Abstract
Background
This review scopes the evidence on the effectiveness and cost-effectiveness of interventions to improve suboptimal use of medicines in order to determine the evidence gaps and help inform research priorities.
Sources of data
Systematic searches of the National Health Service (NHS) Economic Evaluation Database, the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects.
Areas of agreement
The majority of the studies evaluated interventions to improve adherence, inappropriate prescribing and prescribing errors.
Areas of controversy
Interventions tend to be specific to a particular stage of the pathway and/or to a particular disease and have mostly been evaluated for their effect on intermediate or process outcomes.
Growing points
Medicines optimization offers an opportunity to improve health outcomes and efficiency of healthcare.
Areas timely for developing research
The available evidence is insufficient to assess the effectiveness and cost-effectiveness of interventions to address suboptimal medicine use in the UK NHS. Decision modelling, evidence synthesis and elicitation have the potential to address the evidence gaps and help prioritize research.
Keywords: cost-effectiveness, medicines management, medicines optimization, review, economics
Background
Medicines optimization aims to ensure that patients get the most from their medicines.1 Although beneficial and relatively uncontroversial, it is difficult to achieve in practice given the multiple stages in the medicines pathway (prescribing, dispensing, administration, monitoring and record keeping) and complex interactions between the different stakeholders (e.g. clinicians, pharmacists, patients, etc.). As a result, only a proportion of patients get the maximum benefit from their medicines and some suffer avoidable harm. Suboptimal use of medicines can also result in extra costs for healthcare systems, such as in hospitalizations related to prescribing or monitoring errors, or morbidity from low patient adherence. Therefore, addressing suboptimal use of medicines is likely to achieve better health outcomes and to ensure a more efficient use of resources.
The benefits of improving suboptimal use of medicines have long been recognized, both in the UK and internationally.1,2 The UK National Health Service (NHS), in particular, has deployed a number of initiatives to address the issue. The National Prescribing Centre, part of the National Institute for Health and Care Excellence (NICE) since 2011, was established in the late 1990s to support the NHS in improving prescribing and medicine use. NICE has had a large role in improving medicines use by issuing guidance on the effectiveness and cost-effectiveness of new medicines and guidelines on good practice, specifically around medicines reconciliation and discharge and adherence to medicines.3,4 Nonetheless, medicines optimization remains an elusive goal and more needs to be done to improve medicines use.
The challenge is to identify how best to intervene given the differences in effectiveness and cost-effectiveness of potential interventions that could be invested on by the NHS. In other words, which interventions are more likely to deliver the best health outcomes (and/or lower costs)? However, suboptimal use of medicines covers a large number of issues, each specific to different stages in the medicines pathway. Comparing the effectiveness and cost-effectiveness of all possible interventions related to suboptimal use of medicines would be impossible. Therefore, the first step is to gain an understanding of the size and the nature of the evidence base in order to highlight the areas with the largest body of research and areas where new research may be required. As such, a scoping review was conducted on the effectiveness and cost-effectiveness of interventions to address suboptimal use of medicines. Rather than addressing specific research questions on, for example, the comparative effectiveness of interventions, a scoping review seeks to identify gaps in the existing literature to inform where more research may be needed.5 Hence, this review is a ground-clearing exercise that maps out the evidence base on how to improve suboptimal use of medicines and pinpoints where future research would have the most value. The results of this scoping review can be used as a starting point for a systematic review on specific interventions to improve suboptimal use of medicines or as supportive evidence for future primary research.
Methods
Typology of issues affecting the medicines pathway
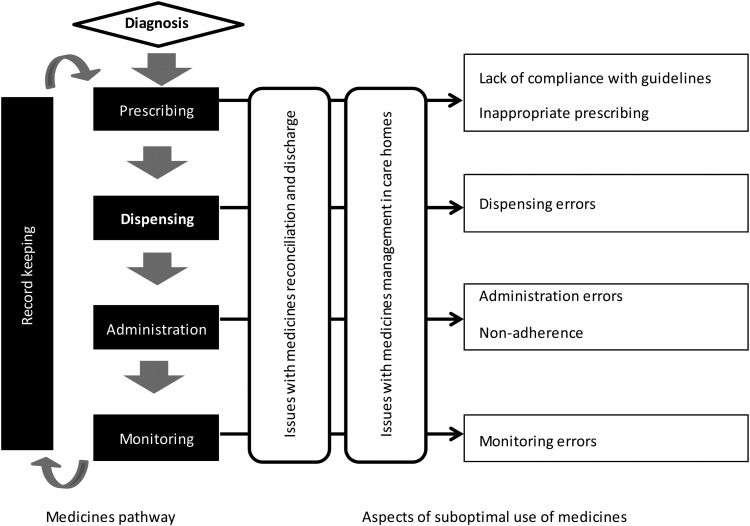
Figure 1 describes the medicines pathway and presents a typology of issues considered throughout this review. The medicines pathway can be seen as a cycle starting at prescribing. Prescribing can be performed by a number of healthcare professionals, including doctors, dentists, nurses, pharmacists, optometrists, etc. depending on their qualifications and the medicines considered. Prescribing is a complex act as it requires consideration of the diagnosis, the response to previous medicines prescribed to treat the problem, guidelines on the condition, and the preferences and characteristics of the patient. Therefore, it can be affected by a number of issues, namely lack of compliance with guidelines, inappropriate prescribing given the patients' characteristics, prescribing errors or insufficient prescribing of low-cost generics. Prescriptions are dispensed in the pharmacy. Dispensing errors can occur at the point of supply, with varying degrees of severity; conversely, prior prescribing errors can be detected and addressed during dispensing. Administration can be by the patient himself or herself or by a carer (informal or formal carer). Two issues can occur at the administration stage: errors (e.g. too much or too little by mistake) and non-adherence. Non-adherence is a complex and important issue. It can be non-intentional (e.g. due to not understanding administration instructions or forgetfulness) or intentional, whereby the patient consciously decides not to take the medicine. Medicines often require patient monitoring, for efficacy and adverse events, sometimes involving laboratory measurement of their effect (e.g. international normalized ratio for warfarin). Information regarding prescribing, dispensing, monitoring and, in some sectors, administration (such as care homes or in hospital) should be appropriately recorded to inform the subsequent stages in the cycle in what is termed as ‘record keeping’.
Fig. 1.
Issues around suboptimal use of medicines.
A number of issues emerge from the stages and complex interactions between stakeholders along the medicines pathway: lack of compliance with guidelines, inappropriate prescribing (including under- or over-prescribing), prescribing errors, insufficient prescribing of generics, dispensing errors, non-adherence, monitoring errors and issues specific to the interface between care sectors in medicines reconciliation and discharge and around medicines management in care homes. This list of issues is not exhaustive but can guide the categorization of the evidence base into topics of research. They were chosen based on rapid review of the literature on the burden of suboptimal use of medicines and discussions with expert researchers in the area and policy advisors (see Acknowledgements for the list of advisors consulted for this study).6
Data sources and searches
Searches were conducted in three databases: the NHS Economic Evaluation Database (NHS EED) for cost-effectiveness studies and the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for systematic reviews. NHS EED contains cost-effectiveness studies of healthcare interventions and is updated weekly. Included studies are published in the database and prioritized for abstract writing. Structured abstracts are written and independently checked by health economists. DARE contains systematic reviews of the effects of healthcare interventions and the delivery and organization of health services and is updated weekly; citations identified as potential systematic reviews are assessed for inclusion by two researchers. Reviews need to meet at least four of five criteria (criteria 1–3 are mandatory) to be included: (1) inclusion/exclusion criteria are reported; (2) adequate search; (3) included studies are synthesized; (4) quality of the studies is assessed; (5) there are sufficient details about the included studies. Reviews are then published in the database and prioritized for abstract writing. Structured abstracts are written by researchers and checked by a technical editor. DARE includes records of all Cochrane reviews and protocols, as well as published papers associated with Cochrane reviews. More details on these databases can be found here http://www.crd.york.ac.uk/CRDWeb/AboutPage.asp (5 August 2014, date last accessed).
The bibliographic search strategies were designed to provide an overview of the literature and to identify any evidence gaps. The strategies were designed by an information specialist in consultation with the researchers. A combination of relevant free text terms, synonyms and subject headings were included. The process was iterative; sample sets of results were screened for relevance and a reasonable level of inclusivity and the findings from this were used to fine tune the strategy. The base search strategy was constructed using the Cochrane Library and then adapted to the other resources searched. The evidence base around effectiveness was assessed from systematic reviews since there was an expectation of a large body of literature in the area. Grey literature (work published in channels other than peer-reviewed journals) was not examined since the objective is to map out the size and the nature of the evidence base rather than an exhaustive review of all the evidence. Searches were conducted in February 2013 and were limited to material published since 2000 written in English. The date limit was applied to increase the likelihood that the evidence identified is relevant to the current context and to the decision problems faced by policy-makers. Full details can be found in the online supplementary data, Appendix.
Study selection
Studies were selected following a stepwise procedure. First, articles with obviously irrelevant titles were excluded. Secondly, abstracts were retrieved, read and assessed based on three criteria: (i) whether the study assessed interventions related to medicines use, (ii) whether the study assessed the effectiveness or cost-effectiveness of interventions (as opposed to theory supporting their use, their development, procedures or experiences of the different stakeholders and (iii) whether the study was published in the English language. Thirdly, the structured abstract from NHS EED or DARE was retrieved, read and assessed to confirm inclusion. Cost-effectiveness studies were included only if two or more interventions were compared in terms of their costs and effects. Any measure of effect was considered, including quality of life, resource use, monetary values or other quantities of interest. Interventions to improve overall patients' management in the whole pathway in a specific disease but which were not directly related to medicine use (e.g. asthma management) were excluded. Quality assessment was not conducted because it was outside the scope of this review.
Data extraction and synthesis
Data were extracted from the NHS EED or DARE structured abstracts using a standardized form in Microsoft Excel. Full-text papers were consulted where the structured abstract was not available or if the abstract did not contain the information required. Data extraction included: objective, issue(s) of suboptimal use of medicines that interventions addressed as defined by the study's objective, type of study, type of intervention, target of the intervention and outcomes. The type of analysis (within trial or model based), the setting and the source of effectiveness data were extracted for cost-effectiveness studies. Extracting the relevant information from the structured abstracted, complemented as required by consulting the full text, was considered the most efficient and appropriate data extraction strategy given the aims of this review.
The studies were classified by the issue of suboptimal medicines use addressed, whether the evidence was generic or specific to a particular condition, the type of intervention and the type of outcome measures included. The classification of studies aimed to facilitate the identification of gaps in the literature and help define future research questions. Particular attention was given to data relevant to cost-effectiveness analysis (i.e. costs, health-related quality of life or quality-adjusted life years (QALYs)) in order to ascertain whether the existing evidence base is sufficient to draw conclusions on the value for money of interventions for the NHS. A narrative synthesis was undertaken.
Results
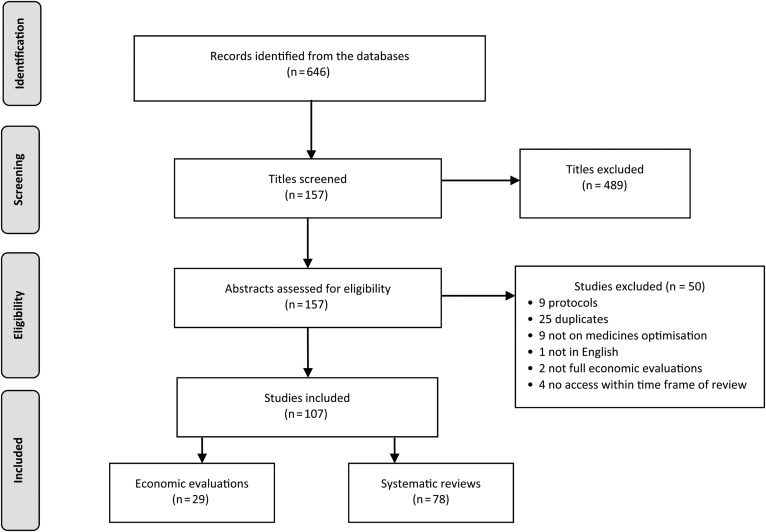
Figure 2 presents the flowchart of the study selection process. Briefly, 646 records were found, of which 157 abstracts were assessed for eligibility. In total, 107 studies were included in the review (29 cost-effectiveness and 78 systematic reviews).
Fig. 2.
PRISMA flow diagram of the study selection process.
Issues and interventions
Table 1 summarizes the disease areas and the type of intervention by issue of suboptimal use of medicines included in the 78 systematic reviews. The interventions were broadly classified based on their names and summary descriptions in one of the following categories: software support, pharmacist-led intervention, nurse-led intervention, multidisciplinary interventions (involving more than one healthcare professional), educational intervention (including interventions involving provision of information leaflets), counselling or behavioural intervention, financial incentives, aids or devices (such as adherence aids, reminders, including dose simplifications with the view of improving adherence), medicines review or reconciliation, protocols or guidelines. The objective was to obtain a manageable number of types of interventions whilst maintaining some detail to assess on which areas was the evidence base more or less prominent. Every one of the issues around suboptimal medicines use was addressed by the systematic reviews with the exception of insufficient prescribing of generics. Most studies evaluated more than one type of intervention as the systematic reviews were typically on any intervention to improve a specific issue related to suboptimal use of medicines. The majority of the studies (51, 65%) focussed on interventions to improve adherence either in any disease area (20; 39%) or for specific conditions (31; 69%). Interventions were mostly educational (35; 69%) such as leaflets or brochures,76 behavioural or counselling (24; 47%) such as group psychotherapy, cognitive behavioural therapy72 or family counselling therapy77 or involving adherence aids (19; 37%) such as unit-of-use packaging,33 reminders such as telephone reminders68 or dose simplifications.29 One review evaluated the effectiveness of incentives of the form of money, goods (such as bus tokens or food) or vouchers redeemable for goods to improve adherence to medications for tuberculosis, substance abuse, human immunodeficiency virus, hepatitis C, schizophrenia and stroke prevention.43 Prescription errors (10, 13%) were the second most frequent issue evaluated. All reviews relating to this issue included interventions involving software support such as computerized order entry,11,20,23–28 computerized reminders,22 automated bedside dispensing22 or computerized advice.21 Two reviews on prescription errors included a variety of interventions in addition to software support.22,24
Table 1.
Disease area and type of studies included by issue of suboptimal medicines use in the systematic review (references in superscript numbers)
| Issue of suboptimal medicines use | N (%) | Disease area |
Type of intervention |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Specific conditions | Software support | Pharmacist -led Intervention | Nurse-led support | Multidisciplinary medicines management | Education (inc. leaflets) | Counselling or behavioural intervention | Financial incentives | Adherence aids, reminders, dose simplification | Medicines review or reconciliation | Protocols or guidelines | ||
| Lack of compliance with guidelines | 6 (8) | 7–11 | URTI12 | 7, 11, 12 | 8 | 9, 12 | 9, 10, 12 | 9 | 9 | 12 | |||
| Inappropriate prescribing (inc. antibiotics) | 9 (12) | 10, 11, 13–18 | Antibiotics19 | 11, 16–18 | 14 | 10, 15, 16, 19 | 13 | 14 | |||||
| Prescription errors | 10 (13) | 11, 20–28 | 11, 20–28 | 22, 24 | 22 | 22, 24 | 22 | 24 | 22, 24 | ||||
| Medicines reconciliation and discharge | 3 (4) | 15, 24, 29 | 24 | 24 | 15, 24, 29 | 24 | 24 | ||||||
| Dispensing errors | 3 (4) | 17, 22, 24 | 17, 22, 24 | 22, 24 | 22 | 22, 24 | 22 | 24 | 22, 24 | ||||
| Administration errors | 3 (4) | 7, 22, 24 | 7, 22, 24 | 22, 24 | 22, 24 | 22, 24 | 22 | 24 | 22, 24 | ||||
| Medicines management in care homes | 4 (5) | 8, 9, 30, 31 | 31 | 8 | 9 | 9, 31 | 9 | 9, 30 | |||||
| Adherence | 51 (65) | 13, 32–50 | Epilepsy51 Depression52–54 HIV55–58 Cardiovascular59–68 Transplantation69 Schizophrenia70–75 Osteoporosis76 Diabetes77 Tuberculosis78 Asthma79, 80 Bipolar disease81 |
34, 37, 47, 57, 59, 68, 70 | 52, 56, 62 | 56 | 36, 46, 47, 56, 68, 79 | 36–41, 44, 46–49, 51, 53–55, 58–69, 74–80, 82 | 34, 35, 37–39, 41, 47, 48, 51, 53, 56, 65, 67, 70–79, 82 |
43 |
13, 32, 33, 36, 37, 41, 42, 45, 47, 50, 56, 57, 59, 62, 63, 68, 70, 74, 76 |
37, 41, 54, 65 | 36, 79 |
| Monitoring | 3 (4) | 15, 17, 83 | 17, 83 | 15 | |||||||||
| Other | 2 (3) | 2 | 84 | 85 | |||||||||
| Total | 78 | 45 (58) | 33 (42) | 24 (31) | 8 (10) | 1 (1) | 9 (12) | 46 (59) | 25 (32) | 1 (1) | 19 (24) | 9 (12) | 5 (6) |
Table 2 summarizes the disease areas by issue of suboptimal use of medicines included in the 29 cost-effectiveness studies. Similarly to the systematic reviews on effectiveness, the majority of the studies (16, 55%) focused on adherence. For example, in Al-Eidan et al. patients were counselled by the hospital pharmacist on the importance on the adherence to therapy105; Desborough et al. assessed a pharmacist-led medication review to help patients manage their medicines103; Schroeder et al. evaluated a nurse-led support intervention to increase adherence and reduce blood pressure.113 Prescription errors were the focus of eight studies (28%), such as Weeks et al.'s quality improvement project to reduce medication errors96 and Sano et al. on standardized chemotherapy order forms to reduce errors in the prescribing of antineoplastic medication.91 Six studies (21%) addressed more than one issue of suboptimal use of medicines.86,88,91,93,94,98 No study evaluated insufficient prescribing of low-cost generics. There was approximately a 50:50 split on whether studies were specific to a disease area or generic. Table 2 also shows the various types of interventions evaluated. The classification of interventions into types followed the rationale used for the classification of interventions in the systematic reviews (see above). Various types of interventions were evaluated. Pharmacist-led interventions were the most frequent (17, 59%), particularly for reducing prescription errors.92–95,98
Table 2.
Issue of suboptimal use of medicines and interventions in cost-effectiveness studies (references in superscript numbers)
| Issue of suboptimal medicines use | N (%) | Disease area |
Software support | Pharmacist -led intervention | Nurse-led support | Multidisciplinary medicines management | Financial incentives | Adherence aids, tools, devices | Dose simplifications | Quality improvement initiative | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Specific conditions | ||||||||||
| Lack of compliance with guidelines | 2 (7) | Cardiovascular86, 87 | 87 | 86 | |||||||
| Inappropriate prescribing (inc. antibiotics) | 4 (14) | 88 | Antibiotic prescribing89, 90 Cancer91 |
88, 90 | 89 | 91 | |||||
| Prescription errors | 8 (28) | 92–97 | Injectables in paediatrics98 Cancer91 |
94, 97, 98 | 92–95, 98 | 98 | 91, 94, 98 | 96 | |||
| Dispensing errors | 2 (7) | 94 | Injectables in paediatrics98 | 94, 98 | 94, 98 | 98 | 94, 98 | ||||
| Administration errors | 2 (7) | Injectables in paediatrics98 Analgesia99 |
98 | 98 | 98 | 98, 99 | |||||
| Medicines management in care homes | 1 (3) | Psychoactive medication100 | 100 | ||||||||
| Adherence | 16 (55) | 88, 93, 101–104 | Erradiation of H.pilory105 HIV106–108 Cardiovascular86, 109–113 |
86, 88, 93, 101–105, 110, 112 | 107, 108, 113 | 111 | 106, 109 | 105 | 111 | ||
| Monitoring | 1 (3) | Anticoagulant monitoring114 | 114 | ||||||||
| Total (%) | - | 12 (41) | 17 (59) | 4 (14) | 17 (59) | 4 (14) | 2 (7) | 2 (7) | 5 (17) | 1 (3) | 1 (3) |
Types of studies
The evidence around interventions to address suboptimal use of medicines was varied but all types of issues were assessed with randomized controlled trials (RCTs). The number of RCTs included in the systematic reviews varied from 1 to 81 (median = 10). Five systematic reviews (6%) included no RCTs.7,26–28,49 Most cost-effectiveness studies (19, 66%) conducted a within-trial cost-effectiveness analysis using data from a single RCT86,87,100,102,105,106,112,113 or a non-randomized study, such as before and after90,96,99,101,103,114 or cohort studies.88,89,91,95,110 Eleven cost-effectiveness studies (38%) used a model, either based on a single study,92,104 a review of the literature93,97,107,108,109,111 or from expert evidence elicitation.94
Outcome measures
Table 3 summarizes the outcome measures used in the studies included in the systematic reviews and in the cost-effectiveness studies. Most studies evaluated the effects of interventions with intermediate outcomes. Intermediate outcomes (e.g. adherence, blood pressure, number of pills taken, error rates) precede and may lead to final outcomes, such as resource use, mortality or quality-adjusted survival. Most systematic reviews report measures of adherence (52, 67%), which is consistent with interventions' objectives. Adherence was measured in a variety of ways. For example, Bärnighausen et al. reports that, of the 26 studies included in their review, 11 used patients' self-reported adherence, 5 used pill counts, 4 used pharmacy refill rates and 17 used clinical indicators of adherence (e.g. CD4 cell count).82 Similarly, Al-Jumah et al. reports that six of the studies used patients' self-reported adherence, three used pill counts, two prescription claims and one an electronic pill container.52 Clinical outcomes measures (24, 31%) and measures of adverse drug events (16, 21%) are also frequent. No study reports QALYs but four (5%) report measures of quality of life. Including final outcome measures such as QALYs can be useful to demonstrate the value of the intervention in improving health. In terms of cost-effectiveness studies, and given the preference for QALYs for decision-making, it is important to distinguish studies that use QALYs as the measure of benefit compared with other measures of quality of life.115 For the cost-effectiveness studies, clinical outcome measures were the most frequently used (8, 28%), such as blood pressure86,110,113 and rate of thrombotic or haemorrhagic events.88,114 QALYs are used in five studies (17%). In De Giorgi et al. outcomes were expressed as point reductions in the criticality index for each safety tool; the criticality index was calculated by multiplying the frequency, severity and detection scores obtained by consensus from a panel of two nurses, one neonatologist and three hospital pharmacists.98
Table 3.
Types of outcome measures (references in superscript numbers)
| Issue of suboptimal medicines use | Type of outcome measure |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality |
Resource use |
Quality of life |
Quality-adjusted life years |
Adverse drug events |
Medication error |
Appropriateness |
Adherence |
Clinical outcome |
Patients' Satisfaction or knowledge |
Discrepancies in records |
Adherence to guidelines |
|||||||||||||
| SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | SR | E | |
| Poor compliance with guidelines | 9 | 9 | 10, 11 | 11 | 7–9, 12 | 86 | 10 | 9, 11 | 86, 87 | 7 | 7, 12 | |||||||||||||
| Inappropriate prescribing (inc. antibiotics) | 14, 15, 17 | 90 | 14, 15 | 10, 11, 15, 16, 19 | 90 | 11, 16–18 | 91 | 14, 16, 17 | 89, 90 | 10, 13, 15 | 88 | 11, 19 | 88, 89 | 15 | ||||||||||
| Prescription errors | 26, 27 | 23, 25 | 94 | 11, 20, 21, 23–28 | 93, 95, 97 | 11, 20, 22–27 | 91, 92, 95, 96 | 98 | 11, 21 | |||||||||||||||
| Medicines reconciliation and discharge | 15 | 15 | 15, 24 | 24 | 15 | 15 | 29 | |||||||||||||||||
| Dispensing errors | 17 | 94 | 24 | 17, 22, 24 | 17 | 98 | ||||||||||||||||||
| Administration errors | 99 | 24 | 22, 24 | 7 | 98 | 7 | 7 | |||||||||||||||||
| Medicines management in care homes | 9, 30 | 9, 30 | 30 | 30, 31 | 8, 9, 30 | 100 | 9, 30 | 30 | ||||||||||||||||
| Adherence | 34, 47, 49, 50, 67, 71, 80 | 102, 112 | 46, 47, 50 | 102, 103 | 107–109, 111 | 93 | 49 | 86 | 13, 32, 33, 35–41, 43–80, 82 | 88, 103, 104, 106, 112, 113 | 32–34, 39, 40, 42, 46, 47, 50, 52, 57, 62, 65, 66, 77, 80, 82 | 86, 88, 105, 110, 113 | 34, 49, 52 | 101, 102 | ||||||||||
| Monitoring | 15, 17 | 15, 83 | 15 | 17 | 17 | 15 | 114 | 15 | ||||||||||||||||
| Other | 84, 85 | 84, 85 | 84 | 84, 85 | 84 | 84 | 84 | 84, 85 | 84 | |||||||||||||||
| Total | 9 (12) | 1 (3) | 16 (21) | 3 (10) | 4 (5) | 2 (7) | 0 | 5 (17) | 16 (21) | 4 (14) | 15 (19) | 4 (14 | 9 (12) | 5 (17) | 52 (67) | 6 (21) | 24 (31) | 8 (28) | 5 (6) | 2(7) | 3 (4) | 0 | 2 (3) | 0 |
SR–systematic reviews; E–cost-effectivenesss.
Countries
The literature on effectiveness appears to span a wide range of countries. Of those structured abstracts reporting the country of origin of the primary effectiveness studies (36; 46%), most studies are based in the USA or Canada (33; 42%), followed by continental Europe (22; 28%) and the UK (19; 24%). A similar picture emerges from the cost-effectiveness studies: the majority are based in the USA or Canada (15; 52%), followed by the UK (7; 24%) and continental Europe (5; 17%).
Discussion
There is a large amount of evidence on interventions to improve suboptimal use of medicines. The largest body of evidence is on the effectiveness of interventions to improve adherence to medication, particularly in a specific disease area. Suboptimal prescribing is also an issue of considerable research, namely interventions aimed at reducing prescription errors and inappropriate prescribing. Interventions to address other issues around suboptimal use of medicines have been evaluated to a much lesser extent. No systematic reviews or cost-effectiveness studies were found on interventions to increase the prescribing of low-cost generics or in improving record keeping. The lack of evidence on interventions to increase prescribing of low-cost generics may be related to the relatively large proportion of generic penetration in some countries such as the UK and that pharmacists, depending on the country, may be allowed to automatically switch a branded medicine to a generic when dispensing.116,117 The literature on cost-effectiveness is much smaller than on effectiveness. Nonetheless, a similar picture emerges: interventions to improve adherence are the focus of the majority of cost-effectiveness studies, followed by interventions to improve prescribing.
Medicines optimization is a growing topic in the policy agenda. The Royal Pharmaceutical Society has recently issued guidance on medicines optimization for pharmacists and pharmacy technicians.1 This guidance sets out four key principles to be adopted by all professionals involved in medicines use: an understanding of patient's experience, evidence-based choice of medicines, safe use of medicines and making medicines optimization part of routine practice. It has been endorsed by NHS England, the Academy of Medical Royal Colleges, the Royal College of General Practitioners, the Royal College of Nursing and the Association of the British Pharmaceutical Industry. In addition, a number of clinical commissioning groups (NHS organizations responsible for the delivery of NHS services in England) have introduced medicines optimization as a tool to achieve better health outcomes and more efficient use of resources. However, and as highlighted by this review, medicines optimization is a wide area with a large number of issues that could be targeted for improvement. Therefore, it is difficult to make definite recommendations on the comparative effectiveness and cost-effectiveness of different interventions in different stages of the medicines pathway. NICE, for example, is developing a guideline to help clarify how to achieve medicines optimization in practice and maximize the benefits obtained from medicines. It will focus on three topics that relate to the all the stages in medicines pathway: reducing medicines-related patient safety incidents, evidence-informed decision-making and professional collaboration. This selection of topics, although well-defined in scope, may prove challenging to cover in one single guideline and some difficult choices may need to be made on which areas to prioritize for systematic review and cost-effectiveness analysis.
The majority of the studies evaluated effectiveness and cost-effectiveness in terms of intermediate outcomes, such as adherence or error rates, in specific diseases. The implication by using these intermediate measures is that patient outcomes are probably affected. However, virtually no studies attempt to link the two, either by empirical measurement or by modelling. This link may be more or less evidence based; for example, restricting the use of non-steroid anti-inflammatories in people with previous gastro-intestinal bleeding has a strong evidence base, whereas avoiding cardio-selective beta-blockers in people with asthma does not.118 Within each of these process categories, there is little standardization of definition or measurement, making further comparison between studies especially problematic. In addition, the link between the same intermediate measure and final outcomes may depend on the disease areas considered. For example, poor adherence to anti-retroviral medication has probably greater short-term impact on health compared with poor adherence to cholesterol-lowering therapies. Therefore, an intervention may be cost-effective in one disease but not cost-effective in another, and it is unclear how would it compare overall with other interventions in other disease areas. For these reasons, the current evidence base does not allow for comparisons of interventions across different diseases and affecting different stages of the medicines pathway in terms of their effects on final outcomes. Therefore, it is not possible to draw conclusions on which interventions should be prioritized for investment and future research. Consequently, more research is needed on which intermediate outcomes are preferred in order to achieve some standardization and comparability between studies. In particular, guidance is needed on how intermediate outcomes relate to final outcomes to help establish the effectiveness and cost-effectiveness of interventions affecting different diseases and issues of suboptimal use of medicines.
Decision analytic modelling, informed by evidence synthesis and expert elicitation, has the potential to address these without the risks, high-costs and long-time frame of RCTs. Decision modelling could simulate the medicines pathway in a specific disease and integrate formal evidence, such as from RCTs or previous systematic reviews, complemented with input from experts in the form of expert elicitation to address the evidence gaps.119 The utility of such a model is 2-fold. First, to evaluate the issues around suboptimal use of medicines with the greatest impact in costs and health. For example, whether the health losses from non-adherence are smaller, larger or equivalent to those from prescribing and dispensing errors. Secondly, to compare the costs and health gains from interventions addressing the different issues and ascertain whether, on balance and given the costs of the interventions, which intervention offers the best value. This information could help inform decisions on which interventions should be prioritized for implementation in the NHS. Finally, the model could indicate the key areas of uncertainty with the greatest impact on costs and health which further research should investigate.
This review has scoped the evidence on effectiveness and cost-effectiveness of interventions to address suboptimal use of medicines. The scoping review was systematic, in terms of the searches, data extraction and presentation of results. It is a valuable resource to researchers starting work in medicines optimization for a number of reasons: (i) provides detailed search strategies on a wide range of issues related to suboptimal use of medicines; (ii) classifies studies by type of issue, disease area, type of intervention and type of outcome measures included and (iii) provides the references of the studies by classification. Therefore, researchers seeking to conduct a review on, for example, interventions to reduce prescription errors, could start by examining the 10 studies referenced in Table 2. Similarly, researchers seeking for parameter inputs for a decision model on, for example, mortality associated with inappropriate prescribing, could start by retrieving the references detailed in Table 3. In addition, this review gives some indication on the research questions most valuable to inform future policies.
This scoping review is affected by some limitations. First, only systematic reviews were included in the review of effectiveness. There are three reasons for this: appropriate because this review aimed to map the existing evidence and highlight evidence gaps, rather than make definitive statements on the effectiveness and cost-effectiveness of specific interventions; efficiency because such a strategy was sufficient to meet these objectives and pragmatic since a review of the entire primary literature or of the grey literature would have been a significant task and impractical within the time available. However, this search strategy risks missing recent peer-reviewed studies not yet included in systematic reviews and the grey literature, such as reports on pilot schemes to improve medicines use. In addition, it may have exaggerated the relative proportion of RCTs since non-experimental designs may be excluded from systematic reviews. Secondly, only three databases were searched for records. These three databases were selected because they hold records of studies that would, in principle, meet our inclusion criteria and therefore limit the number of records found and subsequently excluded. The risk that important evidence on effectiveness and cost-effectiveness of interventions was missed was minimized by discussing the results of the review with experts in the field (see Acknowledgements for their identities). Therefore, although some studies will inevitably have been missed, this review is likely to include the most relevant studies and present a representative picture of the size and nature of evidence base on medicines optimization. A third limitation is that relying on the structured abstracts to extract details on the studies included may have added some inaccuracy to the classification of the types of interventions evaluated. Additionally, it was not possible to ascertain whether interventions were informed by an underpinning theory, as recommended by the Medical Research Council framework for the evaluation of complex interventions.120 However, detailing the exact nature of the intervention was not a major objective of this review, but rather to provide an indication of which types of interventions have been most evaluated for each type of issue on suboptimal medicines use. For these reasons, and despite its limitations, this review meets the objectives specified.
Conclusion
There is a large evidence base on the effectiveness of interventions to improve the suboptimal use of medicines; the cost-effectiveness evidence is much smaller. The evidence base is mostly on interventions for one particular stage of the medicines pathway in terms of their effect on intermediate outcomes. Intermediate outcomes can translate into final health benefits and costs differently, depending on how distant they are from final outcomes and on the strength of this link. Therefore, it is difficult to compare interventions that affect different intermediate outcomes. Evidence on final outcomes, such as mortality or QALYs is limited and typically restricted to cost-effectiveness studies. In addition, a significant proportion of the evidence is specific to certain diseases and its generalizability to others is unclear. For these reasons, it has not been possible to draw conclusions on which issues of suboptimal use of medicines should be prioritized for future research. Nonetheless, some areas for potential research have emerged as potentially valuable for the future and informative for policy decisions. These include methods research on appropriate outcome measures (e.g. for adherence), the relationship between intermediate to final outcomes and how best to compare interventions that affect different stages of the medicines pathway.
Supplementary material
Funding
This work was funded under the Economic Evaluation Policy Research Unit (EEPRU) which receives funding from the Department of Health Policy Research Programme. EEPRU is a collaboration between researchers from two institutions (Centre for Health Economics, University of York and School of Health and Related Studies, University of Sheffield). The views expressed in this article are those of the authors and not necessarily those of the Department of Health. Funding to pay the Open Access publication charges for this article was provided by…
Supplementary Material
Acknowledgements
The authors wish to thank Professor Tony Avery and Professor Nick Barber for their assistance in identifying the main issues related to suboptimal use of medicines and in commenting on the project's report. The authors would like to thank the two anonymous reviewers for their helpful and constructive comments, which much improved the paper. Any errors are the responsibility of the authors.
References
- 1.Royal Pharmaceutical Society. London: Royal Pharmaceutical Society; 2013. Medicines Optimisation: Helping Patients to Make the Most of Medicines. Good Practice Guidance for Healthcare Professionals in England. [Google Scholar]
- 2.World Health Organisation. Geneva: World Health Organisation; 2012. The Pursuit of Responsible Use of Medicines: Sharing and Learning from Countries Experiences. [Google Scholar]
- 3.National Institute for Health and Clinical Excellence and National Patient Safety Agency. London: National Institute for Health and Care Excellence; 2007. NICE Patient Safety Guidance 1. Technical Patient Safety Solutions for Medicines Reconciliation on Admission of Adults to Hospital. [Google Scholar]
- 4.NHS National Institute for Health and Clinical Excellence. NICE CG76. London: National Institute for Health and Clinical Excellence; 2009. Costing Statement: Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence. [Google Scholar]
- 5.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 6.Faria R, Barbieri M, Light K, et al. York: University of York; University of Sheffield; 2014. Economics of Medicines Optimisation. Policy Research Unit in Economic Evaluation in Health and Care Interventions. [Google Scholar]
- 7.Sinnemaki J, Sihvo S, Isojarvi J, et al. Automated dose dispensing service for primary healthcare patients: a systematic review. Syst Rev. 2013;2:1. doi: 10.1186/2046-4053-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother. 2009;43:1096–106. doi: 10.1345/aph.1L700. [DOI] [PubMed] [Google Scholar]
- 9.Forsetlund L, Eike MC, Gjerberg E, et al. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11:16. doi: 10.1186/1471-2318-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;4:345–51. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531–8. doi: 10.1197/jamia.M2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonacker CW, Hoes AW, Dikhoff MJ, et al. Interventions in health care professionals to improve treatment in children with upper respiratory tract infections. Int J Pediatr Otorhinolaryngol. 2010;74:1113–21. doi: 10.1016/j.ijporl.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Coleman CI, Roberts MS, Sobieraj DM, et al. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–80. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- 14.Holland R, Desborough J, Goodyer L, et al. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people: a systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65:303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166:955–64. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 16.Patterson Susan M, Hughes C, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012. CD008165. doi:10.1002/14651858.CD008165.pub2. [DOI] [PubMed]
- 17.Tan K, Dear Peter RF, Newell Simon J. Clinical decision support systems for neonatal care. Cochrane Database Syst Rev. 2005. p. CD004211. doi:10.1002/14651858.CD004211.pub2. [DOI] [PMC free article] [PubMed]
- 18.Wong K, Yu SK, Holbrook A. A systematic review of medication safety outcomes related to drug interaction software. J Popul Ther Clin Pharmacol. 2010;17:e243–55. [PubMed] [Google Scholar]
- 19.Arnold Sandra R, Straus Sharon E. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005. p. CD003539. doi:10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed]
- 20.Ammenwerth E, Schnell-Inderst P, Machan C, et al. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15:585–600. doi: 10.1197/jamia.M2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durieux P, Trinquart L, Colombet I, et al. Computerized advice on drug dosage to improve prescribing spractice. Cochrane Database Syst Rev. 2008. CD002894. doi:10.1002/14651858.CD002894.pub2. [DOI] [PubMed]
- 22.Ioannidis JP, Lau J. Evidence on interventions to reduce medical errors: an overview and recommendations for future research. J Gen Intern Med. 2001;16:325–34. doi: 10.1046/j.1525-1497.2001.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 24.Manias E, Williams A, Liew D. Interventions to reduce medication errors in adult intensive care: a systematic review. Br J Clin Pharmacol. 2012;74:411–23. doi: 10.1111/j.1365-2125.2012.04220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oren E, Shaffer ER, Guglielmo BJ. Impact of emerging technologies on medication errors and adverse drug events. Am J Health Syst Phar. 2003;60:1447–58. doi: 10.1093/ajhp/60.14.1447. [DOI] [PubMed] [Google Scholar]
- 26.Rosse F, Maat B, Rademaker CM, et al. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123:1184–90. doi: 10.1542/peds.2008-1494. [DOI] [PubMed] [Google Scholar]
- 27.van Rosse F, Maat B, Rademaker CM, et al. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123:1184–90. doi: 10.1542/peds.2008-1494. [DOI] [PubMed] [Google Scholar]
- 28.Wolfstadt JI, Gurwitz JH, Field TS, et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med. 2008;23:451–8. doi: 10.1007/s11606-008-0504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayoumi I, Howard M, Holbrook AM, et al. Interventions to improve medication reconciliation in primary care. Ann Pharmacother. 2009;43:1667–75. doi: 10.1345/aph.1M059. [DOI] [PubMed] [Google Scholar]
- 30.Chhabra PT, Rattinger GB, Dutcher SK, et al. Medication reconciliation during the transition to and from long-term care settings: a systematic review. Res Soc Adm Phar. 2012;8:60–75. doi: 10.1016/j.sapharm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Loganathan M, Singh S, Franklin BD, et al. Interventions to optimise prescribing in care homes: systematic review. Age Ageing. 2011;40:150–62. doi: 10.1093/ageing/afq161. [DOI] [PubMed] [Google Scholar]
- 32.Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–9. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Connor J, Rafter N, Rodgers A. Do fixed-dose combination pills or unit-of-use packaging improve adherence: a systematic review. Bull World Health Organ. 2004;82:935–9. [PMC free article] [PubMed] [Google Scholar]
- 34.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et al. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012. CD007459. doi:10.1002/14651858.CD007459.pub2. [DOI] [PMC free article] [PubMed]
- 35.Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010;95:717–23. doi: 10.1136/adc.2009.175125. [DOI] [PubMed] [Google Scholar]
- 36.George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs Aging. 2008;25:307–24. doi: 10.2165/00002512-200825040-00004. [DOI] [PubMed] [Google Scholar]
- 37.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008. CD000011. doi:10.1002/14651858.CD000011.pub3. [DOI] [PubMed]
- 38.Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing. 2004;33:224–9. doi: 10.1093/ageing/afh072. [DOI] [PubMed] [Google Scholar]
- 39.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions (Brief record) Arch Intern Med. 2007;167:540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 40.Mahtani Kamal R, Heneghan Carl J, Glasziou Paul P, et al. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011. CD005025. doi:10.1002/14651858.CD005025.pub3. [DOI] [PubMed]
- 41.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions. JAMA. 2002;288:2868–78. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 42.McGraw C. Multi-compartment medication devices and patient compliance. Br J Community Nurs. 2004;9:285–90. doi: 10.12968/bjcn.2004.9.7.13295. [DOI] [PubMed] [Google Scholar]
- 43.Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: findings from a meta-analysis. Am J Med. 2012;125:888–96. doi: 10.1016/j.amjmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell CL, Conn VS, Jantarakupt P. Older adult medication compliance: integrated review of randomized controlled trials. Am J Health Behav. 2006;30:636–50. doi: 10.5555/ajhb.2006.30.6.636. [DOI] [PubMed] [Google Scholar]
- 45.Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–33. [PubMed] [Google Scholar]
- 46.Smith Susan M, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012. CD006560. doi:10.1002/14651858.CD006560.pub2. [DOI] [PubMed]
- 47.Viswanathan M, Golin CE, Jones CD, et al. Rockville, MD: U.S. Department of Health and Human Services; 2012. Medication adherence interventions: comparative effectiveness. Closing the quality gap: revisiting the state of the science. Evidence Report/Technology Assessment No. 208. [PMC free article] [PubMed] [Google Scholar]
- 48.Wijk BL, Klungel OH, Heerdink ER, et al. Effectiveness of interventions by community pharmacists to improve patient adherence to chronic medication: a systematic review. Ann Pharmacother. 2005;39:319–28. doi: 10.1345/aph.1E027. [DOI] [PubMed] [Google Scholar]
- 49.Wright J, Emerson A, Stephens M, et al. Hospital inpatient self-administration of medicine programmes: a critical literature review. Phar World Sci. 2006;28:140–51. doi: 10.1007/s11096-006-9014-x. [DOI] [PubMed] [Google Scholar]
- 50.Zedler BK, Kakad P, Colilla S, et al. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33:62–73. doi: 10.1016/j.clinthera.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Al-aqeel S, Al-sabhan J. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database Syst Rev. 2011. p. CD008312. doi:10.1002/14651858.CD008312.pub2. [DOI] [PubMed]
- 52.Al-Jumah KA, Qureshi NA. Impact of pharmacist interventions on patients’ adherence to antidepressants and patient-reported outcomes: a systematic review. Patient Pref Adherence. 2012;3:87–100. doi: 10.2147/PPA.S27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chong WW, Aslani P, Chen TF. Effectiveness of interventions to improve antidepressant medication adherence: a systematic review. Int J Clin Pract. 2011;65:954–75. doi: 10.1111/j.1742-1241.2011.02746.x. [DOI] [PubMed] [Google Scholar]
- 54.Rubio-Valera M, Serrano-Blanco A, Magdalena-Belio J, et al. Effectiveness of pharmacist care in the improvement of adherence to antidepressants: a systematic review and meta-analysis. Ann Pharmacother. 2011;45:39–48. doi: 10.1345/aph.1P429. [DOI] [PubMed] [Google Scholar]
- 55.Bain-Brickley D, Butler Lisa M, Kennedy Gail E, et al. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev. 2011. CD009513. doi:10.1002/14651858.CD009513. [DOI] [PMC free article] [PubMed]
- 56.Cote JK, Godin G. Efficacy of interventions in improving adherence to antiretroviral therapy (Structured abstract) Int J STD AIDS. 2005;16:335–43. doi: 10.1258/0956462053888934. [DOI] [PubMed] [Google Scholar]
- 57.Horvath T, Azman H, Kennedy Gail E, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012. CD009756. doi:10.1002/14651858.CD009756. [DOI] [PMC free article] [PubMed]
- 58.Rueda S, Park-Wyllie Laura Y, Bayoumi A, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006. CD001442. doi:10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed]
- 59.Cutrona SL, Choudhry NK, Fischer MA, et al. Modes of delivery for interventions to improve cardiovascular medication adherence. Am J Manag Care. 2010;16:929–42. [PMC free article] [PubMed] [Google Scholar]
- 60.Cutrona SL, Choudhry NK, Fischer MA, et al. Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc. 2012;52:381–97. doi: 10.1331/JAPhA.2012.10211. [DOI] [PubMed] [Google Scholar]
- 61.Molloy GJ, O'Carroll RE, Witham MD, et al. Interventions to enhance adherence to medications in patients with heart failure: a systematic review. Circ Heart Fail. 2012;5:126–33. doi: 10.1161/CIRCHEARTFAILURE.111.964569. [DOI] [PubMed] [Google Scholar]
- 62.Morgado MP, Morgado SR, Mendes LC, et al. Pharmacist interventions to enhance blood pressure control and adherence to antihypertensive therapy: review and meta-analysis. Am J Health Syst Phar. 2011;68:241–53. doi: 10.2146/ajhp090656. [DOI] [PubMed] [Google Scholar]
- 63.Morrison A, Wertheimer AI, Berger ML. Interventions to improve antihypertensive drug adherence: a quantitative review of trials. Formulary. 2000;35:234–55. [Google Scholar]
- 64.Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8:174–80. doi: 10.1111/j.1524-6175.2006.04872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schedlbauer A, Schroeder K, Fahey T. How can adherence to lipid-lowering medication be improved: a systematic review of randomized controlled trials. Fam Pract. 2007;24:380–7. doi: 10.1093/fampra/cmm030. [DOI] [PubMed] [Google Scholar]
- 66.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004. p. CD004804. doi:10.1002/14651858. CD004804. [DOI] [PMC free article] [PubMed]
- 67.Dalem J, Krass I, Aslani P. Interventions promoting adherence to cardiovascular medicines. Int J Clin Pharm. 2012;34:295–311. doi: 10.1007/s11096-012-9607-5. [DOI] [PubMed] [Google Scholar]
- 68.Wal MH, Jaarsma T, Veldhuisen DJ. Non-compliance in patients with heart failure: how can we manage it? Eur J Heart Fail. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Bleser L, Matteson M, Dobbels F, et al. Interventions to improve medication-adherence after transplantation: a systematic review. Transplant Int. 2009;22:780–97. doi: 10.1111/j.1432-2277.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 70.Dodds F, Rebair-Brown A, Parsons S. A systematic review of randomized controlled trials that attempt to identify interventions that improve patient compliance with prescribed antipsychotic medication. Clin Effectiveness Nurs. 2000;4:47–53. [Google Scholar]
- 71.Dolder CR, Lacro JP, Leckband S, et al. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol. 2003;23:389–99. doi: 10.1097/01.jcp.0000085413.08426.41. [DOI] [PubMed] [Google Scholar]
- 72.Ilott R. Does compliance therapy improve use of antipsychotic medication? Br J Community Nurs. 2005;10:514–9. doi: 10.12968/bjcn.2005.10.11.19962. [DOI] [PubMed] [Google Scholar]
- 73.McIntosh A, Conlon L, Lawrie S, et al. Compliance therapy for schizophrenia. Cochrane Database Syst Rev. 2006. CD003442. doi:10.1002/14651858.CD003442.pub2. [DOI] [PMC free article] [PubMed]
- 74.Nose M, Barbui C, Gray R, et al. Clinical interventions for treatment non-adherence in psychosis: meta-analysis. Br J Psychiatry. 2003;183:197–206. doi: 10.1192/bjp.183.3.197. [DOI] [PubMed] [Google Scholar]
- 75.Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653–64. doi: 10.1176/appi.ajp.159.10.1653. [DOI] [PubMed] [Google Scholar]
- 76.Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009;20:2127–34. doi: 10.1007/s00198-009-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hood KK, Rohan JM, Peterson CM, et al. Interventions with adherence-promoting components in pediatric type 1 diabetes: meta-analysis of their impact on glycemic control. Diabetes Care. 2010;33:1658–64. doi: 10.2337/dc09-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.M'Imunya James M, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database Syst Rev. 2012. p. CD006591. doi:10.1002/14651858.CD006591.pub2. [DOI] [PMC free article] [PubMed]
- 79.Moullec G, Gour-Provencal G, Bacon SL, et al. Efficacy of interventions to improve adherence to inhaled corticosteroids in adult asthmatics: impact of using components of the chronic care model. Database Abstracts Rev Effects. 2012;106:1211–25. doi: 10.1016/j.rmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Toelle B, Ram Felix SF. Written individualised management plans for asthma in children and adults. Cochrane Database Syst Rev. 2011. p. CD002171. doi:10.1002/14651858.CD002171.pub3. [DOI] [PMC free article] [PubMed]
- 81.Sajatovic M, Davies M, Hrouda DR. Enhancement of treatment adherence among patients with bipolar disorder (Structured abstract) Psychiatr Serv. 2004;55:264–9. doi: 10.1176/appi.ps.55.3.264. [DOI] [PubMed] [Google Scholar]
- 82.Barnighausen T, Chaiyachati K, Chimbindi N, et al. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis. 2011;11:942–51. doi: 10.1016/S1473-3099(11)70181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer SH, Tjia J, Field TS. Impact of health information technology interventions to improve medication laboratory monitoring for ambulatory patients: a systematic review. J Am Med Inform Assoc. 2010;17:631–6. doi: 10.1136/jamia.2009.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48:923–33. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- 85.Royal S, Smeaton L, Avery AJ, et al. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care. 2006;15:23–31. doi: 10.1136/qshc.2004.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209–16. doi: 10.1592/phco.23.2.209.32096. [DOI] [PubMed] [Google Scholar]
- 87.Cobos A, Vilaseca J, Asenjo C, et al. Cost effectiveness of a clinical decision support system based on the recommendations of the European Society of Cardiology and other societies for the management of hypercholesterolemia: report of a cluster-randomized trial. Dis Manag Health Outcomes. 2005;13:421–32. [Google Scholar]
- 88.Pindolia VK, Stebelsky L, Romain TM, et al. Mitigation of medication mishaps via medication therapy management. Ann Pharmacother. 2009;43:611–20. doi: 10.1345/aph.1L591. [DOI] [PubMed] [Google Scholar]
- 89.Gross R, Morgan AS, Kinky DE, et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. 2001;33:289–95. doi: 10.1086/321880. [DOI] [PubMed] [Google Scholar]
- 90.Zahar JR, Rioux C, Girou E, et al. Inappropriate prescribing of aminoglycosides: risk factors and impact of an antibiotic control team. J Antimicrob Chemother. 2006;58:651–6. doi: 10.1093/jac/dkl288. [DOI] [PubMed] [Google Scholar]
- 91.Sano HS, Waddell JA, Solimando DA, et al. Study of the effect of standardized chemotherapy order forms on prescribing errors and anti-emetic cost. J Oncol Pharm Pract. 2005;11:21–30. doi: 10.1191/1078155205jp149oa. [DOI] [PubMed] [Google Scholar]
- 92.Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379:1310–9. doi: 10.1016/S0140-6736(11)61817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chinthammit C, Armstrong EP, Warholak TL. A cost-effectiveness evaluation of hospital discharge counseling by pharmacists. J Pharm Pract. 2012;25:201–8. doi: 10.1177/0897190011418512. [DOI] [PubMed] [Google Scholar]
- 94.Karnon J, McIntosh A, Dean J, et al. Modelling the expected net benefits of interventions to reduce the burden of medication errors. J Health Serv Res Policy. 2008;13:85–91. doi: 10.1258/jhsrp.2007.007011. [DOI] [PubMed] [Google Scholar]
- 95.Klopotowska JE, Kuiper R, vanKan HJ, et al. On-ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: an intervention study. Crit Care. 2010;14:R174. doi: 10.1186/cc9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weeks WB, Mills PD, Dittus RS, et al. Using an improvement model to reduce adverse drug events in VA facilities. Jt Comm J Qual Improv. 2001;27:243–54. doi: 10.1016/s1070-3241(01)27021-7. [DOI] [PubMed] [Google Scholar]
- 97.Wu RC, Laporte A, Ungar WJ. Cost-effectiveness of an electronic medication ordering and administration system in reducing adverse drug events. J Eval Clin Pract. 2007;13:440–8. doi: 10.1111/j.1365-2753.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 98.de Giorgi I, Fonzo-Christe C, Cingria L, et al. Risk and pharmacoeconomic analyses of the injectable medication process in the paediatric and neonatal intensive care units. Int J Qual Health Care. 2010;22:170–8. doi: 10.1093/intqhc/mzq015. [DOI] [PubMed] [Google Scholar]
- 99.Webster CS, Merry AF, Gander PH, et al. A prospective, randomised clinical evaluation of a new safety-orientated injectable drug administration system in comparison with conventional methods. Anaesthesia. 2004;59:80–7. doi: 10.1111/j.1365-2044.2004.03457.x. [DOI] [PubMed] [Google Scholar]
- 100.Patterson SM, Hughes CM, Cardwell C, et al. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland Study): a cost-effectiveness analysis. J Am Geriatr Soc. 2011;59:586–93. doi: 10.1111/j.1532-5415.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- 101.Benrimoj SI, Peacocke G, Whitehead P, et al. Cognitive pharmaceutical services in emerging health care systems: new patient management and concordance services in community pharmacy. J Soc Admin Pharm. 2003;20:2–12. [Google Scholar]
- 102.Bernsten C, Bjorkman I, Caramona M, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs Aging. 2001;18:63–77. doi: 10.2165/00002512-200118010-00005. [DOI] [PubMed] [Google Scholar]
- 103.Desborough JA, Sach T, Bhattacharya D, et al. A cost-consequences analysis of an adherence focused pharmacist-led medication review service. Int J Pharm Pract. 2012;20:41–9. doi: 10.1111/j.2042-7174.2011.00161.x. [DOI] [PubMed] [Google Scholar]
- 104.Elliott RA, Barber N, Clifford S, et al. The cost effectiveness of a telephone-based pharmacy advisory service to improve adherence to newly prescribed medicines. Pharm World Sci. 2008;30:17–23. doi: 10.1007/s11096-007-9134-y. [DOI] [PubMed] [Google Scholar]
- 105.Al-Eidan FA, McElnay JC, Scott MG, et al. Management of Helicobacter pylori eradication: the influence of structured counselling and follow-up. Br J Clin Pharmacol. 2002;53:163–71. doi: 10.1046/j.0306-5251.2001.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnett PG, Sorensen JL, Wong W, et al. Effect of incentives for medication adherence on health care use and costs in methadone patients with HIV. Drug Alcohol Depend. 2009;100:115–21. doi: 10.1016/j.drugalcdep.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freedberg KA, Hirschhorn LR, Schackman BR, et al. Cost-effectiveness of an intervention to improve adherence to antiretroviral therapy in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;43:S113–8. doi: 10.1097/01.qai.0000248334.52072.25. s. [DOI] [PubMed] [Google Scholar]
- 108.Zaric GS, Bayoumi AM, Brandeau ML, et al. The cost-effectiveness of counseling strategies to improve adherence to highly active antiretroviral therapy among men who have sex with men. Med Decis Making. 2008;28:359–76. doi: 10.1177/0272989X07312714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choudhry NK, Patrick AR, Antman EM, et al. Cost-effectiveness of providing full drug coverage to increase medication adherence in post-myocardial infarction Medicare beneficiaries. Circulation. 2008;117:1261–8. doi: 10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cote I, Gregoire JP, Moisan J, et al. A pharmacy-based health promotion programme in hypertension: cost-benefit analysis. Pharmacoeconomics. 2003;21:415–28. doi: 10.2165/00019053-200321060-00005. [DOI] [PubMed] [Google Scholar]
- 111.Ito K, Shrank WH, Avorn J, et al. Comparative cost-effectiveness of interventions to improve medication adherence after myocardial infarction. Health Serv Res. 2012;47:2097–117. doi: 10.1111/j.1475-6773.2012.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 113.Schroeder K, Fahey T, Hollinghurst S, et al. Nurse-led adherence support in hypertension: a randomized controlled trial. Fam Pract. 2005;22:144–51. doi: 10.1093/fampra/cmh717. [DOI] [PubMed] [Google Scholar]
- 114.Jennings HR, Miller EC, Williams TS, et al. Reducing anticoagulant medication adverse events and avoidable patient harm. Jt Comm J Qual Patient Saf. 2008;34:196–200. doi: 10.1016/s1553-7250(08)34024-0. [DOI] [PubMed] [Google Scholar]
- 115.National Institute for Health and Care Excellence (NICE) Updated Guide to the Methods of Technology Appraisal. London: NICE; 2013. [PubMed] [Google Scholar]
- 116.Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing? Br J Clin Pharmacol. 2010;70:335–41. doi: 10.1111/j.1365-2125.2010.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carone G, Schwierz C, Xavier A. Cost-Containment Policies in Public Pharmaceutical Spending in the EU. Brussels: European Commission; 2012. Economic Papers 461. Directorate-General for Economic and Financial Affaris. [Google Scholar]
- 118.Elliott RA, Putman KD, Franklin M, et al. Cost Effectiveness of a Pharmacist-Led Information Technology Intervention for Reducing Rates of Clinically Important Errors in Medicines Management in General Practices (PINCER) Pharmacoeconomics. 2014;32:573–90. doi: 10.1007/s40273-014-0148-8. [DOI] [PubMed] [Google Scholar]
- 119.Bojke L, Claxton K, Bravo-Vergel Y, et al. Eliciting distributions to populate decision analytic models. Value Health. 2010;13:557–64. doi: 10.1111/j.1524-4733.2010.00709.x. [DOI] [PubMed] [Google Scholar]
- 120.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:8–9. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.