Abstract
Background and Objectives:
Laparoscopic transperitoneal left adrenalectomy (LTLA) has become the standard treatment for adrenal masses <6 cm. LTLA involves the dissection of splenic suspensory ligaments, which replicates their congenital absence or weakening, present in cases of wandering spleen (WS). WS is a rare condition in which the spleen migrates from the left upper quadrant to a more caudal location in the abdomen. A unique case of WS after LTLA was described by Corcione et al. In this prospective study, we investigated the possibility of WS as a consequence of LTLA.
Methods:
Twenty-four patients, 8 men and 16 women, who underwent LTLA with the dissection of splenoparietal and splenorenal ligaments were selected.
Results:
Clinical and ultrasonographic follow-up showed no evidence of postoperative WS.
Conclusions:
In the literature, WS is not commonly reported as a postoperative complication of LTLA. In effect, especially in the case of small adrenal masses, the spleen's repositioning in its seat is autonomous. However, the alarming possibility of WS should not be ignored, especially in the case of extensive dissection of the left colic flexure. It would be useful for other authors to signal this complication, so that different approaches and consequent results may be compared.
Keywords: Abdominal pain, Adrenalectomy, Gastropexy, Stomach volvulus, Wandering spleen
INTRODUCTION
In the era of minimally invasive technologies, laparoscopic transperitoneal left adrenalectomy (LTLA) has become the standard treatment for adrenal masses <6 cm.1 However, masses >6 cm can be successfully treated using the laparoscopic approach as well, replicating principles of open surgical oncology.2,3
Splenorenal and splenoparietal ligaments are dissected during LTLA with the lateral approach, and if necessary, the left colic flexure is mobilized. Then the splenopancreatic block is mobilized and medialized. The middle adrenal vein is identified behind the pancreas so that, after its ligature and dissection, the adrenal gland can be removed. The dissection of splenic ligaments replicates their congenital absence or weakening, present in cases of wandering spleen (WS).
Other possible approaches are laparoscopic transperitoneal anterior adrenalectomy (during which the patient is supine) and posterior retroperitoneal laparoscopic adrenalectomy; neither involves the dissection of splenic ligaments.
WS, also known as splenoptosis or ectopic, free-floating, or aberrant spleen, is the migration of the spleen from the left upper quadrant to a more caudal location in the abdomen.
A unique case of WS after LTLA was described by Corcione et al,4 who reported the case of a 57-year-old woman who underwent LTLA because of a 4-cm nonfunctional mass. Three months later, she was admitted to the surgical emergency department with acute abdominal pain, nausea, and vomiting. She underwent emergency laparotomy, which revealed an organoaxial gastric volvulus caused by the hypermobile spleen.
Another case report, by Le et al,5 showed WS as a rare complication of splenic torsion after laparoscopic Nissen fundoplication.
WS, with an incidence of <0.5% among splenectomies,6 is rare and is often due to the congenital absence or weakening of splenic ligaments.7,8 The spleen develops from a mesenchymal condensation in the dorsal mesogastrium of the lesser sac in the fourth week of gestation. During fetal development, rotation of the intestinal tract and growth of the dorsal mesentery carry the spleen to the left side of the abdominal cavity, where it is anchored to the left kidney and stomach by gastrosplenic and splenorenal ligaments, respectively. WS, as well as intestinal malrotation, congenital short bowel, and other abnormalities, has been associated with mutations of filamin A in male patients affected by X-linked periventricular nodular heterotopia.9 Failure of fusion of the mesogastrium10 to the posterior body wall leads to incomplete formation of splenocolic, splenorenal, and splenophrenic ligaments, and this is considered the mechanism by which WS, defined as the spleen's lying outside the left upper quadrant suspended only by hilar vessels, occurs. Consequently, WS has a long mobile mesenteric pedicle that is prone to torsion and ischemia.11 This mechanism can also be responsible for gastric rotation (volvulus), rarely associated with WS.12
When present in adults, WS may be the delayed onset of congenital conditions or an acquired disease from abdominal wall laxity due to the hormonal effects of pregnancy.13 Some authors have associated it with splenomegaly, although there is no higher incidence of WS in areas of endemic splenomegaly. In adults, it usually occurs as a mobile abdominal mass, whereas in children, it presents as acute or chronic abdominal pain14 and can be associated with a history of congenital diaphragmatic hernia15 or prune belly syndrome.16 The clinical presentation is vague, with symptoms including nausea, vomiting, early satiety, and acute, cramplike abdominal pain17,18 due to splenic ischemia and infarction. Bowel obstruction may occur because of the compression of abdominal organs19; enuresis and hydronephrosis may be other consequences,20 as well as gastric fundal varices,21 mesenteric varices,22 and acute pancreatitis.23 The torsion of the vascular pedicle may result in splenomegaly. The progressive splenic enlargement can lead to rare splenic ruptures and to possible spontaneous massive hemoperitoneum.24 WS can also be an unusual cause of thrombocytopenia.25
Ultrasonography is considered efficacious in diagnosing WS, as it is a noninvasive, inexpensive test that can confirm both the ectopic location of the spleen and blood flow pattern. Both grayscale and color Doppler sonography can easily show the migratory nature and perfusion status of a WS in real time, in the right decubitus position.26 Sometimes it is necessary to examine patients with computed tomography (CT), which provides information on the location, vascular integrity, and size of the spleen as well as the surrounding organs. With WS, CT may show elongated or tortuous splenic vessels and may also reveal multiple collaterals of the hilum of the spleen. With torsion of the splenic pedicle, a “whirled appearance” of the splenic vessels may be noticed on CT.27 Despite the advantages offered by ultrasonography and CT, WS remains a misdiagnosed condition.28
The aim of this prospective study was to determine whether the mobilization of the spleen during LTLA, replicating the congenital condition of absence or weakening of the splenic ligaments, could be recognized as a cause of postoperative WS.
MATERIALS AND METHODS
Between January 2006 and May 2011, 59 patients underwent laparoscopic lateral adrenalectomy; 30 right adrenalectomies and 29 left adrenalectomies were performed.
We took into consideration the 29 patients who underwent LTLA: 9 men (31%) and 20 women (69%). Twenty-four patients (83%) were included in our ultrasonographic follow-up. Five patients were not included, 3 with terminal cancer and the remaining 2 who had undergone multiple upper gastrointestinal surgical procedures.
Therefore, we analyzed 24 patients, 8 men (33%) and 16 women (67%), with a mean age of 59 ± 13 years (range, 37–84 years).
Among these 24 patients, 13 were affected by nonfunctional adenoma (54%), 4 by pheochromocytoma (17%), 3 by metastasis (12%), 3 by adrenocorticotropic hormone—dependent hyperplasia with Cushing's disease (12%), and 1 by Conn's adenoma (4%) (Table 1).
Table 1.
Demographic and Clinical Data of 24 Patients Undergoing LTLA
| No. | Age (y) | Gender | Diagnosis | Complications |
|---|---|---|---|---|
| 1 | 77 | M | Metastases | — |
| 2 | 62 | M | Conn's adenoma | — |
| 3 | 46 | M | Pheochromocytoma | — |
| 4 | 50 | M | ACTH-dependent hyperplasia | — |
| 5 | 80 | M | Adenoma | — |
| 6 | 53 | M | ACTH-dependent hyperplasia | — |
| 7 | 64 | M | Adenoma | — |
| 8 | 65 | M | Adenoma | — |
| 9 | 44 | F | Pheochromocytoma | — |
| 10 | 37 | F | Adenoma | — |
| 11 | 51 | F | Adenoma | — |
| 12 | 62 | F | ACTH-dependent hyperplasia | — |
| 13 | 60 | F | Adenoma | Left pneumothorax |
| 14 | 38 | F | Adenoma | — |
| 15 | 51 | F | Adenoma | — |
| 16 | 50 | F | Adenoma | — |
| 17 | 69 | F | Metastases | Right pleural effusion and hematoma in the left renal region |
| 18 | 70 | F | Metastases | — |
| 19 | 53 | F | Adenoma | — |
| 20 | 55 | F | Pheochromocytoma | — |
| 21 | 51 | F | Pheochromocytoma | — |
| 22 | 72 | F | Adenoma | — |
| 23 | 84 | F | Adenoma | — |
| 24 | 66 | F | Adenoma | — |
ACTH, adrenocorticotropic hormone; LTLA, laparoscopic transperitoneal left adrenalectomy.
Each patient underwent preoperative abdominal ultrasonography, CT, or nuclear magnetic resonance, as well as hormonal laboratory exams. LTLA was performed after informed consent was obtained from patients.
All LTLAs were performed using the same laparoscopic technique: right lateral decubitus pneumoperitoneum induced by a Veress needle; 3 trocars, placed on the midclavicular line (T3, 5 mm for the forceps and divaricators), midaxillary line (T2, 10–12 mm for the radiofrequency dissector, scissors, irrigation-aspiration cannula, clips, etc), and anterior axillary line in the middle of the line joining the umbilicus to the subcostal margin (T1, 10–12 mm for the optic) (Figure 1); dissection of the splenoparietal and splenorenal ligaments; en bloc mobilization and medialization of the spleen and pancreas (Figure 2); sectioning using clips or a radiofrequency dissector of the middle adrenal vein; and removal of the adrenal gland into an Endobag (Covidien, Dublin, Ireland). Mobilization of the left colic flexure was performed only when absolutely necessary, and exposure of the adrenal lodge was limited to the field of view. Furthermore, care was taken in repositioning the spleen and pancreas to their correct seats without using fibrin glue or other devices. The radiofrequency dissector was used during the whole procedure.
Figure 1.
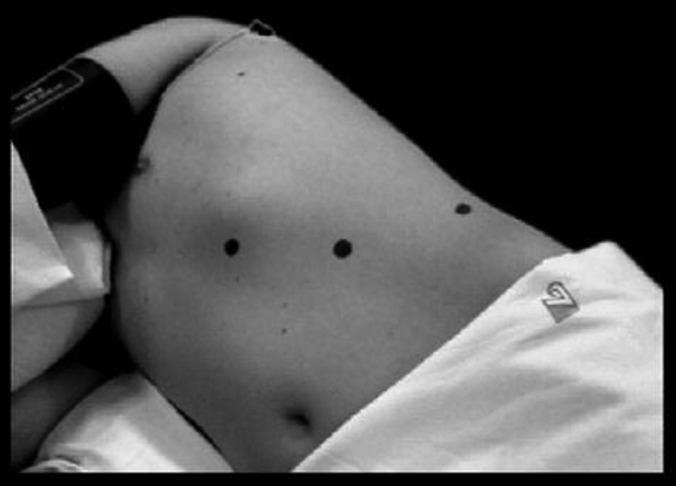
Trocar positions.
Figure 2.
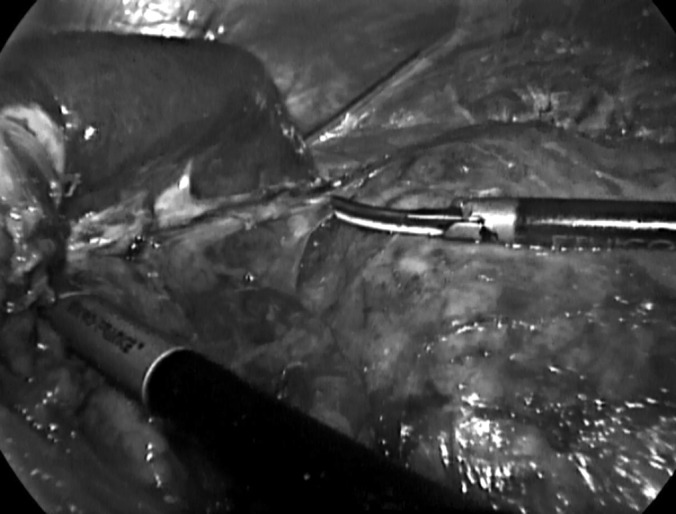
Dissection between left kidney and spleen with pancreas, after their en bloc mobilization.
The 24 selected patients were studied postoperatively using abdominal ultrasonography after 2 and 4 months (4 patients [17%]) and after 1 year (20 patients [83%]), with a 3.5-MHz convex probe, always by the same radiologist, experienced in ultrasonography, who evaluated the following parameters: morphologic and dynamic aspects of the spleen in the supine position, in the right lateral position, and in orthostatism; potential mobilization of the spleen related to the diaphragmatic dome; and flow direction and resistive index at the origin of the splenic artery and at the splenic hilum.
RESULTS
The average operation length was 89 ± 35 minutes (range, 45–180 minutes); the average diameter of the lesions was 4 ± 0 cm (range, 3–8 cm); and the average postoperative stay was 2 ± 1 days (range, 1–5 days).
Postoperative courses were free of complications in 22 patients. In the remaining 2 patients (overall morbidity, 8%), 1 patient had left pneumothorax treated with thoracic drainage, and 1 patient had right pleural effusion with hematoma in the left renal region, conservatively treated.
Clinical and ultrasonographic follow-up showed an absence of symptoms attributable to WS in each patient, confirming normal position of the spleen with normal blood flow in the supine position, in the right lateral position, and in orthostatism; an absence of spleen mobilization related to the diaphragmatic dome; and an absence of free or loculated collections. The average longitudinal spleen diameter was 9.9 ± 1.3 cm (range, 7.8–12.2 cm), and the average anteroposterior spleen diameter was 4.8 ± 0.6 cm (range, 3.8–5.8 cm). The average resistive index at the origin of the splenic artery was 0.6 ± 0.1 (range, 0.4–0.7), and the average resistive index at the splenic hilum was 0.6 ± 0.1 (range, 0.5–0.7). Hepatic steatosis was seen in 3 patients (12%), and ptosis of the right kidney was seen in 1 patient (4%).
DISCUSSION
To evaluate whether WS can be considered a frequent postoperative complication of LTLA, we considered 29 patients who underwent this surgical procedure, but 5 patients were not included in the study. For the remaining 24 patients, postoperative ultrasonographic follow-up was performed to evaluate the morphologic and dynamic aspects of the spleen, its potential mobilization, and the flow direction and resistive index at the origin of the splenic artery and at the splenic hilum. WS was completely absent, despite the dissection of ligaments and the female majority. Although there are surgical techniques that do not involve dissection of the splenic ligaments, LTLA seems to be free of complications related to the supposed hypermobile spleen.
Given the rarity of WS, we are aware that the number of patients in our study is too small to reach any definitive conclusions, and our results should be regarded as purely exploratory. WS should not be considered a common complication of LTLA, especially in patients with small adrenal masses, which generally do not require extensive dissection; in our patients, the spleen was repositioned in its correct seat without the necessity of glue or splenopexy, mobilization of the left colic flexure was performed only when absolutely necessary, and exposure of the adrenal lodge was limited to the field of view. In addition, we have no data regarding postoperative adhesion formation, but we suppose that the healing process of dissected structures may be sufficient to fix the spleen in its natural place. WS may occur if the dissection is extensive; for this reason, in these cases we recommend the use of glue or other methods to reposition the spleen in its correct seat.
CONCLUSIONS
Clinical and ultrasonographic follow-up of 24 patients who underwent LTLA showed no symptoms or signs of migration of the spleen. LTLA remains widely recognized as a safe approach, at least as effective as other techniques, such as laparoscopic transperitoneal anterior adrenalectomy and posterior retroperitoneal laparoscopic adrenalectomy. In any case, dissection should be limited and should not exceed too much in the mobilization of the left colic flexure.
Furthermore, it is necessary to pay attention when repositioning the spleen and pancreas in their correct seats. WS should be therefore considered a very rare complication of LTLA that, however, cannot be ignored. It would be useful for other authors to signal this complication, should it occur, so that different approaches and consequent results may be compared.
Contributor Information
Micaela Piccoli, Department of General Surgery, New S. Agostino Estense Hospital N.O.C.S.A.E. Baggiovara, Modena, Italy..
Giuseppe Massimiliano De Luca, Department of Biomedical Sciences and Human Oncology, Section of General and Oncologic Surgery, Unit of Endocrine, Digestive and Emergency Surgery, University Medical School “A. Moro” of Bari, Bari, Italy..
Alessandro Pasculli, Department of Biomedical Sciences and Human Oncology, Section of General and Oncologic Surgery, Unit of Endocrine, Digestive and Emergency Surgery, University Medical School “A. Moro” of Bari, Bari, Italy..
Marta Angelini, Department of Radiology, Vignola Hospital, Vignola, Italy..
Lorenzo Guicciardi, Department of General Surgery, New S. Agostino Estense Hospital N.O.C.S.A.E. Baggiovara, Modena, Italy..
Barbara Mullineris, Department of General Surgery, New S. Agostino Estense Hospital N.O.C.S.A.E. Baggiovara, Modena, Italy..
Domenico Marchi, Department of General Surgery, New S. Agostino Estense Hospital N.O.C.S.A.E. Baggiovara, Modena, Italy..
Gianluigi Melotti, Department of General Surgery, New S. Agostino Estense Hospital N.O.C.S.A.E. Baggiovara, Modena, Italy..
References:
- 1. Ramacciato G, Mercantini P, LA Torre M, et al. Is laparoscopic adrenalectomy safe and effective for adrenal masses larger than 7 cm? Surg Endosc 2008;22:516–521 [DOI] [PubMed] [Google Scholar]
- 2. Ku JH, Yeo WG, Kwon TG, Kim HH. Laparoscopic adrenalectomy for functioning and non-functioning adrenal tumors: analysis of surgical aspects based on histological types. Int J Urol. 2005;12:1015–1021 [DOI] [PubMed] [Google Scholar]
- 3. Corcione F, Miranda L, Marzano E, et al. Laparoscopic adrenalectomy for malignant neoplasms: our experience in 15 cases. Surg Endosc. 2005;19:841–844 [DOI] [PubMed] [Google Scholar]
- 4. Corcione F, Tricarico F, Barbaros U, Marzano E, Montini F, Trombetti A. Gastric volvulus after laparoscopic left adrenalectomy: case report. Surg Laparosc Endosc Percutan Tech. 2008;18:207–208 [DOI] [PubMed] [Google Scholar]
- 5. Le K, Griner D, Hope WW, Tackett D. Splenic torsion requiring splenectomy six years following laparoscopic Nissen fundoplication. JSLS. 2012;16:184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maàmouri N, Guellouz S, Chebbi F, et al. Asymptomatic chronic torsion of a pelvic wandering spleen. Tunis Med. 2011;89:403. [PubMed] [Google Scholar]
- 7. Lips N, Deroose CM, Bielen D, Bossuyt P, Mortelmans L. Wandering spleen on a 68Ga-DOTATOC-PET/TC scan. Eur J Nucl Med Mol Imaging. 2011;38:982. [DOI] [PubMed] [Google Scholar]
- 8. Yildiz AE, Ariyurek MO, Karcaaltincaba M. Splenic anomalies of shape, size, and location: pictorial essay. Sci World J. 2013;2013:321810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oegema R, Hulst JM, Theuns-Valks SD, et al. Novel no-stop FLNA mutation causes multi-organ involvement in males. Am J Med Genet A. 2013;161:2376–2384 [DOI] [PubMed] [Google Scholar]
- 10. Cripps M, Svahn J. Hand-assisted laparoscopy for wandering spleen. Surg Endosc. 2011;25:312. [DOI] [PubMed] [Google Scholar]
- 11. Montenovo MI, Ahad S, Oelschlager BK. Laparoscopic splenopexy for wandering spleen: case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2010;20:182–184 [DOI] [PubMed] [Google Scholar]
- 12. Lianos G, Vlachos K, Papakonstantinou N, Katsios C, Baltogiannis G, Godevenos D. Gastric volvulus and wandering spleen: a rare surgical emergency. Case Rep Surg. 2013;2013:561752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anyfantakis D, Kastanakis M, Katsougris N, Papadomichelakis A, Petrakis G, Bobolakis E. Acute torsion of a wandering spleen in a post-partum female: a case report. Int J Surg Case Rep. 2013;4:675–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helvind NM, Gögenur I, Stadeager M. Splenic torsion as cause of acute abdomen in children. Ugeskr Laeger. 2013;175:587–588 [PubMed] [Google Scholar]
- 15. Mehta A, Vana PG, Glynn L. Splenic torsion after congenital diaphragmatic hernia repair: case report and review of the literature. J Pediatr Surg. 2013;48:e29–e31 [DOI] [PubMed] [Google Scholar]
- 16. Tran S, Grossman E, Barsness KA. Prune belly syndrome, splenic torsion, and malrotation: a case report. J Pediatr Surg. 2013;48:e41–e43 [DOI] [PubMed] [Google Scholar]
- 17. Romero JR, Barksdale EM. Wandering spleen: a rare cause of abdominal pain. Pediatr Emerg Care. 2003;19:412–414 [DOI] [PubMed] [Google Scholar]
- 18. Leci-Tahiri L, Tahiri A, Bajrami R, Maxhuni M. Acute abdomen due to torsion of the wandering spleen in a patient with Marfan syndrome. World J Emerg Surg. 2013;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heydari MB, Johari HG, Eskandari S. Wandering spleen presenting as small bowel obstruction. Am J Emerg Med. 2013;31:984–985 [DOI] [PubMed] [Google Scholar]
- 20. Okazaki T, Ohata R, Miyano G, Lane GJ, Takahashi T, Yamataka A. Laparoscopic splenopexy and gastropexy for wandering spleen associated with gastric volvulus. Pediatr Surg Int. 2010;26:1053–1055 [DOI] [PubMed] [Google Scholar]
- 21. Irak K, Esen I, Keskin M, et al. A case of torsion of the wandering spleen presenting as hypersplenism and gastric fundal varices. Turk J Gastroenterol. 2011;22:93–97 [DOI] [PubMed] [Google Scholar]
- 22. Zarroug AE, Hashim Y, El-Youssef M, Zeidan MM, Moir CR. Wandering spleen as a cause of mesenteric and portal varices: a new etiology? J Pediatr Surg. 2013;48:e1–e4 [DOI] [PubMed] [Google Scholar]
- 23. Magno S, Nanni L, Retrosi G, Cina A, Gamba PG. An unusual case of acute pancreatitis and gastric outlet obstruction associated with wandering spleen treated by laparoscopic splenopexy. J Laparoendosc Adv Surg Tech A. 2011;21:467–470 [DOI] [PubMed] [Google Scholar]
- 24. Sucandy I, Akmal YM, Gabrielsen JD. Spontaneous massive hemoperitoneum: a potentially life threatening presentation of the wandering spleen. N Am J Med Sci. 2011;3:99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mirkes C, Nguyen G, Cable C. The wandering spleen: an unusual case of thrombocytopenia. J Blood Med. 2011;2:161–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen JW, Yeh DM, Peng SH, et al. Sonographic diagnosis of a subclinical wandering spleen: role of the decubitus position. J Ultrasound Med. 2012;31:483–487 [DOI] [PubMed] [Google Scholar]
- 27. Priyadarshi RN, Anand U, Kumar B, Prakash V. Torsion in wandering spleen: CT demonstration of whirl sign. Abdom Imaging. 2013;38:835–838 [DOI] [PubMed] [Google Scholar]
- 28. Magowska A. Wandering spleen: a medical enigma, its natural history and rationalization. World J Surg. 2013;37:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]


