Abstract
Significance
Electronic cigarettes, also known as e-cigarettes, are devices designed to imitate regular cigarettes and deliver nicotine via inhalation without combusting tobacco. They are purported to deliver nicotine without other toxicants and to be safer alternative to regular cigarettes. However, little toxicity testing has been performed to evaluate the chemical nature of vapor generated from e-cigarettes. The aim of this study was to screen e-cigarette vapors for content of four groups of potentially toxic and carcinogenic compounds: carbonyls, volatile organic compounds, nitrosamines, and heavy metals.
Materials and methods
Vapors were generated from 12 brands of e-cigarettes and the reference product, the medicinal nicotine inhaler, in controlled conditions using a modified smoking machine. The selected toxic compounds were extracted from vapors into a solid or liquid phase and analyzed with chromatographic and spectroscopy methods.
Results
We found that the e-cigarette vapors contained some toxic substances. The levels of the toxicants were 9 to 450 times lower than in cigarette smoke and were, in many cases, comparable to trace amounts found in the reference product.
Conclusions
Our findings are consistent with the idea that substituting tobacco cigarettes with electronic cigarettes may substantially reduce exposure to selected tobacco-specific toxicants. E-cigarettes as a harm reduction strategy among smokers unwilling to quit warrants further study.
Keywords: electronic cigarette, smoking, formaldehyde, acetaldehyde, acrolein, nitrosamines, volatile organic compounds
INTRODUCTION
An electronic cigarette, also known as e-cigarette, is a type of nicotine inhaler, imitating ordinary cigarettes. Although the majority of e-cigarettes look similar to other tobacco products, such as cigarettes or cigars, certain types resemble pens, screwdrivers, or even harmonicas. E-cigarettes contain nicotine solution in a disposable cartridge. The cartridge is replaced when the solution is finished or might be re-filled by the e-cigarette user. In contrast with ordinary cigarettes, which involve tobacco combustion, e-cigarettes use heat to transform nicotine solution into vapor. Processed and purified nicotine from tobacco leaves, suspended in a mixture of glycerin or propylene glycol with water, is vaporized. Nicotine present in such vapor enters the respiratory tract, from where it is absorbed to the bloodstream.[1-4]
Distributors of e-cigarettes promote the product as completely free of harmful substances. The basis for the claim of harmlessness of the e-cigarettes is that they do not deliver toxic doses of nicotine and the nicotine solution lacks harmful constituents. E-cigarettes are new products and, as such, require further testing to assess their toxic properties. Currently, the scientific evidence on the lack or presence of toxic chemicals in the vapor generated from e-cigarettes, and inhaled by their users is very limited. In August 2008, Ale Alwen, the Assistant Director-General for Non-communicable Diseases and Mental Health, stated that ‘the electronic cigarette is not a proven nicotine replacement therapy. WHO has no scientific evidence to confirm the product’s safety and efficacy. However, WHO does not discount the possibility that the electronic cigarette could be useful as a smoking cessation aid. The only way to know is to test.’.[5] Douglas Bettcher, Director of the WHO’s Tobacco Free Initiative stated that only clinical tests and toxicity analysis could permit considering e-cigarettes a viable method of nicotine replacement therapy.[6]
The majority of tests carried out on e-cigarettes until now consist of analyzing the chemicals in the cartridges or nicotine refill solutions.[7-18] The current tests show that the cartridges contain no or trace amounts of potentially harmful substances, including nitrosamines, acetaldehyde, acetone and formaldehyde. However, using e-cigarettes requires heating the cartridges and under such conditions chemical reactions may result in formation of new compounds. Such a situation takes place in the case of ordinary cigarettes, where a number of toxic compounds are formed during combustion. The US Department of Health and Human Services of the FDA agency carried out tests which showed the presence of trace amounts of nitrosamines and diethylene glycol in e-cigarette vapor. These tests were conducted in a manner which simulated the actual use of the products.[19]
We developed analytical methods and measured concentrations of selected compounds in the vapor generated by different brands and types of e-cigarettes. We focused our study on the four most important groups of toxic compounds present in the tobacco smoke: carbonyl compounds, volatile organic compounds (VOCs), tobacco-specific nitrosamines (TSNAs), and metals (Table 1).
Table 1.
| Chemical compounds | Toxic effects |
|---|---|
| Carbonyl compounds | |
| formaldehyde*, acetaldehyde*, acrolein* | cytotoxic, carcinogenic, irritant, pulmonary emphysema, dermatitis |
| Volatile organic compounds (VOCs) | |
| benzene*, toluene*, aniline | carcinogenic, hematotoxic, neurotoxic, irritant |
| Nitrosamines | |
| N’–nitrosonornicotine (NNN)*, 4- (methylonitrosoamino)-1-(3-pirydyl)-l-butanone (NNK) *, N’-nitrosoethylomethyloamine |
carcinogenic |
| Polycyclic aromatic compounds (PAHs) | |
| benzo(a)pyrene, benzo(a)anthracene, dibenzo(a)anthracene |
carcinogenic |
| Free radicals | |
| methyl radical, hydroxyl radical, nitrogen monoxide |
carcinogenic, neurotoxic |
| Toxic gases | |
| carbon monoxide, hydrogen sulfide, ammonia, sulphur dioxide, hydrogen cyanide |
cardiovascular toxicants, carcinogenic, irritant |
| Heavy metals | |
| cadmium (Cd)*, lead (Pb)*, mercury (Hg)* | carcinogenic, nephrotoxic, neurotoxic, hematotoxic |
| Other toxicants | |
| carbon disulphide | neurotoxic |
Note: indicates compounds analyzed in this study
MATERIALS AND METHODS
Electronic cigarettes and reference product (Nicorette® inhalator)
Since the internet is currently the main distribution channel for the products, we searched price comparison websites, online marketplace (Allegro.pl auction service), and internet discussion forums for e-cigarette users to identify the most popular brands of e-cigarettes distributed from within Poland. The searching was limited to web pages from Poland, and only Polish language was allowed for in retrieval options. Some 30 brands were identified. The brands were entered into Google.pl, and ranked according to the number of hits they generated. The number of hits in the search engine for the selected 30 models allowed selection of the 11 most popular e-cigarettes brands. Additionally, one e-cigarette model purchased in Great Britain was used in the study. All e-cigarette models selected for the study were purchased online. Characteristics of the product tested in the study are shown in Table 2.
Table 2.
Characteristics of products tested in the study.
| Product code |
Brand name | Model | Cartridge type |
Flavor | Labeled nicotine content (mg or mg/mL) |
Measured nicotine content (mg) [3] |
Retailer | Country |
|---|---|---|---|---|---|---|---|---|
| EC01 | Joye | 510 | Cartridge | Marlboro | 4 | 4 | Inspired s.c. | Poland |
| EC02 | Janty | eGo | Cartridge | Marlboro | 16 | 5 | Janty | Poland |
| EC03 | Janty | Dura | Cartridge | Marlboro | 16 | 5 | Janty | Poland |
| EC04 | DSE | 901 | Cartridge | Regular | 16 | 9 | Fausee | Poland |
| EC05 | Trendy | 808 | Cartridge | Trendy | 18 | 2 | Damhess | Poland |
| EC06 | Nicore | M401 | Cartridge | Marlboro | 18 | 5 | Atina Poland | Poland |
| EC07 | Mild | 201 | Cartridge | Marlboro | 18 | 19 | Mild | Poland |
| EC08 | Colinss | Age | Cartomizer | Camel | 18 | 11 | Colinss | Poland |
| EC09 | Premium | PR111 | Cartomizer | Tobacco | 16 | 12 | Premium | Poland |
| EC10 | Ecis | 510 | Cartridge | Menthol | 11 | 5 | Arcotech | Poland |
| EC11 | Dekang | Pen | Cartridge | Regular | 18 | 18 | Ecigars Polska | Poland |
| EC12 | Intellicig | Evolution | Cartridge | Regular | 8 | 8 | Intellicig | UK |
The suitable cartridges of the same brand name were used for the study. They were purchased from the same sources that e-cigarette and were matched to selected models. All cartridges were characterized by high nicotine content (16-18 mg). As a reference product the medicinal nicotine inhalator was used (Nicorette® 10 mg, Johnson&Johnson, Poland). The inhalator for the study was purchased in one of the local pharmaceutical warehouses.
Generation of vapor from e-cigarettes and reference product
Vapor from e-cigarettes was generated using smoking machine Palaczbot® (Technical University of Lodz, Poland) as described previously.[3] This is a one-port linear piston-like smoking machine with adjustable puffing regimes in a very wide range, controlled by computer interface.
Pilot samples demonstrated that it was impossible to generate vapor from e-cigarettes in standard laboratory conditions assumed for conventional cigarettes testing (ISO 3808).[24] Inhalation of a volume of 35 mL anticipated in conventional cigarette standard is insufficient for an activation of most of the e-cigarettes. Thus, we decided to generate vapor in conditions reflecting the actual manner of e-cigarettes using, determined based on the results of inhalation topography measurement among 10 ‘e-smokers’, who declared that they regularly use e-cigarettes for a period longer that one month.[3] All testing procedures in this work were carried out using the same averaged puffing conditions: puff duration of 1.8 sec, intervals between puffs of 10 sec, puff volume 70 mL, and number of puffs taken in one puffing session was 15. A total of 150 puffs were taken from each e-cigarette in 10 series of 15 puffs with intervals between series of 5 minutes each. Each e-cigarette was tested three times on three following days after batteries were recharged during nights. A fresh cartridge was placed on the e-cigarettes each day they were tested. Vapor was visibly being produced during the full 150 puffs taken from each product tested.
Analytical chemistry
Note: The details of the sample preparation and analysis are given in the Supplementary Materials.
It was planned to absorb the analyzed vapor components in bulbs containing an organic solvent (extraction to liquid) or on suitable sorbents (extraction to solid phase). This required the modification of the system described above, in such a manner to enable quick connection of desirable sorption system. Carbonyl compounds and organic compounds due to their volatility were trapped in tubes packed with solid adsorbent. Metals and nitrosamines in turn, which are characterized by lower volatility, were to be absorbed in two gas washing bottles with methanol (50 mL in each bottle). Both washing bottles were immersed in acetone-dry ice bath in order to avoid any losses of volatile solvent. A picture of set for vapor generation from e-cigarette and metals or nitrosamines absorption is presented in Supplementary Figure 2.
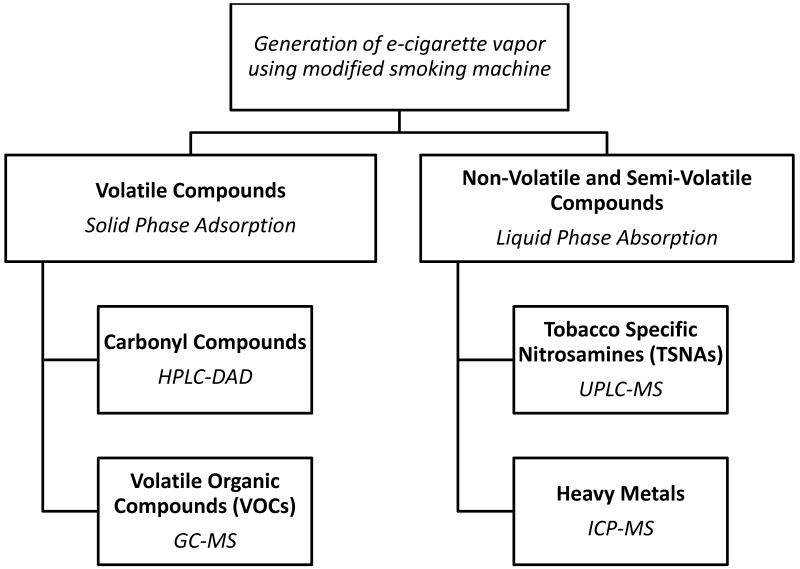
The samples, after preparation and condensation procedure, were analyzed using analytical methods with high specificity and sensitivity allowing detection of even trace amounts of analyzed compounds. Figure 1 shows the sample preparation procedure; and all analytical methods are described in details in the Supplementary Materials. The following carbonyl compounds were analyzed in this work using high-performance liquid chromatography with spectrophotometric detector (HPLC-DAD): formaldehyde, acetaldehyde, acrolein, acetone, propionic aldehyde, crotonaldehyde, butanol, benzaldehyde, isovaleric aldehyde, valeric aldehyde, m-methylbenzaldehyde, o-methylbenzaldehyde, p-methylbenzaldehyde, hexanal, 2,5-dimethylbenzaldehyde. Volatile organic compounds (VOCs) included benzene, toluene, chlorobenzene, ethylbenzene, m,p-xylene, o-xylene, styrene, 1,3-dichlorobenzene, 1,4-dichlorobenzene, 1,2-dichlorobenzene, naphthalene and were analyzed with gas chromatography-mass spectrometry (GC-MS). Among tobacco-specific nitrosamines (TSNAs) two compounds were measured: N’-nitrosonornicotine (NNN) and 4-(methylonitrosoamino)-1-(3-pirydyl)-l-butanone (NNK) with ultra-performance liquid chromatography-mass spectrometry (UPLC-MS). An inductively coupled plasma mass spectrometry technique (ICP-MS) was used to quantify following metals: cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), cadmium (Cd), lead (Pb), arsenic (As), chromium (Cr), selenium (Se), manganese (Mn), barium (Ba), rubidium (Rb), strontium (Sr), silver (Ag), thallium (Tl), and vanadium (V). All analytical methods used in this work were validated as per the International Conference on Harmonization guideline Q2(R1).[25]
Figure 1.
Analytical procedures applied in the study to test carcinogens and selected toxicants in vapor from e-cigarettes
Statistical analysis
Results were presented as mean±SEM levels of selected compounds in vapor generated from e-cigarettes (per 150 puffs). The study aimed to compare the results obtained for aerosol from Nicorette® inhalator with the results obtained for all examined e-cigarettes models. Due to small size of the groups, the difference between mean from two groups was assessed based on t Student’s test. All statistical analyses were conducted using the software for statistical data analysis Statistica 9.0 (StaftSoft, Tulsa, USA). The significance level was established as p<0.05.
RESULTS
Carbonyl compounds
Among 15 carbonyls analyzed, only 4 were found in vapor generated from e-cigarettes (Table 3); and these compounds were identified in almost all examined e-cigarettes. The exception was one e-cigarette marked with code EC09, where acrolein was not detected. Three of the carbonyls have known toxic and irritating properties: formaldehyde, acetaldehyde, and acrolein. The content of formaldehyde ranged from 2.0 to 56.1 μg, acetaldehyde from 1.1 to 13.6 μg, and acrolein from 0.7 to 41.9 μg per one e-cigarette (150 puffs). Trace amounts of formaldehyde, acetaldehyde and o-methylbenzaldehyde were also detected from the Nicorette® inhalator. None of these compounds were detected in blank samples.
Table 3.
Levels of selected compounds in vapor generated from e-cigarettes (per 150 puffs). Values are mean±SEM.
| Compound | BS | Levels in vapor from electronic cigarettes1 | Reference product |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Product code | ||||||||||||||
| EC01 | EC02 | EC03 | EC04 | EC05 | EC06 | EC07 | EC08 | EC09 | EC10 | EC11 | EC12 | Inhalator | ||
| Carbonyl compounds [μg] | ||||||||||||||
| Formaldehyde | ND | 44.2±4.1* | 23.6±8.7* | 30.2±2.3* | 47.9±0.2* | 56.1±1.4* | 35.3±2.7* | 19.0±2.7* | 6.0±2.0 | 3.2±0.8 | 3.9±1.5 | 23.9±11.1 | 46.3±2.1* | 2.0±1.1 |
| Acetaldehyde | ND | 4.6±0.2* | 6.8±3.2 | 8.2±2.5* | 11.5±2.0* | 3.0±0.2* | 13.6±2.1* | 11.1±3.3* | 8.8±1.6* | 3.5±0.3* | 2.0±0.1 | 3.7±1.5 | 12.0±2.4* | 1.1±0.6 |
| Acrolein | ND | 41.9±3.4* | 4.4±2.5 | 16.6±2.5* | 30.1±6.4* | 22.0±1.6* | 2.1±0.4* | 8.5±3.6 | 0.7±0.4 | ND | 2.7±1.6 | 1.1±0.6 | 7.4±3.2* | ND |
| o-methylbenzaldehyde | ND | 1.9±0.5 | 4.4±1.2* | 3.2±1.0* | 4.9±1.2* | 1.7±0.1* | 7.1±0.4* | 1.3±0.8 | 5.5±0.0* | 6.0±0.7* | 3.2±0.5* | 5.1±0.1* | 2.2±0.6* | 0.7±0.4 |
| Volatile Organic Compounds (VOCs) [μg] | ||||||||||||||
| Toluene | ND | 0.5±0.1* | ND | 0.2±0.0* | 0.6±0.1* | 0.2±0.0* | ND | 0.3±0.2 | 0.2±0.1 | 6.3±1.5* | 0.2±0.1* | 0.5±0.1* | 0.5±0.0* | ND |
| p,m-xylene | 0.1 | 0.1±0.0* | ND | 0.1±0.0* | 0.2±0.1* | 0.1±0.0 | ND | 0.1±0.1 | 0.1±0.0 | 0.1±0.0* | 0.1±0.0* | 0.1±0.1* | 0.1±0.0 | ND |
| Tobacco-Specific Nitrosamines (TSNAs) [ng] | ||||||||||||||
| NNN | ND | ND | 2.7±2.2 | 0.8±0.8 | ND | ND | 0.9±0.4 | 4.3±2.4 | 1.9±0.3* | 1.2±0.6 | 2.0±1.1 | 3.2±0.6* | 1.3±0.1 | ND |
| NNK | ND | 2.0±2.0 | 3.6±1.8 | 3.5±1.8 | ND | ND | 1.1±1.1 | 21.1±6.3* | 4.6±0.4* | 28.3±13.2 | 2.1±2.1 | 13.0±1.4* | ND | ND |
| Metals [μg] | ||||||||||||||
| Cd | 0.02 | 0.17±0.08 | 0.15±0.03* | 0.15±0.05 | 0.02±0.01 | 0.04±0.01 | 0.22±0.16 | 0.02±0.01 | 0.08±0.03 | 0.01±0.01 | 0.17±0.10 | 0.03±0.03 | ND | 0.03±0.01 |
| Ni | 0.17 | 0.28±0.22 | 0.29±0.08 | 0.21±0.03 | 0.17±0.07 | 0.14±0.06 | 0.11±0.06 | 0.23±0.09 | 0.26±0.10 | 0.19±0.09 | 0.12±0.04 | 0.11±0.08 | 0.11±0.05 | 0.19±0.04 |
| Pb | 0.02 | 0.06±0.01 | 0.06±0.03 | 0.07±0.01 | 0.03±0.01 | 0.05±0.01 | 0.03±0.01 | 0.04±0.01 | 0.57±0.28 | 0.09±0.04 | 0.06±0.02 | 0.04±0.03 | 0.03±0.03 | 0.04±0.01 |
Note: SE, standard error; DL, detection limit; BS, blank sample; ND, not detected;
significant difference with Nicorette inhalator (p<0.05)
units are μg, except for nitrosamines units are ng
Volatile Organic Compounds (VOCs)
Among 11 volatile organic compounds analyzed, only 2 were found in samples of vapor generated from e-cigarettes (Table 3), and these compounds were identified in almost all examined e-cigarettes. The only one exception was e-cigarette marked with code EC02, where toluene and m,p-xylene were not detected. The content of toluene ranged from 0.2 to 6.3 μg per one e-cigarette (150 puffs). Although the m,p-xylene levels found in analyzed samples of e-cigarette vapors ranged from 0.1 to 0.2 μg, it was also found on the same level in blank samples. In Nicorette® inhalator in turn, none of compounds analyzed in that group was noted.
Tobacco-Specific Nitrosamines (TSNAs)
Both nitrosamines analyzed in the study were identified in all but three vapors generated from e-cigarettes (Table 3). NNN was not found in e-cigarettes marked with code EC01, EC04 and EC05 and NNK was not identified in products EC04, EC05, and EC12. The content of NNN ranged from 0.8 to 4.3 ng, and NNK from 1.1 to 28.3 ng per one e-cigarette (150 puffs). In Nicorette® inhalator nor in blank samples in turn, none of these compounds was noted.
Metals
Among 12 metals analyzed in the study, cadmium, nickel and lead were identified, and were present in all vapors generated from e-cigarettes (except cadmium, which was not detected in a product of code EC12; Table 3). The content of cadmium ranged from 0.01 to 0.22 μg, nickel from 0.11 to 0.29 μg, and lead from 0.03 to 0.57 μg per one e-cigarette (150 puffs). The same metals in trace amounts were detected in Nicorette® inhalator and in blank samples.
DISCUSSION
We examined vapors generated from 12 models of e-cigarettes for the presence of four groups of toxic compounds found in tobacco smoke. The Nicorette inhalator was used as a reference product. Such a choice was dictated by the premise that a therapeutic product like Nicorette® inhalator should fulfill specified safety standards and should not contain significant levels of any of the analyzed toxic compounds.
Our results confirm findings from the previous studies, in which small amounts of formaldehyde and acetaldehyde were detected in cartridges.[9, 18] However, the presence of acrolein in a cartridge or nicotine solution has not been reported so far. Formaldehyde and acetaldehyde were also found in vapor exhaled to test chamber by volunteers who used e-cigarette filled with three various nicotine solutions.[26] Recently, Uchiyama et al. demonstrated that vapor generated from single brand of e-cigarette contained low levels of formaldehyde, acetaldehyde, and acrolein.[27] There is a possibility that acrolein is present in vapor only, since this compound may be formed as a result of heating glycerin which is a component of the solution. Pyrolysis of glycerin has been studied in steam with acrolein, formaldehyde, and acetaldehyde observed as the major products.[28, 29] These products appear to result from dehydration and fragmentation of glycerin. Although energy calculations of the dehydration of glycerin by the neutral mechanisms indicate that these processes can only occur at relatively high temperatures such as occur in pyrolysis or combustion, the addition of acids allows substantially lower dehydration temperatures.[30]
All three carbonyls compounds found in the study and discussed above have been shown to be toxic in numerous studies: formaldehyde is classified as carcinogenic to humans (group 1 by IARC);[31] acetaldehyde as possibly carcinogenic to humans (group 2B),[31] and acrolein causes irritation to the nasal cavity, and damage to the lining of the lungs and is thought to contribute to cardiovascular disease in cigarette smokers.[32] Exposure to carbonyl compounds found in vapor might cause mouth and throat irritation which are the most frequently reported adverse events among e-cigarette users.[1, 33] A study by Cassee et al. showed that sensory irritation in rats exposed to mixtures of formaldehyde, acetaldehyde and acrolein is more pronounced than that caused by each of the compounds separately.[34] Future studies should evaluated possible adverse health outcomes of short and long term exposure to these compounds among users of e-cigarettes and people involuntary exposed to exhaled vapors.
We found that vapor of some e-cigarettes contains traces of the carcinogenic nitrosamines NNN and NNK, whereas neither was detected in aerosol from the Nicorette® inhalator. The studies conducted previously reported the presence of NNN and NNK in e-cigarette cartridges in amount of 3.9-8.2 ng per cartridge,[18, 19] which corresponds to the results in vapor obtained in the present paper. However some other studies have reported that some cartridges are free of nitrosamines.[12] This inconsistency of findings of various studies might be due to different analytical methodologies of variable sensitivity applied in the studies discussed above.
Two of analyzed volatile organic compounds were detected: toluene and m,p-xylene. None of the studies conducted until now reported the presence of these compounds in a cartridge, nicotine solution, or e-cigarette vapor. None of these compounds were found in a study by Schripp et al. on passive exposure to e-cigarette vapors.[26] Three toxic metals, cadmium, nickel and lead, were detected in vapor of analyzed e-cigarettes. Since the same elements were also detected in trace amounts in Nicorette® inhalator and in blank samples it is possible that there were other sources of these metals. This limitation of the study does not allow us to conclude with whether e-cigarette alone may be significant source of exposure to these chemicals.
Recently, we published a study on tests for nicotine delivery of Polish and UK e-cigarettes brands.[3] Many of the same brands in that paper have been also included in this study and tested for toxicants delivery. It should be mentioned that the leading brands with the highest nicotine delivery did not have the highest yields for toxicant delivery. This is important as selecting the brands for nicotine the worst brands for toxicants generally can be avoided.
The results allowed us to compare the content of harmful substances between various e-cigarettes models and conventional cigarettes (based on literature data).[35] To compare levels of selected toxins in e-cigarette vapor and mainstream smoke of conventional cigarette we assumed that users of e-cigarettes smoker take on overage 15 puffs during one session of product use, and it would correspond to smoking one conventional cigarette. In our study the vapors from e-cigarettes were generated from 150 puffs (10 series of 15 puffs each). For comparison purposes, we assumed that 150 puffs of e-cigarette correspond to smoking 10 cigarettes. The comparison of toxic substances levels between conventional cigarette and e-cigarette is presented in Table 4.
Table 4.
Comparison of toxicants levels between conventional and electronic cigarettes.
| Toxic compound |
Conventional cigarette (μg in mainstream smoke) [35] |
Electronic cigarette (μg per 15 puffs) |
Average ratio (conventional vs. electronic cigarette) |
|---|---|---|---|
| Formaldehyde | 1.6-52 | 0.20-5.61 | 9 |
| Acetaldehyde | 52-140 | 0.11-1.36 | 450 |
| Acrolein | 2.4-62 | 0.07-4.19 | 15 |
| Toluene | 8.3-70 | 0.02-0.63 | 120 |
| NNN | 0.005-0.19 | 0.00008-0.00043 | 380 |
| NNK | 0.012-0.11 | 0.00011-0.00283 | 40 |
As shown in Table 4 levels of selected toxic compounds found in the smoke from a conventional cigarette were from 9 to 450-fold higher than levels in in the vapor of an e-cigarette. Smoking (also referred to as ‘vaping’) an e-cigarette can result in comparable exposure to carcinogenic formaldehyde as that received from cigarettes smoking. Formaldehyde was also found in the vapor of medicinal inhalator, at levels that overlapped with those found in e-cigarette vapor. Exposure to acrolein, an oxidant and respiratory irritant thought to be a major contributor to cardiovascular disease from smoking, is 15 times lower on average in e-cigarette vapor compared to cigarette smoke. The amounts of toxic metals and aldehydes in e-cigarettes are trace and comparable to amounts contained in examined therapeutic product.
The results of the study support the proposition that the vapor from e-cigarette is less injurious than the smoke from cigarettes. Thus one would expect that if a person switched from conventional cigarettes to e-cigarettes that exposure to toxic chemicals and related adverse health effects would be reduced. The confirmation of that hypothesis requires however further studies involving people using e-cigarette devices.
The primary limitation of our research is that the puffing profile we used may not reflect actual user puff topography. Hua et al. reported that e-cigarette users take longer puffs, and that puff duration varied significantly among e-cigarette brands and users.[36] This suggests that actual doses of toxicants inhaled by e-cigarette users might be higher than measured in our study. Similarly to results of tobacco cigarette testing with smoking machines (ISO, FTC) the values obtained in our study should be interpreted with caution. The other limitation of our research is that we have tested only 12 brands of e-cigarettes. There are numerous different brands on the market, and there is little information on their quality control.
CONCLUSIONS
The vapor generated from e-cigarettes contains potentially toxic compounds. However, the levels of potentially toxic compounds in e-cigarette vapor is from 9 to 450-fold lower than those in the smoke from conventional cigarette, and in many cases comparable to the trace amounts present in pharmaceutical preparation. Our findings are support the idea that substituting tobacco cigarettes with electronic cigarettes may substantially reduce exposure to tobacco-specific toxicants. The use of e-cigarettes as a harm reduction strategy among cigarettes smokers who are unable to quit warrants further study.
Supplementary Material
Supplementary Figure 1. Various models and brands of e-cigarettes evaluated in the study with Nicorette inhalator (product in horizontal position).
Supplementary Figure 2. Set for extraction of analyzed toxicants (TSNAs and metals) into liquid solvent.
Supplementary Table 1. Detection and quantitation limits of compounds evaluated in vapor of e-cigarettes (per 150 puffs).
WHAT THIS PAPER ADDS.
Distributors of e-cigarettes promote the product as completely free of harmful substances. Currently, there is no comprehensive research on the presence of toxic chemicals in the vapor generated from e-cigarettes and inhaled by their users.
This study of chemical composition of vapor generated from 12 brands of e-cigarettes revealed that the vapor contained some toxic substances.
The levels of potentially toxic compounds in e-cigarette vapor were found to be from 9 to almost 450-fold lower compared to smoke from conventional cigarette, and in many cases comparable to trace amounts present in pharmaceutical preparation.
FUNDING
This study was conducted while the first author was at Medical University of Silesia, Poland and was supported by the Ministry of Science and Higher Education of Poland under grant number N N404 025638. The study sponsor had no involvement in the study design, collection, analysis and interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication. Analysis of nitrosamines at the University of California, San Francisco was supported by grants P30 DA012393 and S10 RR026437 from the National Institutes of Health.
Footnotes
CONTRIBUTORS
MLG and NB designed the study and wrote the paper. JK, MG and LK tested the products using smoking machine. AS and JK developed the analytical method and measured carbonyl compounds and VOCs. AP, MJ, and CRD developed the analytical method and measured metals. CH and PJ developed the analytical method and measured TSNAs. MLG and JK analyzed the data. All contributors approved final version of the manuscript.
COMPETING INTEREST
Dr. Goniewicz received research funding from Pfizer, manufacturer of stop smoking medication and is currently funded by the UK Center for Tobacco Control Studies, UK Public Health Centre of Excellence (UKCTCS). UKCTCS receives it funding from the Economic and Social Research Council (ESRC), British Heart Foundation (BHF), Cancer Research UK, National Institute for Health Research (NIHR), and Medical Research Council (MRC). Dr. Benowitz is a consultant for several companies that market smoking cessation medications and has been a paid expert in litigation against tobacco companies. The other authors declare they have no actual or potential competing financial interests.
DATA SHARING STATEMENT
Data could be made available to qualified researchers by request to the corresponding author.
REFERENCES
- 1.Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e-cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 2.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat past mistakes? J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 3.Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–66. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 4.Vansickel AR, Cobb CO, Weaver MF, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–53. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Marketers of electronic cigarettes should halt unproven therapy claims. News release. Geneva, Switzerland: [accessed 2 Oct 2012]. Sep 19, 2008. http://www.who.int/mediacentre/news/releases/2008/pr34/en/index.html. [Google Scholar]
- 6.World Health Organization (WHO) WHO says there is no evidence that the electronic cigarette helps smokers to quit smoking. WHO this week asked manufacturers and marketers to stop their unproved therapy claims. Transcript of WHO podcast. Geneva, Switzerland: [accessed 2 Oct 2012]. Sep 26, 2008. http://www.who.int/mediacentre/multimedia/podcasts/2008/transcript_48/en/ [Google Scholar]
- 7.Laugesen M. Ruyan nicotine electronic inhaler/e-cigarette: bench-top tests; Poster POS5-11. Poster presented at the 2009 Joint Conference of SRNT and SRNT-Europe; Saggart, Co. Dublin, Ireland. 27-30 April 2009; [accessed 1 Oct 2012]. http://www.healthnz.co.nz/DublinEcigBenchtopHandout.pdf. [Google Scholar]
- 8.Alliance Technologies LLC [accessed 16 March 2012];Characterization of liquid “smoke juice” for electronic cigarettes. 2009 http://truthaboutecigs.com/science/4.pdf.
- 9.Coulson H. Analysis of components from Gamucci electronic cigarette cartridges, tobacco flavor regular smoking liquid 2009. Report number: E98D. LPD Lab Service; [accessed 16 March 2012]. Mar 3, 2009. http://truthaboutecigs.com/science/7.pdf [Google Scholar]
- 10. [accessed 16 March 2012];Exponent. NJOY e-cigarette health risk assessment. http://truthaboutecigs.com/science/5.php.
- 11.Alliance Technologies LLC [accessed 16 March 2012];Characterization of Regal cartridges for electronic cigarettes. 2009 http://truthaboutecigs.com/science/8.pdf.
- 12.Alliance Technologies LLC [accessed 16 March 2012];Characterization of Regal cartridges for electronic cigarettes - Phase II. 2009 http://truthaboutecigs.com/science/9.pdf.
- 13.Ellicott M. Analysis of components from “e-juice XX high 36mg/ml rated nicotine solution” ref S 55434. Report number: E249A. LPD Lab Service; [accessed 16 March 2012]. Jun 11, 2009. http://truthaboutecigs.com/science/11.pdf. [Google Scholar]
- 14.Valance C, Ellicott M. Analysis of chemical components from high, med and low nicotine cartridges. Report number: D318. LPD Lab Service; [accessed 16 March 2012]. Sep 10, 2008. http://truthaboutecigs.com/science/12.pdf. [Google Scholar]
- 15.Alliance Technologies LLC [accessed 16 March 2012];Chemical composition of “Instead” electronic cigarette smoke juice and vapor. 2009 http://truthaboutecigs.com/science/13.pdf.
- 16.Cai X, Kendall MW. Gas chromatography mass spectrometry (GC-MS) analysis report. Job number C09Y8961. EAG Evans Analytical Group; [accessed 16 March 2012]. Jul 21, 2009. http://truthaboutecigs.com/science/14.pdf. [Google Scholar]
- 17.Tytgat J. “Super Smoker” Expert report. Final report. Toxicology Laboratory, Catholic University; Leuven: [accessed 16 March 2012]. Jun 29, 2007. http://truthaboutecigs.com/science/15.pdf. [Google Scholar]
- 18.Laugesen M. Safety report on the Ruyan® e-cigarette cartridge and inhaled aerosol. Health New Zealand Ltd.; Christchurch, New Zealand: [accessed 21 May 2012]. Oct 30, 2008. http://www.healthnz.co.nz/RuyanCartridgeReport30-Oct-08.pdf. [Google Scholar]
- 19.Westenberger BJ. Evaluation of e-cigarettes. Department of Healthand Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis; St Louis, MO: [accessed: 23 May 2012]. May 4, 2009. http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf. [Google Scholar]
- 20.International Agency for Research on Cancer (IARC) Evaluation of the carcinogenic risks to humans. Tobacco smoke and involuntary smoking. IARC Monographs. Vol. 38. Lyon, France: [accessed 3 Oct 2012]. 2004. http://monographs.iarc.fr/ENG/Monographs/vol83/mono83-1.pdf. [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: [accessed 3 Oct 2012]. 2004. http://www.surgeongeneral.gov/library/reports/smokingconsequences/index.html. [Google Scholar]
- 22.Perfetti TA, Rodgman A. The complexity of tobacco and tobacco smoke. Beitrage zur Tabakforschung International. 2011;24:215–32. [Google Scholar]
- 23.Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of IARC group I carcinogens reported in mainstream cigarette smoke. Food Chem Toxicol. 1997;35:1107–30. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 24.International Organization for Standardization (ISO) Routine analytical cigarette-smoking machine – Definitions and standard conditions. ISO 3308:2000. Geneva, Switzerland: 2000. [Google Scholar]
- 25.International Conference on Harmonization (ICH) Technical requirements for registration of pharmaceuticals for human use. Topic Q2 (R1): Validation of analytical procedures: text and methodology. Geneva, Switzerland: [accessed: 8 November 2011]. 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. [Google Scholar]
- 26.Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. doi: 10.1111/j.1600-0668.2012.00792.x. Published Online First 2 June 2012. doi:10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 27.Uchiyama S, Inaba Y, Kunugita N. Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. J Chromatogr A. 2010;1217:4383–8. doi: 10.1016/j.chroma.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Antal MJ, Mok WSL, Roy JC, et al. Pyrolytic sources of hydrocarbons from biomass. J Anal Appl Pyrolysis. 1985;8:291–303. [Google Scholar]
- 29.Stein YS, Antal MJ, Jones MJ. A study of the gas-phase pyrolysis of glycerol. Anal Appl Pyrolysis. 1983;4:283–96. [Google Scholar]
- 30.Nimlos MR, Blanksby SJ, Qian X, et al. Mechanisms of glycerol dehydration. J Phys Chem A. 2006;110:6145–56. doi: 10.1021/jp060597q. [DOI] [PubMed] [Google Scholar]
- 31.International Agency for Research on Cancer (IARC) Agents classified by the IARC Monographs. 1-105. Geneva, Switzerland: [accessed: 10 September 2012]. 2012. http://monographs.iarc.fr/ENG/Classification/index.php. [Google Scholar]
- 32.U.S. Environmental Protection Agency (EPA) Toxicological review of acrolein. Washington, DC: [accessed: 10 September 2012]. May, 2003. http://www.epa.gov/iris/toxreviews/0364tr.pdf. [Google Scholar]
- 33.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An Internet survey. Drug Alcohol Rev. doi: 10.1111/j.1465-3362.2012.00512.x. Published Online First 20 September 2012. doi:10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassee FR, Arts JH, Groten JP, et al. Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Arch Toxicol. 1996;70:329–37. doi: 10.1007/s002040050282. [DOI] [PubMed] [Google Scholar]
- 35.Counts ME, Morton MJ, Laffoon SW, et al. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Hua M, Yip H, Talbot P. Mining data of usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. doi: 10.1136/tobaccocontrol-2011-050226. Published Online First 24 November 2011. doi:10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Various models and brands of e-cigarettes evaluated in the study with Nicorette inhalator (product in horizontal position).
Supplementary Figure 2. Set for extraction of analyzed toxicants (TSNAs and metals) into liquid solvent.
Supplementary Table 1. Detection and quantitation limits of compounds evaluated in vapor of e-cigarettes (per 150 puffs).