Abstract
Background and Objectives:
Aerosolized droplets of blood can travel considerable distances on release of intra-abdominal pressure during laparoscopic surgery. This creates an environmental hazard for members of the surgical team. This study describes and provides a method of measurement of aerosolized blood contamination during evacuation of the pneumoperitoneum in laparoscopic surgery.
Methods:
Samples were measured by removing a trocar from the abdomen while a pneumoperitoneum of 15 mm Hg was present. A white poster board was placed 24 inches above the incision to catch the released blood spatter. By use of machine vision, luminol fluorescence, and computerized spatial analysis, data from the boards were recorded, analyzed, and scored based on the distance, size, and quantity of particulate contamination.
Results:
We analyzed 27 boards. Spatter was present on every board. The addition of luminol to the boards increased the amount of visible spatter. Most tests created <1000 blood spatters. Fluids are typically ejected as a fine mist. Every test included at least 1 blood spatter. The range of the average blood spatter size was 0.53 × 10–3 to 7.11 × 10–3 sq in. The amount of spatter detected did not show any apparent correlation with the patient's body mass index, the estimated blood loss, or the type of operation performed.
Conclusions:
Evacuation of the pneumoperitoneum during laparoscopic surgery results in consistent contamination. Most blood spatter is not visible to the naked eye. Our results suggest that all surgical participants should wear appropriate protective barriers and conscious measures should be undertaken to prevent environmental contamination during pneumoperitoneal evacuation.
Keywords: blood spatter, aerosolized blood, laparoscopy safety
INTRODUCTION
Laparoscopic surgery is known to provide many advantages to patients by reducing postoperative pain and incision-related comorbidities, shortening hospital stays, and making it possible to return to work earlier. Surgeon and staff exposure to blood and body fluids has generally been reduced without the need to “open” the abdominal cavity. There is, however, a potential increase in the risk of aerosolized droplets or tissue traveling considerable distances on release of intra-abdominal pressure as can commonly occur with specimen extraction in laparoscopic surgery.
Nearly every surgeon has at one time laparoscopically extracted a gallbladder through a tight fascial opening and noticed dramatic expulsion of pneumoperitoneum and droplets of body fluid. This clearly creates an environmental hazard for members of the surgical and anesthesia teams.
Few studies have attempted to describe blood spatter contamination during laparoscopic surgery. Organizations such as the Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, Joint Commission on Accreditation of Healthcare Organizations, and Association of Perioperative Registered Nurses (AORN) recommend use of protective eyewear and barrier precautions during surgery; however, the recommendations are extrapolated from data regarding blood splashes during open surgery.1–3 The few studies that have been performed were observational studies that looked at blood spray contamination across both open and laparoscopic surgery. They did not look at contamination that may not be visible to the naked eye.4,5
This study aims to describe and provide a method of measurement of aerosolized blood and tissue contamination during evacuation of the pneumoperitoneum in laparoscopic surgery. It also aims to show that the degree of blood spatter contamination that occurs during laparoscopic surgery is much greater than typically assumed by the operative team.
METHODS
Patients were selected at random from a cohort of patients undergoing laparoscopic bariatric surgery. The operations performed included laparoscopic Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and laparoscopic complex revision bariatric surgery. During the operation, a 15-mm trocar was placed in the left mid abdomen. It was removed near the end of the case to enable extraction of the gastric specimen or passage of an end to end anastamotic stapler. Just before extraction of the 15-mm trocar, the abdomen was insufflated to a pressure of 15 mm Hg. A white 18 × 24–inch poster board was placed horizontally 24 inches above the patient's abdomen. The trocar was removed, and the pneumoperitoneum was allowed to completely evacuate. The board was examined for particulate spatter by multiple methods.
Each test board was photographed with a high-definition camera under white-light conditions (Figure 1). The test boards were then treated with luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), placed under a black light, and re-photographed (Figure 2). Luminol is a chemiluminescent compound that glows blue when mixed with an oxidizing agent.6 Its use has been well established in the field of forensics to detect trace amounts of blood as it reacts with the iron in hemoglobin.
Figure 1.

Original board with visible light.
Figure 2.

Luminol-treated board.
After the luminol-treated board was photographed, the digital photograph was converted to grayscale and cropped to standardize size measurements. The image size was then retrieved, and the background intensity was averaged and subtracted from the original. This was accomplished by developing an image map of the background to accommodate variations in contrast and illumination. A threshold value for high-intensity outliers was established on a pixel-by-pixel basis, and a binary image was created. The threshold value was adjusted as needed to optimize the image. Objects were labeled with connected white pixels and then generated a maximum, minimum, and total objects list. These data were used to determine the average object size. The image was then normalized to scale. The image analysis was completed by use of first-order statistics and Monte Carlo simulation to provide categorization. Normalized results were created by approximating the zone of interest, estimating edge detection, and estimating the centroid. Assumptions that were made in the computational analysis included uniform linear gradients, fixed maximum lighting, zero acceleration, uniform inserts for each insert type, and parameters tuned to give average cycle times matching current production data.
After computational analysis, a colorized image was produced with colors based on object size (Figure 3). We calculated the number of spatters on the test board, as well as the largest and smallest diameters of each spatter particle. The cross-sectional area of spatter was measured, and on the basis of a distance of 24 inches from the site of origination, a 3-dimensional model was calculated to estimate the general area contaminated by blood spatter.
Figure 3.

Board with colorized representation.
Data calculated from each sample board were then correlated with previously collected patient-specific data, which included the patient's body mass index (BMI), the estimated blood loss during the operation, and the type of operation performed.
RESULTS
A total of 27 boards were collected and analyzed from separate patients; 7 patients underwent Roux-en-Y gastric bypass, 15 patients underwent sleeve gastrectomy, and 5 patients underwent revision bariatric surgery. Body fluids are typically ejected as a fine mist. Particulate spatter was present on every board collected. On 26 of 27 boards, there was blood spatter present that was visible to the naked eye. With the addition of luminol to the boards, the amount of visible spatter was significantly increased. In all samples, blood spatter was visible after the addition of luminol with black-light visualization.
The measured number of blood spatters per board ranged from 31 to 2750 spatters. Most tests created <1000 blood spatters (Figure 4). The maximum individual blood spatter cross-sectional area ranged from 0.031 × 10–3 to 1246 × 10–3 sq in (Figure 5). Typically, the individual blood spatter cross-sectional areas were <400 × 10–3 sq in. The cross-sectional area of the smallest blood spatter was 0.031 × 10–3 sq in. Every test included at least 1 blood spatter measuring 0.031 × 10–3 sq in. The range of the average blood spatter size was 0.53 × 10–3 to 7.11 × 10–3 sq in. Typically, the individual blood spatter measured <400 × 10–3 sq in (Figure 6). Notably, a marked portion of the spray was not visible to the human eye.
Figure 4.
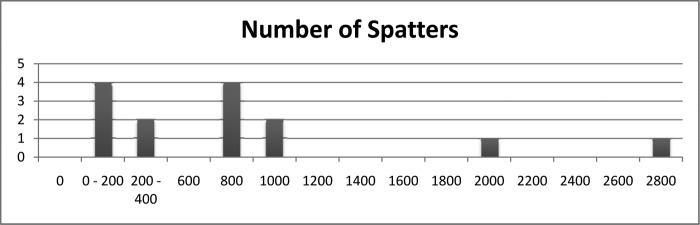
Number of blood spatters measured.
Figure 5.
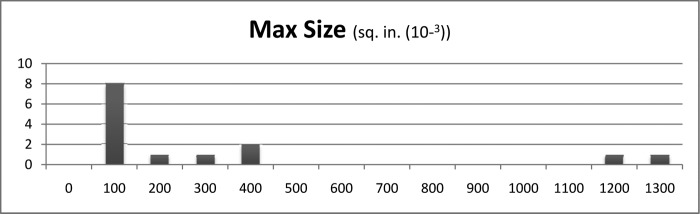
Size of largest measured blood spatter. Max = maximum.
Figure 6.
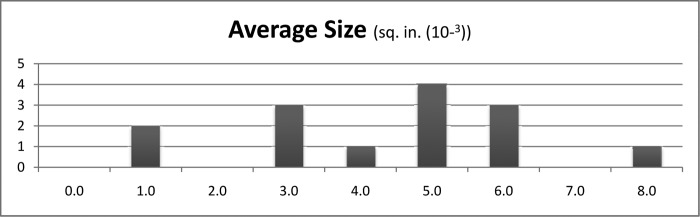
Average size of blood spatter measured.
Among gastric bypass patients in the study, the number of spatter particles observed ranged from 31 to 704 (mean, 316). In the group of sleeve patients, the number of spatter particles observed ranged from 58 to 2750 (mean, 983), and among revision bariatric surgery patients, the number of particles ranged between 98 and 709 particles (mean, 404). Patients with a BMI <40 (n = 11) had a range of 156 to 2750 spatters per board (mean, 983), whereas those with a BMI between 40 and 50 (n = 9) had a range of 31 to 929 spatters per board (median, 316) and those with a BMI >50 (n = 7) had a range of 279 to 761 (mean, 561). In cases in which the estimated blood loss was >40 mL (n = 4), the average number of spatter particles was 542 (range, 279–704), whereas in cases with <40 mL of blood loss (n = 23), the average number of spatter particles was 780 (range, 31–2750).
Among the patients undergoing the various operations, gastric bypass patients emitted the largest individual particles (mean, 400 × 10–3 sq in). The group of patients with a BMI between 40 and 50 had the largest mean diameter of largest particles, at 479 × 10–3 sq in. The average spatter particle size among the sleeve gastrectomy patients was 4.14 × 10–3 sq in. Among the gastric bypass patients, it was 2.26 × 10–3 sq in, and the revision bariatric surgery group averaged 4.49 × 10–3 sq in. The average spatter particle measured 4.46 × 10–3 sq in among patients with a BMI <40 versus 3.81 × 10–3 sq in among those with a BMI of 40 to 50 and 2.69 × 10–3 sq in among those with a BMI >50.
DISCUSSION
This study shows consistent environmental contamination of blood and body fluid during rapid evacuation of the pneumoperitoneum. Previous studies have sought to measure blood spatter contamination on masks and glasses.4 These studies reported eyeglass contamination in 25% to 51% of surgical cases, with vascular surgeries having the most frequent contamination.4,7–9 Of these studies, only one looked specifically at laparoscopic surgery, noting visible blood splash on the surgical team's glasses in 50% of cases.4 The results of our study show a significantly higher incidence of blood spatter associated with laparoscopic surgery. The differences in the observed incidences are likely a result of this study's ability to detect blood spatter that may not be apparent to the naked eye. By implementing the use of machine vision, as well as luminol enhancement of the blood spatter, the ability to detect very fine blood particles is greatly enhanced.
In this study a distance of 24 inches from the patient's incision was chosen as a point of measurement because this was found to be at face level with the tallest members of the operating room staff. Some early tests were conducted at a distance of 18 inches from the patient's abdomen, which showed even higher degrees of blood spatter. The results of the samples collected at 18 inches from the patient were not included in this study to provide uniform testing conditions. Those results will be made available subsequently in a follow-up study looking at the effect of variables such as lower pneumoperitoneal pressures, smaller trocar-site incisions, and variable positions of the incision on the abdomen. On the basis of our findings at 24 inches from the patient, it can reasonably be concluded that this blood spatter would be coming in contact with the operating team members' faces and eyes if adequate safety measures are not observed.
In addition, given the amount of blood spatter routinely observed at 24 inches, as well as the size of the particles observed, blood spatter—though small—may be contaminating as large as a 6-foot radius from the surgical site. This would support current Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, Joint Commission on Accreditation of Healthcare Organizations, and AORN safety measures, which include facemasks and eye shields for all members of the operative team, as well as the use of appropriate barrier gowns and gloves.1,2 Additional measures such as decreasing the pneumoperitoneal pressure and halting carbon dioxide flow at the time of specimen extraction may also decrease blood spatter. Moreover, operative techniques or instruments that result in less active bleeding into the trocar wounds could be used to decrease the amount of blood susceptible to aerosolization.
The use of protective wraparound-style glasses and masks with face shields has been shown to effectively prevent blood splash contact with the eyes.1,2,4 The use of regular eyeglasses does not provide the same level of protection as wraparound glasses. Studies have shown a 5% rate of contamination of side flaps on wraparound eyeglasses that are not present on everyday-use eyeglasses.9
It should be noted that although the risk of transmission of blood-borne viruses through contact with the eyes or mucous membranes is low, such transmission nonetheless presents a risk that health care workers need to be aware of. The risk of transmission of human immunodeficiency virus (HIV) through a percutaneous hollow-bore needle stick is 0.5%. This risk is decreased to 0.1% with mucous membrane exposure.10 Cases of HIV transmission as a result of blood contact with the eye have been reported.11
Hepatitis C carries a risk of transmission that is similar to, if not slightly higher than, that of HIV. An epidemiologic study in France of patients undergoing interventional radiologic procedures showed that as many as 10% of patients (91 of 944) tested positive for hepatitis C; moreover, among those, 90% (n = 82) had positive viremia titers, suggesting a high potential for transmission through blood-based contacts.12 These studies further emphasize the importance of adequate protective clothing, as well as the need for conscious measures, to reduce the risk of aerosolized spread of blood.
Some simple practices such as decreasing the pneumoperitoneal pressure or evacuating the pneumoperitoneum through the port valve before specimen extraction would likely significantly decrease the amount of blood spatter and the area of blood spatter involved. Additional measures such as placing a towel over the incision or implementing the use of surgical techniques that limit the amount of bleeding in the incision may be effective ways to reduce the amount of aerosolized blood exposure.
CONCLUSIONS
Evacuation of the pneumoperitoneum during laparoscopic surgery results in consistent, visible contamination at a distance of 24 inches from the patient's incision. An even larger portion of the blood contamination that occurs is not visible to the naked eye. If efforts are not made to control the aerosolized spread of particles during release of the pneumoperitoneum, significant contamination to the surgical team can occur. These droplets are of variable size and may not be noticed when making contact with one's skin. Pressurized droplets can also make contact with the eyes of surgical personnel, even those wearing eye protection.
The results of this study support current AORN recommendations regarding the use of universal precautions and suggest not only that all members of the surgical team, during a laparoscopic case, should wear appropriate protective barriers to prevent body fluid contact but also that conscious measures should be undertaken to prevent environmental contamination during pneumoperitoneal evacuation. Simple steps such as reducing the intra-abdominal pressure during extraction can dramatically reduce the amount of airborne fluid and tissue, making the operating room safer for all. In addition, this study establishes a method of measurement of aerosolized blood spatter, and it offers a tool to measure the efficacy of this method in monitoring future innovations in operating room safety.
Contributor Information
Richard K. Englehardt, Bariatric Medical Institute of Texas, San Antonio, TX, USA.; Department of Surgery, University of Texas at Houston Medical School, Houston, TX, USA.
Brent M. Nowak, Department of Mechanical Engineering, University of Texas at San Antonio, San Antonio, TX, USA..
Michael V. Seger, Bariatric Medical Institute of Texas, San Antonio, TX, USA..
Frank D. Duperier, Bariatric Medical Institute of Texas, San Antonio, TX, USA..
References:
- 1. Recommended Practices For Surgical Attire. In: Perioperative Standards and Recommended Practices. Denver, CO: Association of Perioperative Registered Nurses; 2012:57–72 [Google Scholar]
- 2. Recommended Practices for Prevention of Transmissible Infections in the Perioperative Practice Setting. In: Perioperative Standards and Recommended Practices. Denver, CO: Association of Perioperative Registered Nurses; 2012:341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wines MP, Lamb A, Argyropoulos AN, Caviezel A, Gannicliffe C, Tolley D. Blood splash injury: an underestimated risk in endourology. J Endourol. 2008;22(6):1183–1187 [DOI] [PubMed] [Google Scholar]
- 4. Davies CG, Khan MN, Ghauri ASK, Ranaboldo CJ. Blood and body fluid splashes during surgery-the need for eye protection and masks. Ann R Coll Surg Engl. 2007;89:770–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marasco S, Woods S. The risk of eye splash injuries in surgery. Aust N Z J Surg. 1998;68:785–787 [DOI] [PubMed] [Google Scholar]
- 6. Nieman T. Detection Based on Solution-Phase Chemiluminescence Systems. In: Birks JW, ed. Chemiluminescence and Photochemical Reaction Detection in Chromatography. New York, NY: VCH; 1989:99–123 [Google Scholar]
- 7. Brearley S, Buist LJ. Blood splashes: an underestimated hazard to surgeons. BMJ. 1989;299:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berridge DC, Lees TA, Chamberlain J, Jones NAG. Eye protection for the vascular surgeon. Br J Surg. 1993;80:1379–1380 [DOI] [PubMed] [Google Scholar]
- 9. Bell KM, Clement DA. Eye protection for the surgeon. J R Coll Surg Edinb. 1992;37:60–61 [PubMed] [Google Scholar]
- 10. Saltzman DJ, Williams RA, Gelfand DV, Wilson SE. The surgeon and AIDS: twenty years later. Arch Surg. 2005;140:961–967 [DOI] [PubMed] [Google Scholar]
- 11. Italian nurse in AIDS compensation test case. Nurs Stand. 1995;46:12 [Google Scholar]
- 12. Baffoy-Fayard N, Maugat S, Sapoval M, Cluzel P. Potential exposure to hepatitis C virus through accidental blood contact in interventional radiology. J Vasc Intervent Radiol. 2003;14:173–179 [DOI] [PubMed] [Google Scholar]


