Abstract
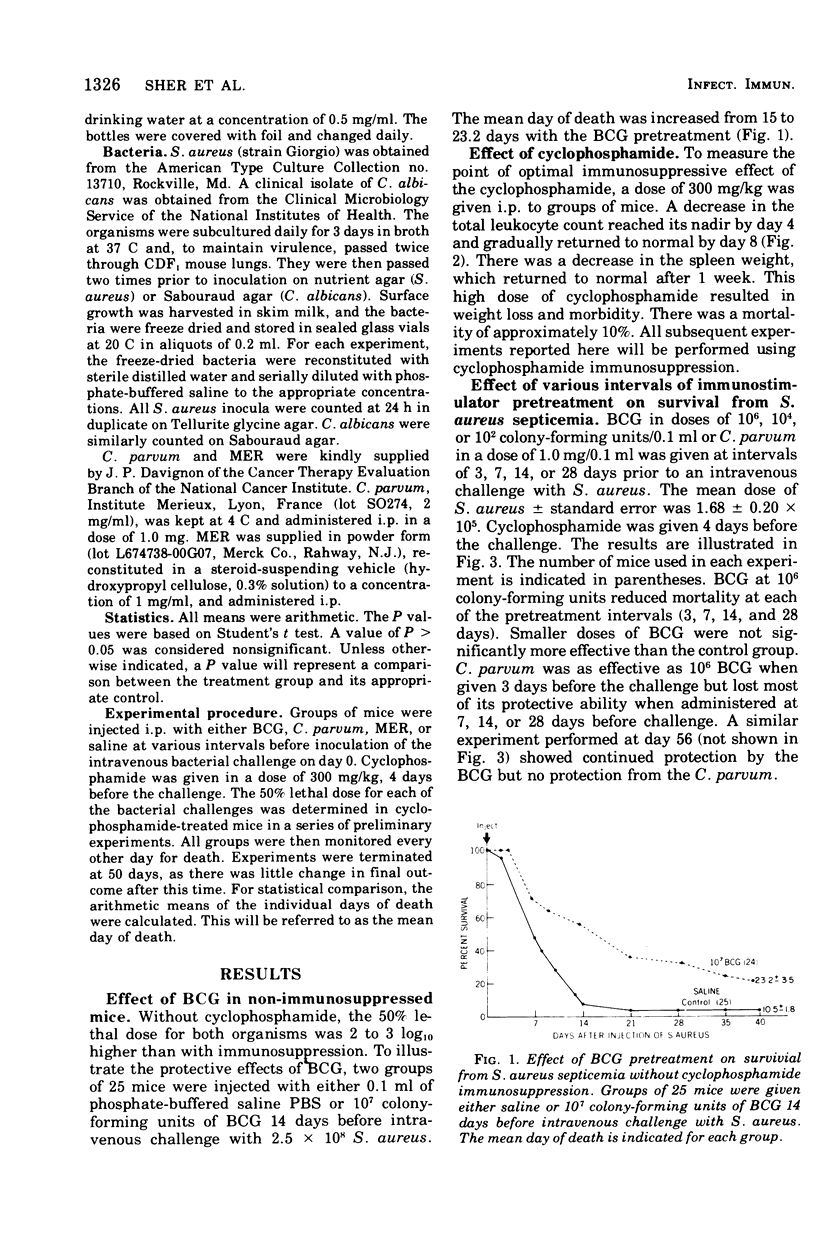
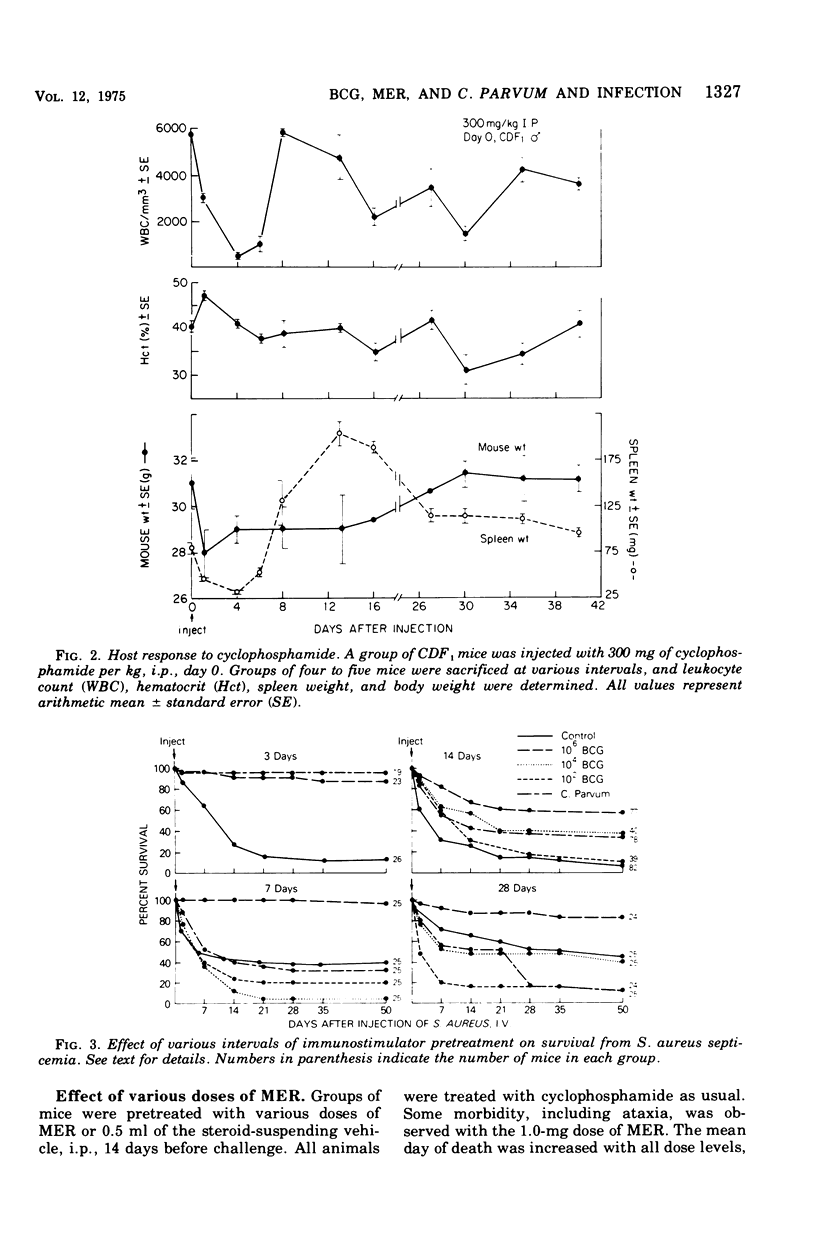
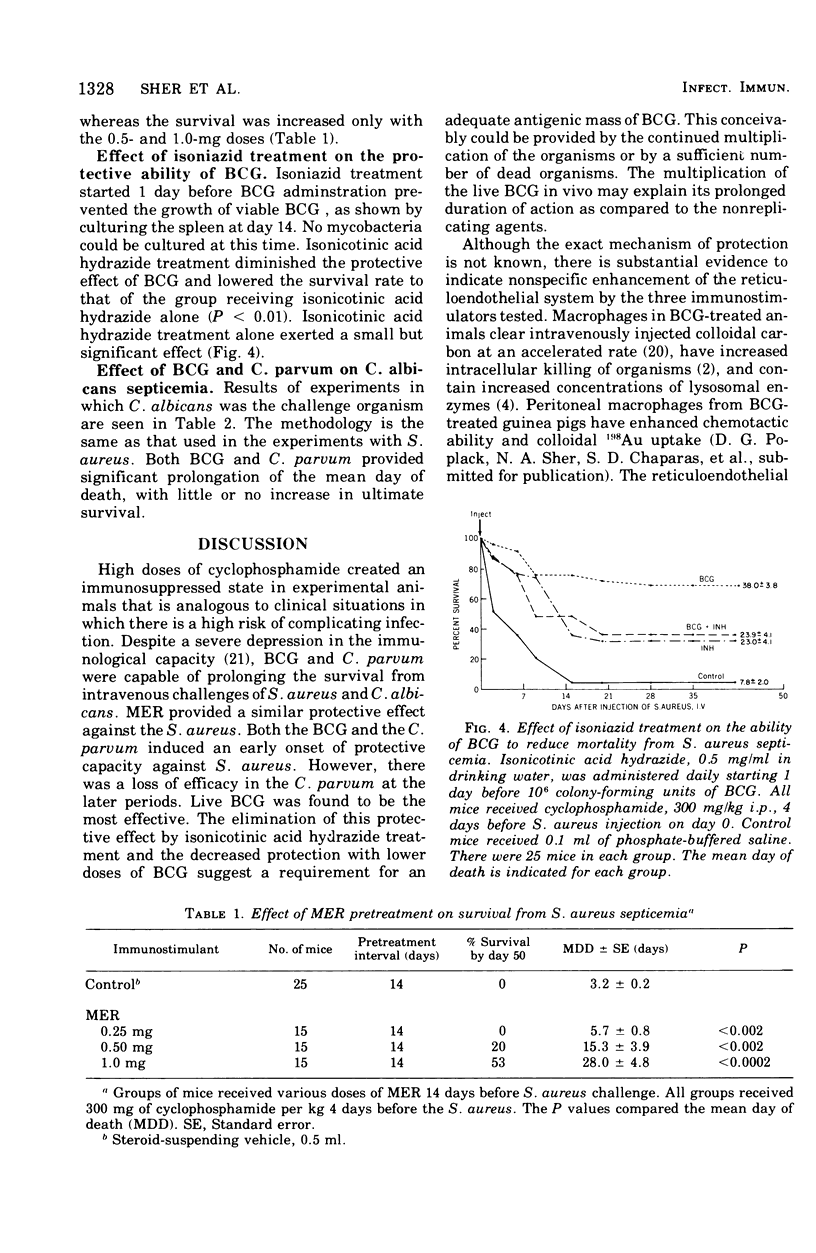
An immunosuppressed mouse model was devised to test the effects of immunopotentiators on the prevention of bacterial and fungal infections. The effects of BCG and Corynebacterium were tested against Staphylococcus aureus and Candida albicans infection. The effect of methanol-extraction residue (MER-BCG) was tested against S. aureus septicemia. CDF mice were given various doses of BCG, 1.0 mg of C. parvum, or 0.5 mg of MER intraperitoneally at varying intervals before injection of an intravenous bacterial challenge. Four days before challenge, 300 mg of cyclophosphamide per ml was given intraperitoneally. BCG (106 colony-forming units) reduced mortality due to S.aureus at pretreatment intervals of 3, 7, 14, and 28 days. Isonicotinic acid hydrazide treatment elimated the protective effect of the live BCG. C. parvum was as effective as BCG against S. aureus septicemia when given 3 days before infection, but lost most of its protective effect after that time. MER protected at doses as small as 0.25 mg when given 25 days prior to challenge. Both BCG and C. parvum exerted a protective effect against Candida albicans infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effects of cellular constituents of mycobacteria on the resistance of mice to heterologous infections I. Protective effects. J Exp Med. 1957 Nov 1;106(5):703–717. doi: 10.1084/jem.106.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN B. N., PREVOT A. R., BIOZZI G., STIFFEL C., MOUTON D., MORARD J. C., BOUTHILLIER Y., DECREUSEFOND C. STIMULATION DE L'ACTIVIT'E PHAGOCYTAIRE DU SYST'EME R'ETICULOENDOTH'ELIAL PROVOQU'EE PAR CORYNEBACTERIUM PARVUM. J Reticuloendothel Soc. 1964 Jan;1:77–96. [PubMed] [Google Scholar]
- Hughes W. T. Fatal infections in childhood leukemia. Am J Dis Child. 1971 Oct;122(4):283–287. doi: 10.1001/archpedi.1971.02110040067003. [DOI] [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Karim R., Baker M. B., Baker R. E. Herpesvirus hominis type 2 infections in rabbits: effect of prior immunization with attenuated Mycobacterium bovis (BCG) cells. Infect Immun. 1972 Oct;6(4):465–468. doi: 10.1128/iai.6.4.465-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. S., Graw R. G., Jr, Young R. C. Management of infections in patients with leukemia and lymphoma: current concepts and experimental approaches. Semin Hematol. 1972 Apr;9(2):141–179. [PubMed] [Google Scholar]
- Levine A. S., Siegel S. E., Schreiber A. D., Hauser J., Preisler H., Goldstein I. M., Seidler F., Simon R., Perry S., Bennett J. E. Protected environments and prophylactic antibiotics. A prospective controlled study of their utility in the therapy of acute leukemia. N Engl J Med. 1973 Mar 8;288(10):477–483. doi: 10.1056/NEJM197303082881001. [DOI] [PubMed] [Google Scholar]
- McBride W. H., Jones J. T., Weir D. M. Increased phagocytic cell activity and anaemia in Corynebacterium parvum treated mice. Br J Exp Pathol. 1974 Feb;55(1):38–46. [PMC free article] [PubMed] [Google Scholar]
- Pollard M., Sharon N. Chemotherapy of spontaneous leukemia in germfree AKR mice. J Natl Cancer Inst. 1970 Oct;45(4):677–680. [PubMed] [Google Scholar]
- Powles R. Immunotherapy for acute myelogenous leukaemia. Br J Cancer Suppl. 1973 Aug;1:262–265. [PMC free article] [PubMed] [Google Scholar]
- ROSENTHAL S. R., KENNIEBREW A. H., RAISYS N. A modified semi-solid medium and technique for determining viability of BCG. Acta Tuberc Pneumol Scand. 1962;42:167–172. [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W. Salmonellosis in Mice Infected with Mycobacterium bovis BCG II. Resistance to Infection. Infect Immun. 1970 Jun;1(6):583–586. doi: 10.1128/iai.1.6.583-586.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Pearson J., Chirigos M. Virulence of six strains of Mycobacterium bovis (BCG) in mice. Infect Immun. 1973 Nov;8(5):736–742. doi: 10.1128/iai.8.5.736-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. H., Woodruff M. F. Comparative effect of two strains of C. parvum on phagocytic activity and tumour growth. Nature. 1968 Jul 13;219(5150):197–198. doi: 10.1038/219197a0. [DOI] [PubMed] [Google Scholar]
- Stiffel C., Mouton D., Bouthillier Y., Decreusefond C., Biozzi G. Réponse du SRE au Mycobacterium tuberculosis (BCG) et au Corynebacterium parvum chez des souris de différentes lignées. J Reticuloendothel Soc. 1970 Feb;7(2):280–293. [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Vogler W. R., Chan Y. K. Prolonging remission in myeloblastic leukemia by tice-strain bacillus Calmette-Guérin. Lancet. 1974 Jul 20;2(7873):128–131. doi: 10.1016/s0140-6736(74)91556-6. [DOI] [PubMed] [Google Scholar]
- WEISS D. W., BONHAG R. S., PARKS J. A. STUDIES ON THE HETEROLOGOUS IMMUNOGENICITY OF A MENTHANOL-INSOLUBLE FRACTION OF ATTENUATED TUBERCLE BACILLI (BCG). I. ANTIMICROBIAL PROTECTION. J Exp Med. 1964 Jan 1;119:53–70. doi: 10.1084/jem.119.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS D. W. Enhanced resistance of mice to infection with Pasteurella pestis following vaccination with fractions of phenol-killed tubercle bacilli. Nature. 1960 Jun 25;186:1060–1061. doi: 10.1038/1861060a0. [DOI] [PubMed] [Google Scholar]
- Weiss D. W. Nonspecific stimulation and modulation of the immune response and of states of resistance by the methanol-extraction residue fraction of tubercle bacilli. Natl Cancer Inst Monogr. 1972 Dec;35:157–171. [PubMed] [Google Scholar]



