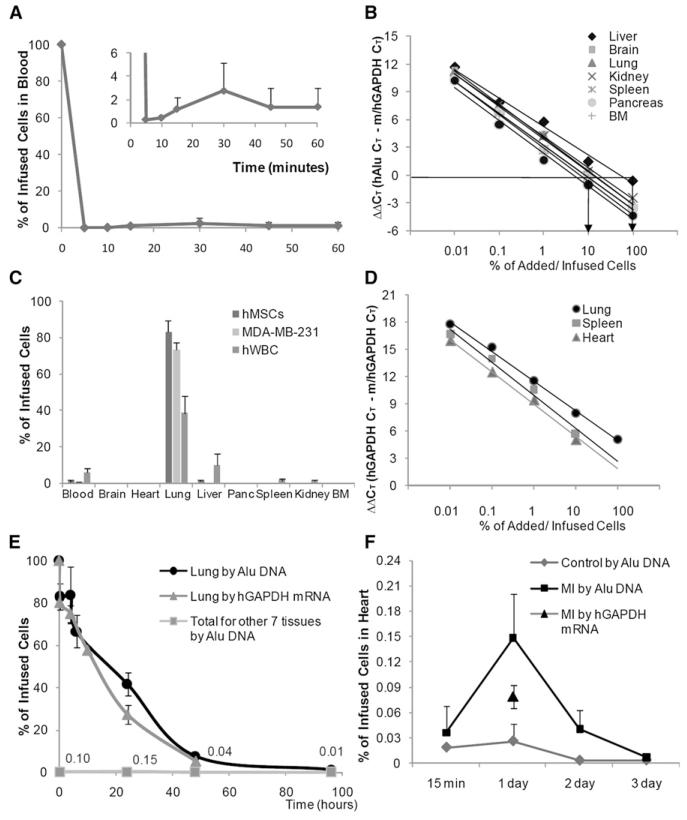
To follow the fate of hMSCs infused i.v. into mice, we used realtime PCR assays for human-specific Alu sequences (McBride et al., 2003). After i.v. infusion of 2 × 106 hMSCs, the Alu assay indicated that 99% ± 1.07% SD of the cells were cleared from the circulation within 5 min (Figure 1A). From 2% to 3% of the cells (4–6 × 104) reappeared in the circulation after a lag period of about 10 min, apparently after release from the lung. To verify that the small number of Alu sequences detected in blood reflected hMSCs, 50 μl of peripheral blood recovered after 15 min was plated on plastic culture dishes in hMSC medium and incubated for 14 days. The cultures generated typical colonies of spindle-shaped hMSCs that were labeled with antibodies to both human nuclei antigen and human β2-microglobulin (see Figure S1 available online). To follow the distribution of the cells in tissues, we developed individual standard curves for each tissue by adding varying numbers of hMSCs to the tissues from naive mice just before homogenization (Figure 1B). The use of tissue-specific standard curves minimized variations introduced by differences in yields of extracted DNA, cell numbers of the organs, or efficiencies of the PCR reactions. The sensitivity of the assay was about 100 human cells per mouse organ assayed. To facilitate the assay, a quantitative colorimetric assay for DNA in extracts (Burton, 1956) was used instead of UV absorbance to select appropriate aliquots for the PCR reactions. As expected (Gao et al., 2001; Schrepfer et al., 2007; Lee et al., 2009), most of the cells cleared from the circulation were trapped in the lung. In mice sacrificed after 15 min, 83% ± 6.3% SD of the human DNA was recovered in lung, and only trace amounts were recovered in other tissues (Figure 1C). Similar results were obtained in control experiments with i.v. infusions of a line of metastatic breast carcinoma cells (MDA-MB-231 in Figure 1C). After i.v. infusion of human white blood cells, a smaller fraction was recovered in lung after 15 min, and larger numbers both remained in circulation and appeared in liver (Figure 1C). The fraction of hMSCs trapped in the lung was not significantly reduced by decreasing the number of infused MSCs to as little as 10,000 in the same volume of vehicle (150 μl), pretreating the cells with antibodies to integrin-α4 or integrin-a6 (Qian et al., 2006), or preincubating the cells with rat white blood cells (Chute, 2006). To examine effects of arterial infusion, 2 × 106 hMSCs were infused into the left ventricle of the heart. Most of the cells were again cleared from the blood in 5 min (Figure S2A), but there again was a small recirculation of about 1.72% ± 1.81% SD of the infused cells for 15–60 min (Figure S2A). Also, in comparison to i.v. infusions, larger numbers of the cells were recovered in organs such as brain, heart, lung, liver, and kidney 15 min after the infusions (Figure S2B). Control experiments with breast metastatic cancer cells produced a similar pattern of tissue distribution (Figure S2B).
Figure 1. Assays for Fate of hMSCs Infused into Mice.
(A) Clearance of human Alu sequences from blood after i.v. infusion of about 2 × 106 hMSCs into mice. Values are means ± SD; n = 6.
(B) Standard curves for real-time PCR assays of human Alu sequences in seven organs. Values indicate ΔΔCt for primers for mouse/human GAPDH genes and Alu sequences on same samples.
(C) Tissue distribution of human Alu sequences 15 min after i.v. infusion of about 2 × 106 hMSCs into mice. Values are means ± SD; n = 6.
(D) Standard curves for real-time RT-PCR assays of human mRNA for GAPDH. Values indicate ΔΔCt for primers for mouse/human GAPDH genes and cDNA for human-specific GAPDH on the same samples.
(E) Kinetics of hMSCs in lung and six other tissues after i.v. infusion of about 2 × 106 hMSCs. Values are means ± SD; n = 6.
(F) Appearance of hMSCs in heart after i.v. infusion of about 1 × 106 hMSCs 1 day after permanent ligation of the left anterior descending coronary artery.
For a semiquantitative assay for viable cells (Nishida et al., 2006), a similar strategy was used to develop a quantitative RT-PCR assay specific for human GAPDH mRNA (Figure 1D). The assay had about the same sensitivity as the Alu assay but required more manipulation of the samples. Data developed with the assay indicated that the distribution of Alu sequences largely reflected live cells (Figures 1E and 1F).
Kinetics and Redistribution of hMSCs Trapped in Lung
To examine the redistribution with time of the cells from lung, 2 × 106 hMSCs were infused i.v., and seven tissues of mice were assayed for up to 4 days (Figure 1E). Assays for Alu sequences indicated that the cells initially trapped in lung disappeared with a half-life of about 24 hr. Similar values were obtained by assays for viable human cells by the levels of human mRNA for GAPDH (Figure 1E). Histological sections of lung demonstrated that the hMSCs trapped in lung formed emboli in afferent blood vessels (Lee et al., 2009), with many of the cells undergoing apoptosis (data not shown). The cells that disappeared from lung did not appear in any significant numbers in the six other tissues: a total of 0.04% of the infused Alu sequences (equivalent to about 4000 cells) were recovered in the six tissues after 48 hr and 0.01% after 96 hr (Figure 1E).
Trapping of hMSCs in Infarcted Heart
To determine whether larger numbers of i.v.-infused hMSCs appeared in the heart after MI, hMSCs were infused into the tail veins for NOD/SCID mice 1 day after MIs were produced by permanent ligation of the anterior descending coronary artery (LAD). Assays for Alu sequences indicated that 0.04% ± 0.03% SD of the infused cells (400 cells ± 300 SD; n = 5) were recovered in the infarcted hearts 15 min after the infusion (Figure 1F). One day after i.v. infusions, the Alu sequences in heart increased about 5-fold to 0.148% ± 0.053% SD, equivalent to about 1480 cells ± 530 SD (n = 5). Similar values were obtained by assays for human GAPDH mRNA (792 cells ± 140 SD; n = 5) 1 day after the infusions.
Changes in the Mouse and Human Transcriptome Produced by Embolization
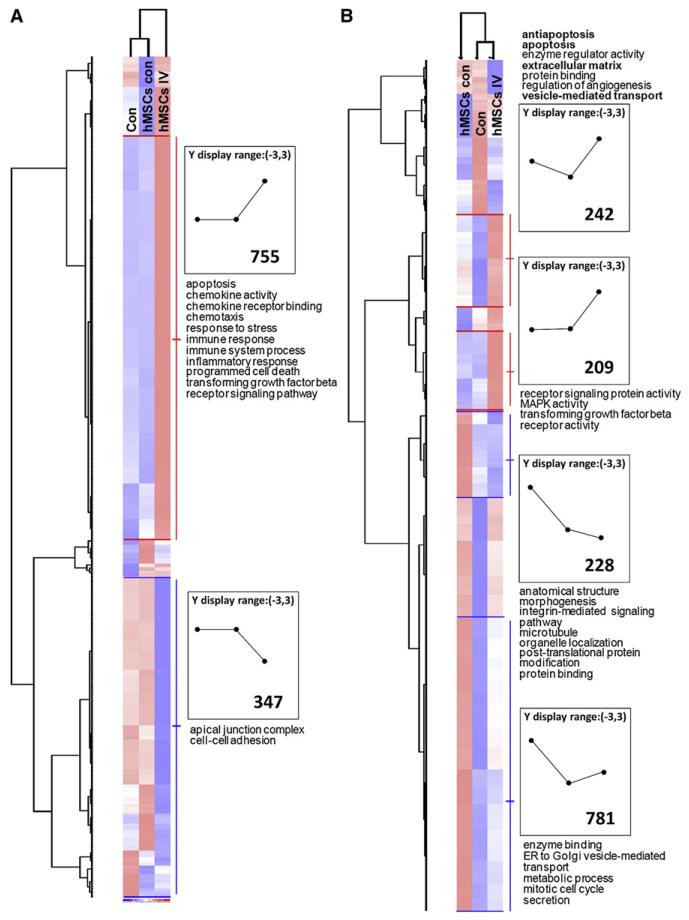
To assay both transcriptomes, about 2 × 106 hMSCs were infused into the tail veins of mice, and RNA was extracted from lung 10 hr later, a time at which assays for human GAPDH mRNA indicated there were adequate amounts of human mRNA for assays (Figure 1E). After filtering for cross-hybridization with human mRNA (see the Supplemental Data), the data indicated that embolization with the hMSCs upregulated expression of 755 mouse transcripts and downregulated expression of 347 mouse transcripts 2-fold or more (Figure 2A). Also, the data indicated that after embolization in lung, 451 human transcripts were upregulated and 1009 transcripts were downregulated (Figure 2B).
Figure 2. Heat Map of Microarray Assays of Mouse Lungs after I.v. Infusion of hMSCs.
About 2 × 106 hMSCs were infused i.v., and lung RNA was recovered 10 hr later for assays on both mouse-specific and human-specific microarrays (Affymetrix, Santa Clara, CA). Data were filtered for cross-hybridization (CV > 0.5 and call > 33%), analyzed with the Microarray Suite 5.0 program, and normalized to a value of 1 and variance of 3 SD (+3, red; 3, blue). Gene ontology categories of genes are indicated. The number of genes with expression differences is indicated in the boxes.
(A) Assay on mouse-specific chip.
(B) Assay of same RNA on human-specific chip. Symbols: con, lung from control mouse; hMSCs con, sample of hMSCs added to lung from control mouse before extraction of RNA; hMSCs i.v., sample from mouse lung 10 hr after i.v. infusion of hMSCs.
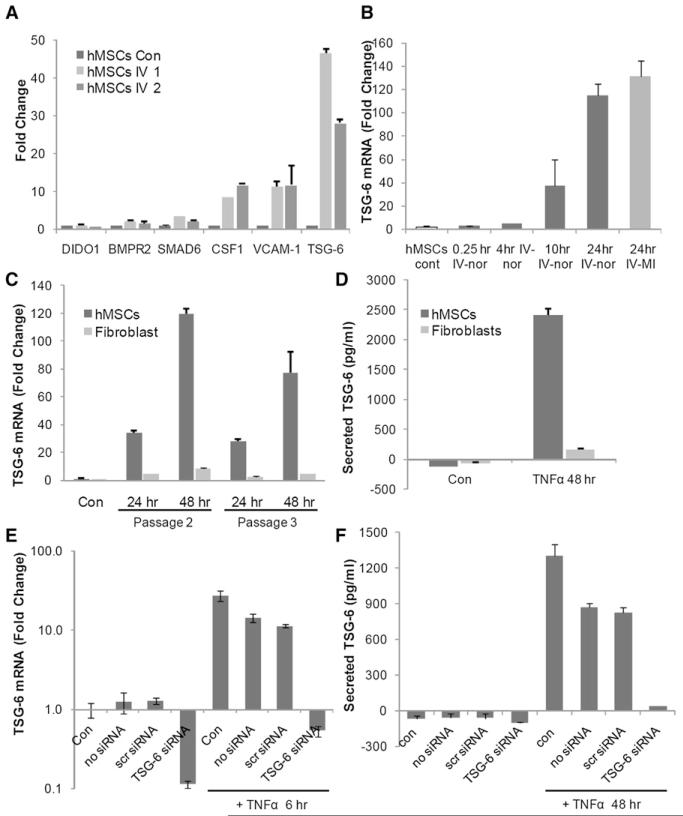
The upregulated 451 human transcripts were subjectively examined for candidate genes of interest, and human-specific real-time RT-PCR assays were used to confirm the microarray data (Figure 3A). The results confirmed 2-fold or greater increases in the transcripts for SMAD6, CSF1, VCAM-1, and TNFAIP6 (TSG-6). The increases in TSG-6 were 28-fold and 47-fold or considerably larger than the 7.5-fold increase detected by the microarrays (Table S1). As recently reported (Ylostalo et al., 2008), real-time RT-PCR assays frequently demonstrated larger changes in transcripts than microarray assays with the system employed here.
Figure 3. Activation of hMSCs to Express TSG-6.
(A) Real-time RT-PCR assays for human-specific mRNAs in lung 10 hr after i.v. infusion of 2 × 106 hMSCs. Values are fold increase over values for cultured hMSCs, normalized by ΔΔCt for hGAPDH. Symbols: hMSCs con, sample of hMSCs added to lung from control mouse before extraction of RNA; hMSCs i.v. 1 and 2, samples from lungs of two mice 10 hr after i.v. infusion of hMSCs.
(B) Real-time RT-PCR assays for human TSG-6 in mouse lung. About 2 × 106 hMSCs were infused i.v. into naive mice (IV-nor) or mice at 1 hr after MI (IV-MI), and lungs were recovered 0.25-24 hr after the infusions. Values are ± SD; n = 2 or 3 for normal mice; n = 6 for MI mice.
(C) Real-time RT-PCR assays for TSG-6 in hMSCs and human fibroblasts from the same donor incubated in serum-free medium with 10 ng/ml TNF-α for 24 or 48 hr. Results with two passages of the same cells are shown. Values are ± SD; n = 3.
(D) ELISA assays for TSG-6 in medium from hMSCs and human fibroblasts incubated in serum-free medium with 10 ng/ml TNF-α for 48 hr. Values are ± SD; n = 3.
(E) Real-time RT-PCR assays TSG-6 of control hMSCs (Con), hMSCs treated with transfection reagents only (no siRNA), hMSCs transfected with a scrambled siRNA (scr siRNA), or hMSCs transduced with TSG-6 siRNA (TSG-6 siRNA). Cells were incubated with or without 10 ng/ml TNF-α for 6 hr. Values are ± SD; n = 3.
(F) ELISA assays for TSG-6 in medium after incubation of cells with or without TNF-α for 48 hr. Symbols are as in (E). Values are ± SD; n = 3.
MSCs In Vitro Are Activated to Secrete TSG-6
The increase in TSG-6 was of particular interest because the protein was previously shown to be a powerful anti-inflammatory factor (Forteza et al., 2007; Getting et al., 2002; Wisniewski and Vilcek, 2004; Milner et al., 2006). Real-time RT-PCR assays demonstrated that human TSG-6 mRNA in lung was increased at 10 hr and further increased at 24 hr after i.v. infusions of hMSCs (Figure 3B). There was no difference in expression of TSG-6 in lungs from naive mice and mice with MIs (Figure 3B). TSG-6 was discovered by analysis of cDNA clones from skin fibroblasts that were incubated with TNF-α (Lee et al., 1992). Therefore, hMSCs and fibroblasts from same donor were incubated with TNF-α, and the mRNAs were assayed by real-time RT-PCR. The transcript for TSG-6 in hMSCs was increased about 120-fold after incubation with 10 ng/ml TNF-α for 48 hr and increased about 80-fold with a further passage of the hMSCs (Figure 3C). ELISAs indicated that incubation with TNF-α for 48 hr increased the secretion of TSG-6 protein from undetectable levels to over 2000 pg/ml per 105 cells per 48 hr (Figure 3D). Surprisingly, the response of hMSCs to TNF-α far exceeded the response of human fibroblasts. In parallel experiments, mouse MSCs incubated with TNF-α under the same conditions upregulated expression of the transcript for TSG-6 3.94-fold (±0.49 SD; n = 4).
Transient transduction of hMSCs with TSG-6 siRNA abrogated the effects of TNF-α on TSG-6 transcription (Figure 3E) and secretion (Figure 3F). Expression of TSG-6 was partially reduced by a mock transduction or transduction with a scrambled siRNA.
Both I.v. MSCs and rhTSG-6 Decrease Proinflammatory Proteases in Mice with MI
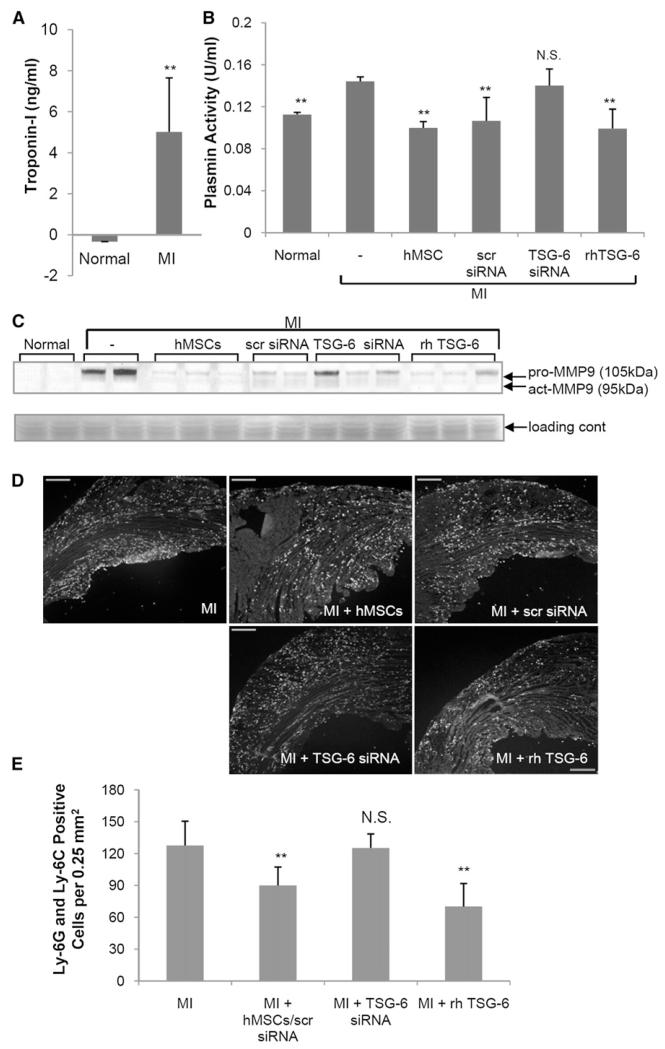
Acute MI produces an acute inflammatory response in which infiltrating neutrophils generate MMPs that degrade the myocardium (Fang et al., 2007; Lindsey et al., 2001). The permanent LAD increased serum levels of both cardiac troponin I (Figure 4A), a biomarker for myocardial injury (Chapelle, 1998; Pervaiz et al., 1997), and plasmin activity (Figure 4B), a marker for inflammatory responses (Heymans et al., 1999; Griffin et al., 2005). The plasmin activity was decreased by two infusions of rhTSG-6, an observation consistent with its known inhibitory effects (Bardos et al., 2001; Milner et al., 2006). The plasmin activity was also decreased by i.v. infusion of hMSCs and hMSCs with a scrambled siRNA, but not hMSCs transduced with siRNA for TSG-6.
Figure 4. Assays of Serum and Heart.
(A) Assay for cardiac troponin I in serum 48 hr after MI. Values are ± SD; **p < 0.01 with n = 3 (normal) or six mice (MI) per group.
(B) Plasmin activity in serum 48 hr after MI. Symbols: Normal, naive mice; −, MI only; hMSCs, 2 × 106 hMSCs infused i.v. 1 hr after MI; scr siRNA, 2 × 106 hMSCs transduced with scrambled siRNA infused i.v. 1 hr after MI; TSG-6 siRNA, 2 × 106 hMSCs transduced with TSG-6 siRNA infused i.v. 1 hr after MI; rhTSG-6, 30 μg rhTSG-6 protein infused i.v. 1 hr and again 24 hr after MI. Values are ± SD; **p < 0.01 with n = 3 mice per group. N.S., not significant.
(C) Hearts assayed for pro- and active-matrix MMP9 on a gelatin zymogen gel 48 hr after MI. Image is reversed. Symbols are as in (B).
(D and E) Granulocyte and monocyte infiltration in the heart 48 hr after MI. Sections stained with anti-Ly-6G and Ly-6C. Symbols are as in (B), except that 100 μg rhTSG-6 protein was infused i.v. 1 hr and again 24 hr after MI. Magnification ×4. Scale bars, 250 μm. Values are ± SD; n = 3 or 4 for each group. **p < 0.001; N.S., not significant.
As expected (Fang et al., 2007), the enzymic activities of both pro-MMP9 and active MMP9 were increased in heart 2 days after MI (Figure 4C). I.v. infusion of hMSCs or hMSCs transduced with a scrambled siRNA decreased both activities (Figure 4C). The effects of hMSCs were partially negated by knockdown of the TSG-6 gene prior to infusion of the cells. Also, the effects of hMSCs were partially duplicated by the two infusions of human recombinant TSG-6. The decreases in pro-MMP activities were reflected in decreases in granulocyte and monocyte infiltration in the heart (Figures 4D and 4E).
Effects of TSG-6 on Infarct Size and Heart Function in MI
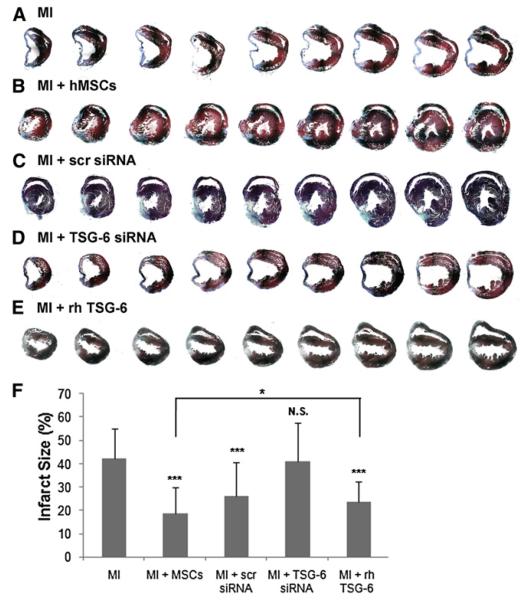
As reported previously (Iso et al., 2007), i.v. infusion of hMSCs decreased infarct size examined 3 weeks after MI (Figures 5A, 5B, and 5F and Figure S3). hMSCs with an siRNA knockdown of the TSG-6 gene had no effect on infarct size (Figures 5D and 5F). hMSCs transduced with the scrambled siRNA produced an intermediate effect on infarct size (Figures 5C and 5F), apparently because the scrambled siRNA had a partial effect on TSG-6 secretion (Figures 3E and 3F). In addition, i.v. infusion of 100 μg of rhTSG-6 immediately following the surgery and at 24 hr also decreased infarct size (Figures 5E and 5F and Figure S3). However, the effect of rhTSG-6 was somewhat less than the decrease in infarct size following administration of the hMSCs (p < 0.05).
Figure 5. Assays of Infarct Size.
Each heart was cut from the apex through the base into over 400 sequential 5 μm sections and stained with Masson Trichrome. Every twentieth section is shown. Additional heart samples are shown in Figure S3.
(A–E) Symbols are as in Figure 4B, except that 100 μg rhTSG-6 protein was infused i.v. 1 hr and again 24 hr after MI.
(F) Infarct size measurements (%) obtained by midline length measurement from tenth section of the infarct area for a total of 20 sections per heart (Takagawa et al., 2007). Values are ± SD; n = 3 or 4 mice in each group; ***p < 0.0001 compared to MI controls; N.S., not significant compared to MI controls; *p < 0.05 for MI + MSCs versus MI + rhTSG-6.
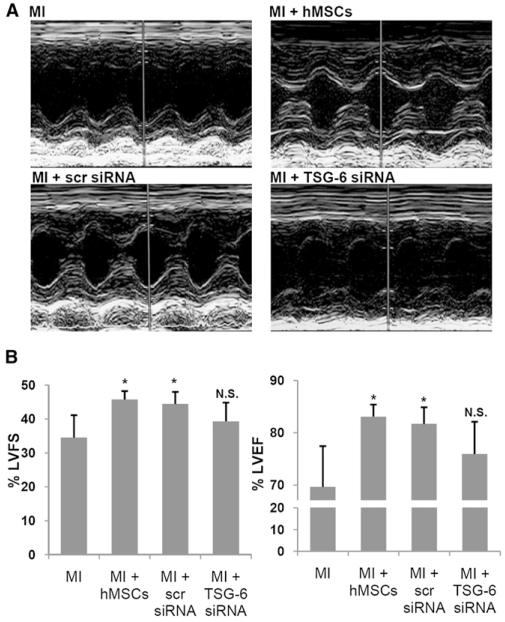
Assays by echocardiography demonstrated comparable effects on heart function. I.v. infusions of 2 × 106 hMSCs or hMSCs with a scrambled siRNA 1 hr after MI produced significant improvements in percent left ventricular fractional shortening and left ventricular ejection fraction in hearts assayed 3 weeks later (Figure 6 and Table S3). Infusions of hMSCs with a knockdown of TSG-6 had no effect.
Figure 6. Echocardiographic Assays 3 Weeks after MI.
(A) Representative M-mode echocardiograms. Symbols are as in Figure 4B.
(B) Left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF) from echocardiographic data. Values are ± SD; n = 5 or 6 for each group; *p < 0.05 versus MI; N.S., not significant.
DISCUSSION
The hMSCs trapped in mouse lung after i.v. infusion underwent major changes in their patterns of gene expression in response to the injury to the lung produced by microembolization of the cells in the pulmonary vasculature (Furlani et al., 2009; Lee et al., 2009). The upregulation of the human TSG-6 was of special interest because of the known anti-inflammatory effects of the protein (Milner et al., 2006; Wisniewski and Vilcek, 2004) and because excessive inflammatory responses contribute to the pathological changes produced by MI (Ovechkin et al., 2005; Paolocci et al., 2006; Carvalho et al., 2006; Fang et al., 2007; Moshal et al., 2008). Therefore, the results suggested a possible explanation for the observations that i.v. infusions of MSCs improved cardiac function in models for MI (Halkos et al., 2008; Iso et al., 2007; Krause et al., 2007; Wolf et al., 2007). In the mouse model for MI, knockdown of TSG-6 expression in hMSCs largely negated the improvements in inflammatory responses, infarct size, and cardiac function produced by i.v. fusions of hMSCs. In addition, i.v. infusions of rhTSG-6 largely duplicated the therapeutic effects of the hMSCs on inflammatory responses and infarct size. Therefore, the results indicated that the hMSCs that were trapped in the lung were activated to secrete TSG-6, and the TSG-6 suppressed the excessive inflammatory response to LAD so as to decrease the proteolytic damage to the heart and the subsequent fibrotic scarring and decrease in cardiac function. The 1500 or so of hMSCs transiently appeared in the infracted heart after infusion of 106 hMSCs may also have contributed to the anti-inflammatory effects.
The upregulation of TSG-6 was detected by the cross-species strategy of infusing hMSCs into NOD/SCID mice. Similar strategies of using hMSCs in animal models previously proved useful, because the hMSCs provided numerous endogenous markers for the cells and no obvious cross-species artifacts were encountered (Hwang et al., 2008; Lu et al., 2009; Bai et al., 2009; Gonzalez-Rey et al., 2009; Sasportas et al., 2009), apparently because of the immune modulatory effects of the cells (Uccelli et al., 2008). Also, the strategy of using hMSCs avoids the technical difficulties of isolating mouse MSCs (Baddoo et al., 2003; Gnecchi and Melo, 2009; Peister et al., 2004; Sung et al., 2008) and the marked tendency of mouse MSCs to develop genomic instability and become tumorgenic as they are expanded in culture (Sung et al., 2008; Tolar et al., 2007). Permanent LAD ligation in mice does not mimic human MI as closely as ischemia and reperfusion models in larger animals. However, permanent LAD ligation in NOD/SCID mice provided a useful model for testing the effects on hMSCs because the mice retained the excessive inflammatory responses to MI (Iso et al., 2007).
TSG-6 is a 30 kDa glycoprotein (Heng et al., 2008; Milner et al., 2006) that was shown to produce three distinct anti-inflammatory effects (Milner et al., 2006; Wisniewski and Vilcek, 2004): (1) it inhibits the inflammatory network of proteases primarily by increasing the inhibitory activity of inter-α-inhibitor, (2) it binds to fragments of hyaluronan and thereby blunts their proinflammatory effects, and (3) it inhibits neutrophil infiltration into sites of inflammation. In transgenic mice, inactivation of the gene increased inflammatory responses (Szanto et al., 2004), and overexpression of the gene decreased inflammatory responses (Mindrescu et al., 2002). Also, administration of the recombinant protein improved arthritis in several murine models (Bardos et al., 2001; Mindrescu et al., 2000). Although TSG-6 was originally discovered by screening cDNA libraries from fibroblasts incubated with TNF-α (Lee et al., 1992), the results here demonstrated that hMSCs produced far more TSG-6 in response to TNF-α than dermal fibroblasts.
The results do not exclude the possibility that the hMSCs trapped in lung secreted additional cardioprotective factors in addition to TSG-6. The effects of rhTSG-6 on infarct size in the mice were slightly less than the effects of i.v. infusions of hMSCs. MSCs in culture and in response to chemokines or injured cells secrete large amounts of therapeutic factors such as TGF-β, HGF, IL-4, IL-10, PGE2, and stanniocalcin-1 (Caplan, 2009; Gnecchi et al., 2008; Block et al., 2009; Ohtaki et al., 2008). TSG-6 may, however, play a key role in many beneficial effects of MSCs. Inflammatory responses to sterile tissue injury are frequently excessive and require active suppression (Schwab et al., 2007). Also, chronic inflammation plays a key role in diseases such as diabetes, stroke, Alzheimer’s disease, and Parkinson’s disease (Bergsbaken et al., 2009; McCombe and Read, 2008; Shoelson et al., 2006; Theuma and Fonseca, 2004). Therefore, secretion of TSG-6 by MSCs trapped as emboli in lung may in part explain the therapeutic effects observed after i.v. infusions of MSCs in animal models for these and other diseases (Uccelli et al., 2008; Ezquer et al., 2008; Parr et al., 2007). Secretion of TSG-6 may also play a role in therapies for heart disease with other cells such as skeletal myoblasts, fetal myoblasts, and embryonic stem cells (Jolicoeur et al., 2007).
EXPERIMENTAL PROCEDURES
Cell Preparations
hMSCs and mouse MSCs from bone marrow were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (formerly http://www.som.tulane.edu/gene_therapy/distribute.shtml; currently available by contacting msc@medicine.tamhsc.edu). The center has supplied standardized preparations of MSCs enriched for early progenitor cells to over 250 laboratories under the auspices of a National Institutes of Health/National Center for Research Resources (NIH/NCRR) grant (P40 RR 17447-06). The hMSCs were expanded to passage 3 and 70% confluency and the mouse MSCs cultures as indicated in the Supplemental Data. Source and conditions for culture of human breast carcinoma cells and fibroblasts are also presented in the Supplemental Data.
I.v. Infusion of hMSCs
Mice were anesthetized, and 150 μl of a suspension of about 1 or 2 × 106 hMSCs was infused with a 28 gauge needle either through a tail vein or through the chest wall into the left ventricle. Successful i.v. infusion was monitored by lack of extravasation at the site and recoveries of about 80% of the Alu sequences in lung within the first hour of infusion (Figure 1C). Prior to infusion, the cells were maintained at 4°C, and they were gently resuspended with a pipette to ensure they were not aggregated before infusion.
Isolations of DNA and RNA
Blood samples of 50 μl were withdrawn with a needle and syringe from the left ventricle of the heart and adjusted to 2 mM EDTA. The mice were then perfused through the left ventricle with 20 ml of PBS and then through the right ventricle with 5 ml of PBS. Brain, heart, lung, liver, pancreas, spleen, kidney tissues, and bone marrow were isolated by dissection and stored at −80°C. To extract DNA, the samples were thawed and added to in 5 ml buffer (10 mM Tris HCl [pH 8.0] containing 20 μl Proteinase K [10 mg/ml], 0.1 mM EDTA [rO 8.0], 0.5% SDS, and 20 ug/ml RNase A). The samples were homogenized (PowerGen Model 125 Homogenizer; Fisher Scientific) and incubated in a shaker at 200 rpm and 50°C overnight. DNA was extracted by mixing 0.5 ml of sample with 0.5 ml phenol/chloroform solution (pH 6.7) and centrifugation at 15,300 × g for 5 min in 2 ml phase lock gel tubes (Phase Lock Gel; Eppendorf/Brinkmann Instruments, Inc). DNA was precipitated with half volume of 2.5 M ammonium acetate and same volume of 100% ethanol overnight at 4°C. The precipitates were washed with ice-cold 75% ethanol and resuspended in sterile water.
RNA was isolated from the same mouse tissues and from cell cultures using Trizol (Invitrogen) and cleaned by RNeasy Mini Kit (QIAGEN).
Real-Time PCR Assays for Alu Sequences
Because assays by UV absorbance of DNA extracts from several tissues did not provide values accurate enough for the PCR assays, DNA concentration was measured by diphenylamine reaction (Burton, 1956). Samples of 40 μl were digested for 1 hr at 37°C with 3 μl DNase I (Fisher Scientific) in 5 μl DNase buffer and 2 μl of sterile water. Each sample was diluted with 50 μl of sterile water, and 200 μl of a stock solution of diphenylamine reagent was added (1 g diphenylamine [Fisher Scientific] in 100 ml glacial acetic acid [Fisher Scientific] and 2.75 ml H2SO4 [Sigma]). The samples were incubated for 21 min at 100°C, and absorbance was measured at 595 nm. Standard curves were prepared with 0.039–1.25 mg/ml calf thymus DNA (Sigma).
Real-time PCR assays for Alu sequences (McBride et al., 2003) were performed in a volume of 50 μl that contained 25 μl Taqman Universal PCR Master Mix (Applied Biosystems), 900 nM each of the forward and reverse primers, 250 nM TaqMan probe, and 200 ng target template (see the Supplemental Data for sequences of primers and probes). Reactions were incubated at 50°C for 2 min and at 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Standard curves were generated by adding serial dilutions of hMSCs into mouse tissue samples just prior to homogenization. Real-time PCR assays for human and mouse genes for GAPDH were performed in a volume of 50 μl that contained 25 μl SYBR Green Master Mix (Applied Biosystems), 200 nM each of the forward and reverse primers, and 200 ng target template. All real-time PCR assays were performed in duplicate or triplicate, and average values are presented. The final value for total DNA in the sample was corrected by parallel real-time PCR assays with primers that amplified both the human and the mouse gene for GAPDH (NCBI home page, http://www.ncbi.nlm.nih.gov/geo/; Supplemental Data).
Real-Time RT-PCR Assays for mRNA for Human GAPDH
Standard curves were generated by adding serial dilutions of hMSCs to mouse tissue samples just prior to homogenization. About 200 ng of total RNA was used to synthesize double-stranded cDNA by reverse transcription (Super-Script III; Invitrogen). cDNA was analyzed by real-time PCR (ABI 7900 Sequence Detector, Applied Biosystems) with human-specific GAPDH primers and probe (TaqMan Gene Expression Assays ID, Hs00266705_g1) using Taqman Universal PCR Master Mix (Applied Biosystems). The final value for total cDNA in the sample was corrected by parallel real-time PCR assays with primers that amplified both the human and the mouse genes for GAPDH (see the Supplemental Data).
Assays of mRNAs in Lung by Microarrays
RNA was isolated from lungs of mice, assayed on both mouse (MG-430 2.0) and human (HG-U133 Plus 2.0) microarrays (Affymetrix, Santa Clara, CA), and the data filtered as described in the Supplemental Data.
Real-Time RT-PCR Analysis for Selected mRNAs
About 200 ng of total RNA was used to synthesize double-stranded cDNA by reverse transcription (SuperScript III; Invitrogen). cDNA was analyzed by realtime PCR using Taqman Universal PCR Master Mix (Applied Biosystems). For the assays, reactions were incubated at 50°C for 2 min, 95°C for 10 min, and then 40 cycles at 95°C for 15 s followed by 60°C for 1 min. For relative quantitation of gene expression, human-specific GAPDH primers and probe (Taq-Man Gene Expression Assays ID, Hs00266705_g1) were used. All other PCR primer and probe sequences are listed in the Supplemental Data.
Transfections with TSG-6 siRNA
Target hMSCs for the transfections were prepared with viable passage 1 hMSCs that were plated at 50,000 cells/well in CCM in 6-well plates. After incubation for 1 day, cells were transfected with 10 or 20 nM siRNA for TSG-6 (sc-39819; Santa Cruz Biotechnology, Santa Cruz, CA) or RNAi-negative control (Stealth RNAi negative Control; Invitrogen) using a commercial kit (Lipofectamine RNAiMAX reagent; Invitrogen). Six hours later, the medium was replaced with 3 ml per well of CCM lacking antibiotics, and hMSCs were incubated for 16-20 hr.
TSG-6 ELISA
TSG-6 protein levels in medium from TNF-α-treated MSCs were determined by ELISA. A 96-well plate (Maxisorp; Nunc) was coated overnight at 4°C with 50 μl of 10 μg/ml monoclonal antibody specific for TSG-6 (clone A38.1.20; Santa Cruz Biotechnology, Inc.) in 0.2 M sodium bicarbonate buffer (pH 9.2). The plates were washed with PBS and blocked with 0.25% (wt/vol) BSA and 0.05% (vol/vol) Tween 20 in PBS for 30 min at room temperature. Plates were again washed with PBS. Samples of 50 μl or standards of recombinant human TSG-6 protein (R&D Systems) in blocking buffer were added. After 2 hr at room temperature, wells were washed with PBS followed by 50 μl/well of 0.5 μg/ml biotinylated anti-human TSG-6 (TSG-6 Biotinylated PAb Detection Antibody; R&D Systems). After 2 hr, plates were washed with PBS. Fifty microliters streptavidin-HRP (R&D Systems) was added to each well. The plate was covered and incubated for 20 min at room temperature. One hundred microliters substrate solution (R&D Systems) was added, and the sample was incubated for 10 min at room temperature. Absorbance was read at 450 nm (Fluostar Optima; BMG Labtechnologies).
Permanent Ligation of the Anterior Descending Coronary Artery
Male immunodeficient NOD/SCID mice (NOD.CB17-Prkdcscid/J; The Jackson Laboratory) 7–8 weeks of age were ventilated mechanically under anesthesia with isoflourine, the chest was opened, the left anterior descending coronary artery was ligated, and the chest was closed. The effectiveness of the LAD was established in preliminary experiments by the demonstration that serum cardiac troponin I levels were elevated in 7 mice 48 hr after the surgery (Figure 4A).
Other Assays
As indicated in the Supplemental Data, commercial kits were used to assay mouse cardiac troponin I in serum (ELISA kit; Life Diagnostics, Inc.), plasmin activity in serum (Roche Applied Science), and MMPs in heart by zymography (10% Zymogram Gelatin Gels; Invitrogen/Novex).
Leukocyte Infiltration Assay in Heart
Frozen heart sections of 5 μm from MI-induced mice were stained with anti-Ly-6G and Ly-6C (RB6-8C5, BD Biosciences), and Ly-6G- and Ly-6C-positive cells were counted with a software program (ImageJ, NIH Image).
Microscopic Examination of the Myocardium
Paraffin-embedded heart samples at 21 days after MI were cut into over 400 sequential 5 μm sections and stained with Masson Trichrome. Quantitative assays for infarct size were performed as described by Takagawa et al. (2007). In brief, images of every tenth section covering the region of infarct (total of 20 sections per heart) were examined with a spinning disc microscopy (Olympus) using a ×4 objective and captured with Stereo Investigator software (Stereo Investigator version 7; MBF Bioscience). Stereological quantification software was used to measure midline infarct length of heart.
Echocardiography
Echocardiography (Acuson Sequoia C512 echocardiography system, Siemens Medical Solutions USA, Inc.) was performed 21 days after MI.
Statistical Analyses
Comparisons between two groups were made with the use of unpaired and two-tailed Student’s t tests. p < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported in part by grants from the NIH (HL073252, P40 RR 17447, P01 HL 075161, and 1R01HL080682-01A2). We thank Nickolay Bazhanov and Ulf Krause for helping with mouse surgery.
Footnotes
ACCESSION NUMBERS
Microarray data have been deposited in the Gene Expression Omnibus (GEO;http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE15969.
Supplemental Data include Supplemental Experimental Procedures, four tables, and three figures and can be found with this article online at http://www.cell.com/cell-stem-cell/supplemental/S1934-5909(09)00212-4.
REFERENCES
- Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009 doi: 10.1002/glia.20841. Published online February 3, 2009. 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am. J. Pathol. 2001;159:1711–1721. doi: 10.1016/s0002-9440(10)63018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, Dimattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J. Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RF, Dariolli R, Justulin Junior LA, Sugizaki MM, Politi OM, Cicogna AC, Felisbino SL, Dal Pai-Silva M. Heart failure alters matrix metalloproteinase gene expression and activity in rat skeletal muscle. Int. J. Exp. Pathol. 2006;87:437–443. doi: 10.1111/j.1365-2613.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelle JP. Troponin, a new myocardial infarction marker. Rev. Med. Liege. 1998;53:619–624. [PubMed] [Google Scholar]
- Chute JP. Stem cell homing. Curr. Opin. Hematol. 2006;13:399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc. Natl. Acad. Sci. USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dooner M, Cerny J, Colvin G, Demers D, Pimentel J, Greer D, Abedi M, McAuliffe C, Quesenberry P. Homing and conversion of murine hematopoietic stem cells to lung. Blood Cells Mol. Dis. 2004;32:47–51. doi: 10.1016/j.bcmd.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J. Mol. Cell. Cardiol. 2007;43:535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Forteza R, Casalino-Matsuda SM, Monzon ME, Fries E, Rugg MS, Milner CM, Day AJ. TSG-6 potentiates the antitissue kallikrein activity of inter-alpha-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 2007;36:20–31. doi: 10.1165/rcmb.2006-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, et al. Is the intravascular administration of mesenchymal stem cells safe? Microvasc. Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Mahoney DJ, Cao T, Rugg MS, Fries E, Milner CM, Perretti M, Day AJ. The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-alpha -inhibitor-independent manner. J. Biol. Chem. 2002;277:51068–51076. doi: 10.1074/jbc.M205121200. [DOI] [PubMed] [Google Scholar]
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009 doi: 10.1136/gut.2008.168534. Published online January 9, 2009. gut.2008.168534v1. [DOI] [PubMed] [Google Scholar]
- Griffin MO, Jinno M, Miles LA, Villarreal FJ. Reduction of myocardial infarct size by doxycycline: a role for plasmin inhibition. Mol. Cell. Biochem. 2005;270:1–11. doi: 10.1007/s11010-005-2540-3. [DOI] [PubMed] [Google Scholar]
- Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res. Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- Heng BC, Gribbon PM, Day AJ, Hardingham TE. Hyaluronan binding to link module of TSG-6 and to G1-domain of aggrecan is differently regulated by pH. J. Biol. Chem. 2008;283:32294–32301. doi: 10.1074/jbc.M804155200. [DOI] [PubMed] [Google Scholar]
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat. Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Natl. Acad. Sci. USA. 2008;105:20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur EM, Granger CB, Fakunding JL, Mockrin SC, Grant SM, Ellis SG, Weisel RD, Goodell MA. Bringing cardiovascular cell-based therapy to clinical application: perspectives based on a National Heart, Lung, and Blood Institute Cell Therapy Working Group meeting. Am. Heart J. 2007;153:732–742. doi: 10.1016/j.ahj.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat. Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- Koc ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, Kolodny EH, Yoseph YB, Gerson SL, Lazarus HM, et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp. Hematol. 1999;27:1675–1681. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- Krause U, Harter C, Seckinger A, Wolf D, Reinhard A, Bea F, Dengler T, Hardt S, Ho A, Katus HA, et al. Intravenous delivery of autologous mesenchymal stem cells limits infarct size and improves left ventricular function in the infarcted porcine heart. Stem Cells Dev. 2007;16:31–37. doi: 10.1089/scd.2006.0089. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr. Opin. Immunol. 2006;18:586–591. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lonnies H, Nava S, Ringden O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103:2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hu X, Zhu C, Wang D, Zheng X, Liu Q. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J. Neuro-immunol. 2009;206:58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Read SJ. Immune and inflammatory responses to stroke: good or bad? Int. J. Stroke. 2008;3:254–265. doi: 10.1111/j.1747-4949.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem. Soc. Trans. 2006;34:446–450. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- Mindrescu C, Thorbecke GJ, Klein MJ, Vilcek J, Wisniewski HG. Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 2000;43:2668–2677. doi: 10.1002/1529-0131(200012)43:12<2668::AID-ANR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mindrescu C, Dias AA, Olszewski RJ, Klein MJ, Reis LF, Wisniewski HG. Reduced susceptibility to collagen-induced arthritis in DBA/1J mice expressing the TSG-6 transgene. Arthritis Rheum. 2002;46:2453–2464. doi: 10.1002/art.10503. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Rodriguez WE, Sen U, Tyagi SC. Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiol. Res. 2008;57:379–384. doi: 10.33549/physiolres.931221. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Sugahara-Kobayashi M, Takahashi Y, Nagata T, Ishikawa K, Asai S. Screening for control genes in mouse hippocampus after transient forebrain ischemia using high-density oligonucleotide array. J. Pharmacol. Sci. 2006;101:52–57. doi: 10.1254/jphs.fp0050881. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovechkin AV, Tyagi N, Rodriguez WE, Hayden MR, Moshal KS, Tyagi SC. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J. Appl. Physiol. 2005;99:2398–2405. doi: 10.1152/japplphysiol.00442.2005. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found. Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Paolocci N, Tavazzi B, Biondi R, Gluzband YA, Amorini AM, Tocchetti CG, Hejazi M, Caturegli PM, Kajstura J, Lazzarino G, Kass DA. Metalloproteinase inhibitor counters high-energy phosphate depletion and AMP deaminase activity enhancing ventricular diastolic compliance in subacute heart failure. J. Pharmacol. Exp. Ther. 2006;317:506–513. doi: 10.1124/jpet.105.099168. [DOI] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S, Anderson FP, Lohmann TP, Lawson CJ, Feng YJ, Waskiewicz D, Contois JH, Wu AH. Comparative analysis of cardiac troponin I and creatine kinase-MB as markers of acute myocardial infarction. Clin. Cardiol. 1997;20:269–271. doi: 10.1002/clc.4960200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant. Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transplant. Proc. 2008;40:2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J. Appl. Physiol. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuma P, Fonseca VA. Inflammation, insulin resistance, and atherosclerosis. Metab. Syndr. Relat. Disord. 2004;2:105–113. doi: 10.1089/met.2004.2.105. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis MA, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–146. doi: 10.1016/j.cytogfr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Wolf D, Reinhard A, Krause U, Seckinger A, Katus HA, Kuecherer H, Hansen A. Stem cell therapy improves myocardial perfusion and cardiac synchronicity: new application for echocardiography. J. Am. Soc. Echocardiogr. 2007;20:512–520. doi: 10.1016/j.echo.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun Z, Sun HS, Wu J, Weisel RD, Keating A, Li ZH, Feng ZP, Li RK. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 2008;16:993–1005. [PubMed] [Google Scholar]
- Ylostalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp. Hematol. 2008;36:1390–1402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.