Abstract
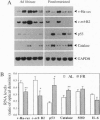
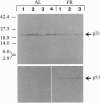
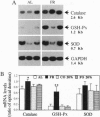
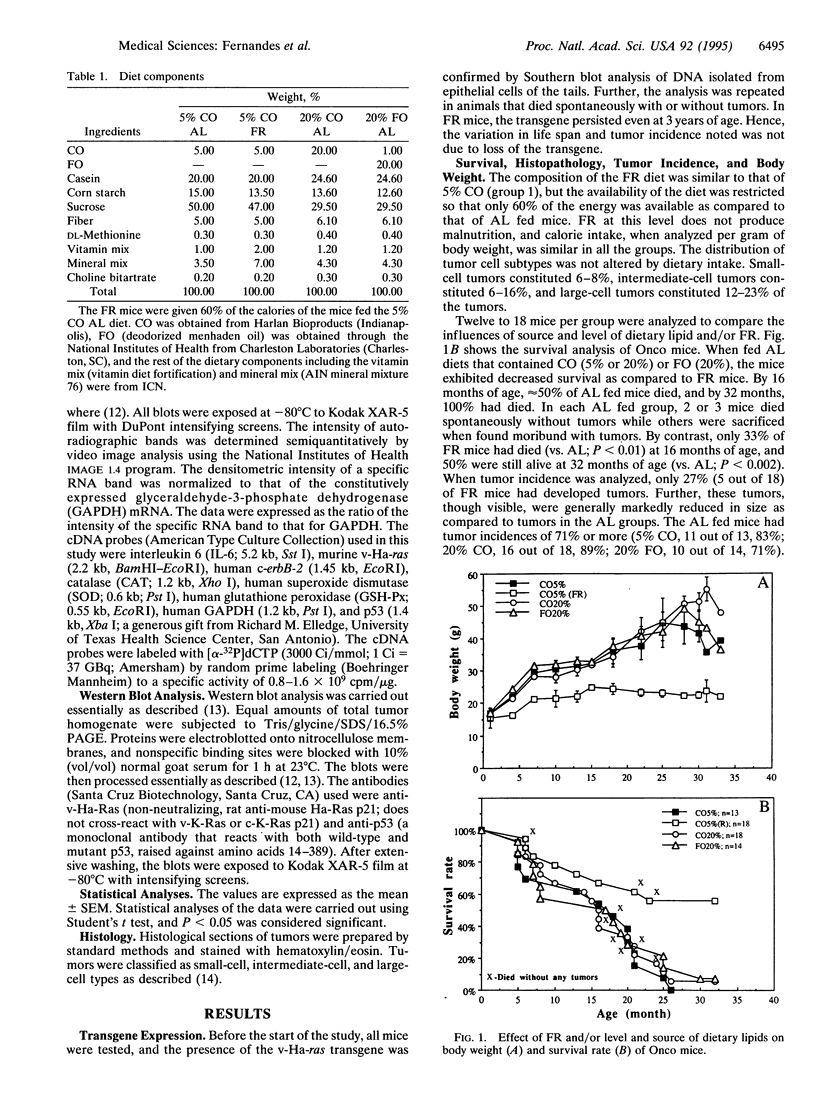
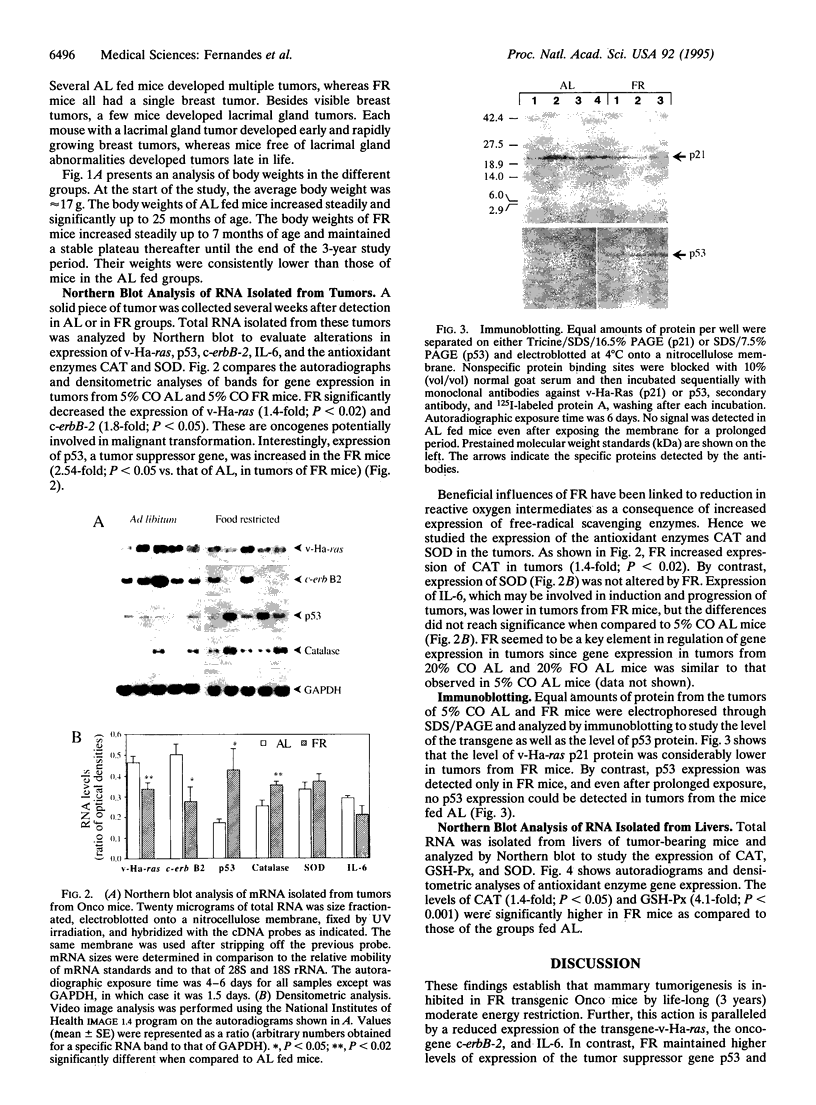
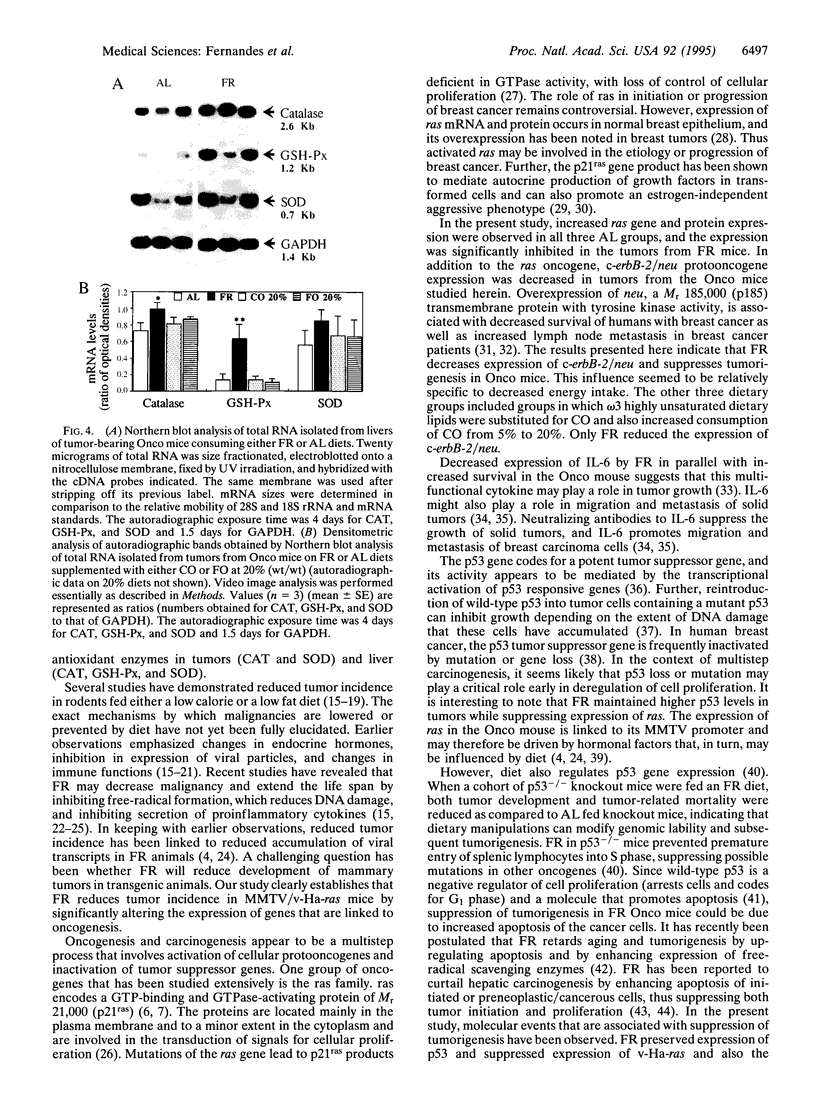
We have studied the effects of food restriction (FR) and substitution of fish oil (FO; omega 3) for corn oil (CO; omega 6) on breast tumor incidence and survival in mouse mammary tumor virus/v-Ha-ras transgenic (Onco) mice. The diets were as follows: group 1, 5% (wt/wt) CO fed ad libitum (AL); group 2, 5% CO, restricted calories (40% fewer calories than AL; FR); group 3, 20% CO fed AL; and group 4, 20% FO fed AL. After 3 years, 40% of FR Onco (group 2) mice were alive, whereas there were no survivors in the other three groups. Similarly, tumor incidence was reduced to 27% (5 out of 18) in FR animals (group 2), whereas it was 83% (11 out of 13) in group 1 mice, 89% (16 out of 18) in group 3 mice, and 71% (10 out of 14) in group 4 mice. These protective effects of FR on survival and tumor incidence were paralleled by higher expression of the tumor suppressor gene p53 (wild type) and free-radical scavenging enzymes (catalase and superoxide dismutase) in breast tumors. Immunoblotting showed less ras gene product, p21, and increased p53 levels in the tumors of FR mice. In addition, FR decreased RNA levels of c-erbB-2, interleukin 6, and the transgene v-Ha-ras in tumors. In contrast, analysis of hepatic mRNA from tumor-bearing FR mice revealed higher expression of catalase, glutathione peroxidase, and superoxide dismutase. Survival and tumor incidence were not influenced significantly by dietary supplementation with FO in place of CO. Taken together, our studies suggest that moderate restriction of energy intake significantly inhibited the development of mammary tumors and altered expression of cytokines, oncogenes, and free-radical scavenging enzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardiff R. D., Sinn E., Muller W., Leder P. Transgenic oncogene mice. Tumor phenotype predicts genotype. Am J Pathol. 1991 Sep;139(3):495–501. [PMC free article] [PubMed] [Google Scholar]
- Carroll K. K., Braden L. M. Dietary fat and mammary carcinogenesis. Nutr Cancer. 1984;6(4):254–259. doi: 10.1080/01635588509513831. [DOI] [PubMed] [Google Scholar]
- Cave W. T., Jr Dietary n-3 (omega-3) polyunsaturated fatty acid effects on animal tumorigenesis. FASEB J. 1991 May;5(8):2160–2166. doi: 10.1096/fasebj.5.8.1673664. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B., Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994 Apr 29;200(2):893–898. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Djuric Z., Lu M. H., Lewis S. M., Luongo D. A., Chen X. W., Heilbrun L. K., Reading B. A., Duffy P. H., Hart R. W. Oxidative DNA damage levels in rats fed low-fat, high-fat, or calorie-restricted diets. Toxicol Appl Pharmacol. 1992 Aug;115(2):156–160. doi: 10.1016/0041-008x(92)90318-m. [DOI] [PubMed] [Google Scholar]
- Engelman R. W., Fukaura Y., Hamada N., Good R. A., Day N. K. Dietary restriction permits normal parturition and lactation but suppresses mouse mammary tumor virus proviral transcription even after mammary involution. Cancer Res. 1991 Oct 1;51(19):5123–5128. [PubMed] [Google Scholar]
- Erickson K. L., Hubbard N. E. Dietary fat and tumor metastasis. Nutr Rev. 1990 Jan;48(1):6–14. doi: 10.1111/j.1753-4887.1990.tb02871.x. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Bysani C., Venkatraman J. T., Tomar V., Zhao W. Increased TGF-beta and decreased oncogene expression by omega-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J Immunol. 1994 Jun 15;152(12):5979–5987. [PubMed] [Google Scholar]
- Fernandes G. Nutritional factors: modulating effects on immune function and aging. Pharmacol Rev. 1984 Jun;36(2 Suppl):123S–129S. [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976 Oct 7;263(5577):504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- Freedman L. S., Clifford C., Messina M. Analysis of dietary fat, calories, body weight, and the development of mammary tumors in rats and mice: a review. Cancer Res. 1990 Sep 15;50(18):5710–5719. [PubMed] [Google Scholar]
- Grasl-Kraupp B., Bursch W., Ruttkay-Nedecky B., Wagner A., Lauer B., Schulte-Hermann R. Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9995–9999. doi: 10.1073/pnas.91.21.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N., Engelman R. W., Tomita Y., Chen R. F., Iwai H., Good R. A., Day N. K. Prolactin effects on the dietary regulation of mouse mammary tumor virus proviral DNA expression. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6733–6737. doi: 10.1073/pnas.87.17.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting S. D., Perkins S. N., Phang J. M. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting S. D., Switzer B. R., French J. E., Kari F. W. The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res. 1993 Jun 15;53(12):2750–2757. [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- James S. J., Muskhelishvili L. Rates of apoptosis and proliferation vary with caloric intake and may influence incidence of spontaneous hepatoma in C57BL/6 x C3H F1 mice. Cancer Res. 1994 Nov 1;54(21):5508–5510. [PubMed] [Google Scholar]
- Kamata T., Feramisco J. R. Epidermal growth factor stimulates guanine nucleotide binding activity and phosphorylation of ras oncogene proteins. Nature. 1984 Jul 12;310(5973):147–150. doi: 10.1038/310147a0. [DOI] [PubMed] [Google Scholar]
- Kasid A., Lippman M. E., Papageorge A. G., Lowy D. R., Gelmann E. P. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science. 1985 May 10;228(4700):725–728. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- Krueger J., Ray A., Tamm I., Sehgal P. B. Expression and function of interleukin-6 in epithelial cells. J Cell Biochem. 1991 Apr;45(4):327–334. doi: 10.1002/jcb.240450404. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- McAndrew P. F. Fat metabolism and cancer. Surg Clin North Am. 1986 Oct;66(5):1003–1012. doi: 10.1016/s0039-6109(16)44037-5. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- McIntosh J. K., Jablons D. M., Mulé J. J., Nordan R. P., Rudikoff S., Lotze M. T., Rosenberg S. A. In vivo induction of IL-6 by administration of exogenous cytokines and detection of de novo serum levels of IL-6 in tumor-bearing mice. J Immunol. 1989 Jul 1;143(1):162–167. [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro J. S., el-Naggar A., Ro J. Y., Blick M., Frye D., Fraschini G., Fritsche H., Hortobagyi G. c-erbB-2 amplification in node-negative human breast cancer. Cancer Res. 1989 Dec 15;49(24 Pt 1):6941–6944. [PubMed] [Google Scholar]
- Rogers A. E., Zeisel S. H., Groopman J. Diet and carcinogenesis. Carcinogenesis. 1993 Nov;14(11):2205–2217. doi: 10.1093/carcin/14.11.2205. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Fernandes G., Telang N. T., Kourides I. A., Good R. A. Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7758–7762. doi: 10.1073/pnas.79.24.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. Interleukin-6 enhances motility of breast carcinoma cells. EXS. 1991;59:178–193. doi: 10.1007/978-3-0348-7494-6_12. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Van de Vijver M. J., Nusse R. The molecular biology of breast cancer. Biochim Biophys Acta. 1991 Apr 16;1072(1):33–50. doi: 10.1016/0304-419x(91)90005-6. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Walker R. A., Wilkinson N. p21 ras protein expression in benign and malignant human breast. J Pathol. 1988 Oct;156(2):147–153. doi: 10.1002/path.1711560209. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]