Abstract
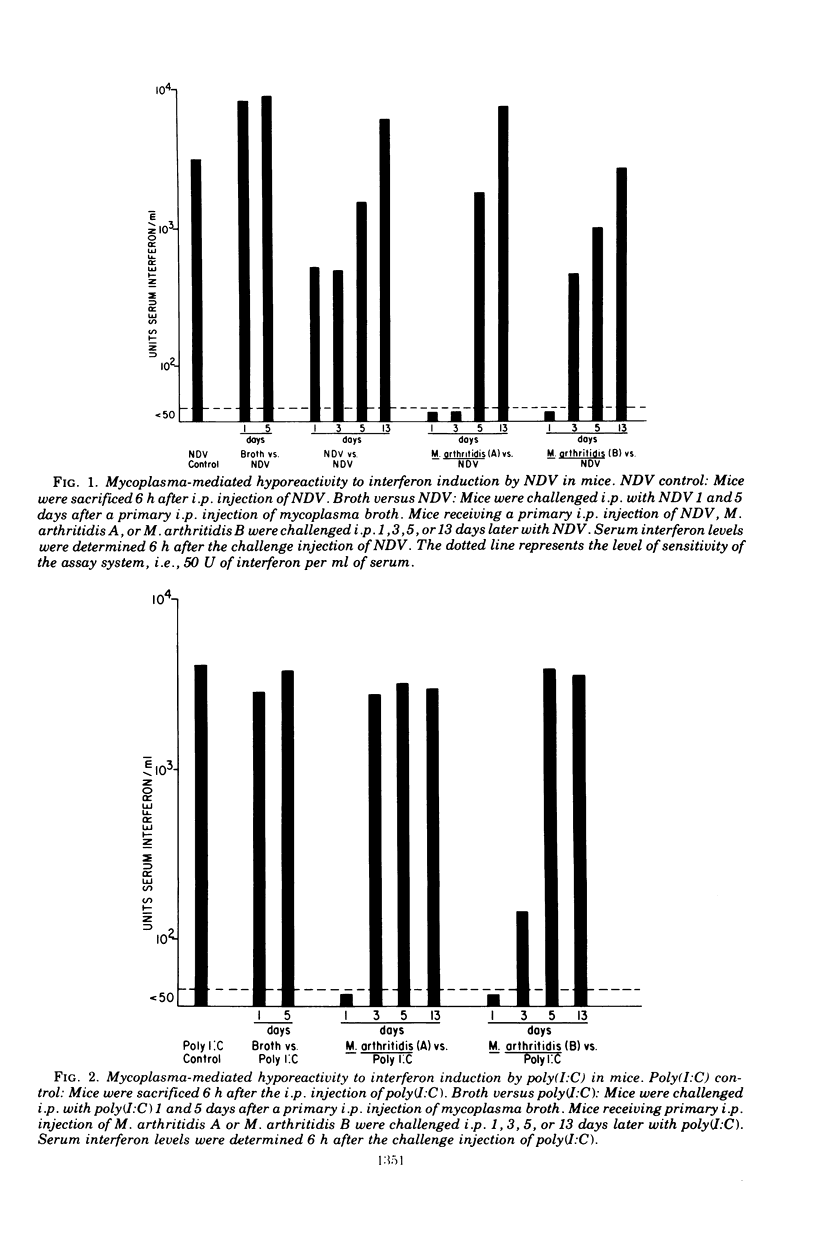
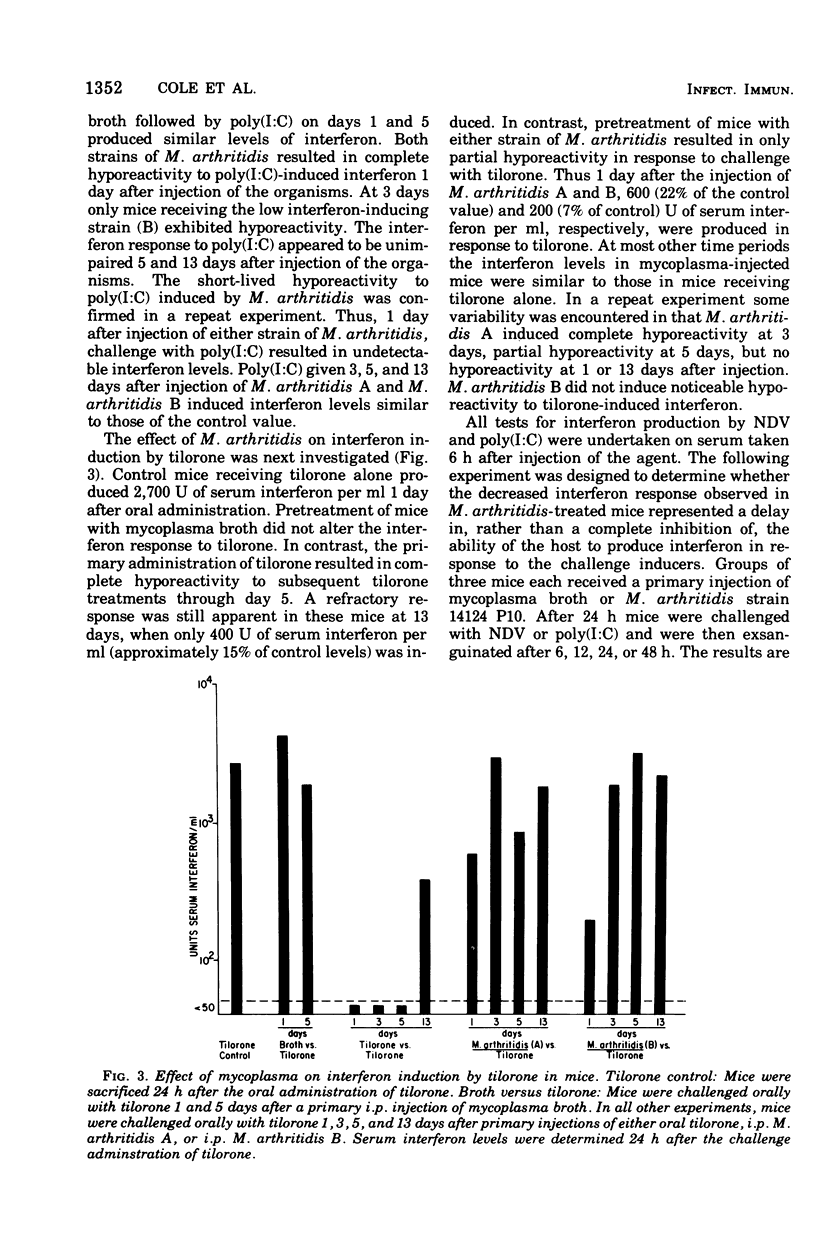
Three strains of Mycoplasma arthritidis were shown to induce marked hyporeactivity in mice to interferon induction by both Newcastle disease virus and poly(I:C). In contrast, the interferon response of mice to tilorone was only partially suppressed by pretreatment of the animals with mycoplasms. Hyporeactivity to Newcastle disease virus was maximal 1 and 3 days after mycoplasms treatment, but the interferon response was maximal 1 day after injection of the mycoplasmas and was no longer apparent by 5 days. No relationship was found between the ability of the mycoplasms themselves to induce interferon and the degree of hyporeactivity produced. These results suggest that mycoplasmas may alter virus-host relationships in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Paucker K. Effect of mycoplasma on interferon production and interferon assay in cell cultures. J Bacteriol. 1966 Jul;92(1):97–101. doi: 10.1128/jb.92.1.97-101.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden E. C., Prochownik E. V., Carter W. A. The interferon refractory state. II. Biological characterization of a refractoriness-inducing protein. J Immunol. 1975 Feb;114(2 Pt 2):752–756. [PubMed] [Google Scholar]
- Buckler C. E., DuBuy H. G., Johnson M. L., Baron S. Kinetics of serum interferon response in mice after single and multiple injections of polyI-poly C. Proc Soc Exp Biol Med. 1971 Feb;136(2):394–398. doi: 10.3181/00379727-136-35272. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Fate of intravenously injected Mycoplasma arthritidis in rodents and effect of vaccines. Infect Immun. 1973 Mar;7(3):416–425. doi: 10.1128/iai.7.3.416-425.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Martin C. H. Hemolysin and peroxide activity of Mycoplasma species. J Bacteriol. 1968 Jun;95(6):2022–2030. doi: 10.1128/jb.95.6.2022-2030.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer-Guignard J. Mouse leukemia: depression of serum interferon production. Science. 1972 Sep 1;177(4051):797–799. doi: 10.1126/science.177.4051.797. [DOI] [PubMed] [Google Scholar]
- Fauconnier B., Wroblewski H. Propriétés inductrices d'interféron et activité antivirale "in vivo" d'une souche de mycoplasma d'origine végétale: Acholeplasma sp. phiG1. Ann Microbiol (Paris) 1974 May-Jun;125(4):469–476. [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Heishman J. O., Olson N. O., Cunningham C. J. Transmission of Mycoplasma gallisepticum, Newcastle disease, infectious bronchitis, and combinations in a three-phase broiler house. Avian Dis. 1969 Feb;13(1):1–6. [PubMed] [Google Scholar]
- Holtermann O. A., Havell E. A. Reduced interferon response in mice congenitally infected with lymphocytic choriomeningitis virus. J Gen Virol. 1970 Oct;9(1):101–103. doi: 10.1099/0022-1317-9-1-101. [DOI] [PubMed] [Google Scholar]
- Kasza L., Hodges R. T., Betts A. O., Trexler P. C. Pneumonia in gnotobiotic pigs produced by simultaneous inoculation of a swine adenovirus and mycoplasma hyopneumoniae. Vet Rec. 1969 Mar 15;84(11):262–267. doi: 10.1136/vr.84.11.262. [DOI] [PubMed] [Google Scholar]
- Morgunova T. D., Postnikova Z. A., Rakovskaia I. V., Kagan G. Ia. Smeshannaia infektsiia myshee linii BAL/B/CDE virusom Raushera i Mycoplasma laidlawii. Vopr Virusol. 1974 Mar-Apr;(2):144–148. [PubMed] [Google Scholar]
- Murphy B. R., Glasgow L. A. Factors modifying host resistance to viral infection. 3. Effect of whole body x-irradiation on experimental encephalomyocarditis virus infection in mice. J Exp Med. 1968 May 1;127(5):1035–1052. doi: 10.1084/jem.127.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Suppression of interferon and antibody and multiplication of Newcastle disease virus in cytomegalovirus infected mice. Proc Soc Exp Biol Med. 1967 Feb;124(2):347–353. doi: 10.3181/00379727-124-31740. [DOI] [PubMed] [Google Scholar]
- Ranck F. M., Jr, Grumbles L. C., Hall C. F., Grimes J. E. Serology and gross lesions of turkeys inoculated with an avian influenza A virus, a paramyxovirus, and Mycoplasma gallisepticum. Avian Dis. 1970 Feb;14(1):54–65. [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Cole B. C., Overall J. C., Jr, Glasgow L. A. Induction of interferon in mice by mycoplasmas. Infect Immun. 1974 Dec;10(6):1296–1301. doi: 10.1128/iai.10.6.1296-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Cole B. C., Overall J. C., Jr, Ward J. R., Glasgow L. A. Induction of interferon in ovine leukocytes by species of mycoplasma and acholeplasma. Proc Soc Exp Biol Med. 1974 Jun;146(2):613–618. doi: 10.3181/00379727-146-38158. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Overall J. C., Jr, Cole B. C., Glasgow L. A. Mycoplasma-associated induction of interferon in ovine leukocytes. Infect Immun. 1973 Nov;8(5):796–803. doi: 10.1128/iai.8.5.796-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. H., Barile M. F., Kirschstein R. L. Enhanced virus yields and decreased interferon production in mycoplasma-infected hamster cells. Proc Soc Exp Biol Med. 1969 Sep;131(4):1129–1134. doi: 10.3181/00379727-131-34053. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. Mycoplasmas and cell cultures. Bacteriol Rev. 1971 Jun;35(2):206–227. doi: 10.1128/br.35.2.206-227.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Hyporeactivity of infection: potential limitation to therapeutic use of interferon-inducing agents. Infect Immun. 1972 Nov;6(5):743–747. doi: 10.1128/iai.6.5.743-747.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Tilorone hydrochloride: an oral interferon-inducing agent. Antimicrob Agents Chemother. 1972 Aug;2(2):73–78. doi: 10.1128/aac.2.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A. Hyporeactivity to interferon induction: characterization of a hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1975 Feb;11(2):294–302. doi: 10.1128/iai.11.2.294-302.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILCEK J., RADA B. Studies on an interferon from tickborne encephalitis virus-infected cells (IF). III. Antiviral action of IF. Acta Virol. 1962 Jan;6:9–16. [PubMed] [Google Scholar]
- Yershov F. I., Zhdanov V. M. Influence of PPLO on production of interferon in virus-infected cells. Virology. 1965 Nov;27(3):451–453. doi: 10.1016/0042-6822(65)90132-7. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]



