Abstract
Introduction
Addiction to cocaine is a major problem around the world, but especially in developed countries where the combination of wealth and user demand has created terrible social problems. Although only some users become truly addicted, those who are often succumb to a downward spiral in their lives from which it is very difficult to escape. From the medical perspective, the lack of effective and safe, non-addictive therapeutics has instigated efforts to develop alternative approaches for treatment, including anticocaine vaccines designed to block cocaine’s pharmacodynamic effects.
Areas covered
This paper discusses the implications of cocaine pharmacokinetics for robust vaccine antibody responses, the results of human vaccine clinical trials, new developments in animal models for vaccine evaluation, alternative vaccine formulations and complementary therapy to enhance anticocaine effectiveness.
Expert opinion
Robust anti-cocaine antibody responses are required for benefit to cocaine abusers, but since any reasonably achievable antibody level can be overcome with higher drug doses, sufficient motivation to discontinue use is also essential so that the relative barrier to cocaine effects will be appropriate for each individual. Combining a vaccine with achievable levels of an enzyme to hydrolyze cocaine to inactive metabolites, however, may substantially increase the blockade and improve treatment outcomes.
Keywords: addiction, adjuvant, antibody, butyrylcholinesterase, cocaine, gene therapy, hydrolase, vaccine
1. Introduction
Addiction to cocaine continues to be a major problem in many parts of the world [1], but especially in developed countries where the combination of criminality and social allure has brewed up an unholy alliance between the greed and violence of drug traffickers [2,3] and the consumption demands of individual users [4,5], who may be the naturally rebellious young or those seeking a temporary escape from their life circumstances. Although only 6 – 18% of cocaine exposed individuals become truly addicted [6,7], those who are so affected often succumb to a downward spiral in their lives from which it is very difficult to escape. Despite efforts in developing novel medications, small-molecule agonists and antagonists have not offered any clinically effective, nonaddictive treatment options and no medical therapy has yet been approved for cocaine addiction [8]. Attacking the physiological effects of stimulant drugs by inhibiting or blocking neuronal pathways has been difficult, reflecting the overriding physiological importance and robustness of the reward and motivation pathways that are coopted by cocaine. Potential treatments developed to date have sometimes also had toxic effects or caused unwanted behavioral changes [8].
Consequently, we and others have sought to harness the immune system to produce antibodies that can entrap small molecules like cocaine and both slow and reduce their translocation into the central nervous system [9,10]. Animal studies showed that vaccination with a drug conjugate vaccine produced antibodies that bound small molecule drugs. However, in published clinical trials of both cocaine [11] and nicotine [12] conjugate vaccines, only about a third of the immunized subjects produced sufficient levels of antibody to effectively reduce uptake of the drug by the central nervous system. One approach for improving vaccines would be to markedly enhance the antibody response to immunization, either by modulating T cell regulation of antibody responses [13] and/or by using novel adjuvants or combinations thereof to enhance activation and increase antibody production. The quantitative requirements for antibody levels will be discussed in the pharmacokinetics section, and potential improvements to immune stimulation will be discussed in the section on alternative vaccine formulations.
But why do some individuals produce much lower antibody responses to vaccines than others? Obviously, in the human population, vaccine responses can be influenced by the patient’s general state of health, concomitant immune responses (e.g., to infections or allergies), treatment with immunosuppressive drugs and the differences in vaccine formulation and delivery [14]. In addition, antibody response may also be strongly influenced by the polymorphisms of an individual’s signaling and regulatory genes governing immune reactions to antigenic stimulation [15,16]. Vaccines have been enormously successful in reducing the incidence of many infectious diseases both by direct protection against infection in individuals and through herd immunity [17], so that even those who fail to respond with protective antibodies or T cells (often 10% or more of the population with some routine childhood vaccines, e.g., with measles and mumps vaccines [18]) can be protected by the reduced prevalence of the microbes. However, vaccines for some new applications will require high levels of antibody responses to benefit any individual. In the cocaine conjugate vaccine trial [11], a screen of single-nucleotide polymorphisms (SNPs) in the major histocompatibility complex (MHC) region of chromosome 6 were examined for associations with antibody responses that reduce cocaine use. Several SNPs were strongly associated with reduced drug use and one with increased antibody responses (Nielsen, Kosten, in preparation). Undoubtedly other genes involved in the immune response (cytokines, receptors, chemokines and the many immune activation pathway molecules) will have polymorphisms that influence the level and persistence of antibodies to cocaine conjugates, as has been observed for other antigenic stimuli [18–21]. Increased knowledge of the genetic control of antibody responses to conjugate vaccines would enable selection of candidates most likely to respond effectively to current vaccine formulations. Equally importantly, it would also help us to improve antibody responses by selective modification of the vaccine formulations for individualized application.
A fundamental problem for human vaccine development is the expense and time required for clinical trials. Clinical trials designed to identify and study genes that influence responses to vaccines would be especially problematic. For example, in an informative study of standard HBV vaccine responses by Chen et al. [19], subjects were selected from 37,221 vaccinated individuals for antibody levels 10-fold lower than the mean, yielding 24 low responders. These were compared with 46 high responders to evaluate the effects of toll-like receptor (TLR) and cytokine/cytokine receptor polymorphisms. Even with such small groups the costs were high. Given fiscal reality, it is currently infeasible to investigate such issues systematically in clinical trials comparing different vaccine formulations. Rodent models have been used for decades to foreshorten the expense and time required by human studies. Mice are especially useful to investigate mechanisms that regulate fundamental immune cell interactions, most particularly genetically modified mice in which specific genes have been knocked out or inserted in certain tissues [22]. Unfortunately, experience shows that rodent models do not always yield results that predict human responses, reflecting the 60 million years since the immunoregulatory genes in these two species diverged from a common ancestor [23]. A potential solution for current vaccine research has been the development of a ‘humanized’ mouse model using immunodeficient mice engrafted with human stem cells (humanized immune system[HIS] mice), so that their immune responses can reflect those of the stem cell donor. The advantages and limitations of HIS-mice versus conventional mice will be discussed further below (see Section ‘3.1’).
Finally, the Expert Opinion will address the potential of cocaine vaccines as therapeutic agents for addicts. Included will be the limitations of antibodies alone and the promise of combining vaccines with hydrolytic enzymes that degrade cocaine to inactive products.
2. Cocaine pharmacokinetics and antibody binding
Simple calculations based on drug concentrations and antibody binding capacities suggest that only robust vaccine responses provide the minimum antibody concentrations needed for useful cocaine sequestration [9,24]. A vaccine needs to elicit anticocaine antibodies exceeding 250 nM (~ 40 µg/ml) in order to bind a large fraction of a typical recreational cocaine dose, which produces peak plasma cocaine concentrations of ~ 500 nM [25]. As binding to small drug molecules rapidly establishes an equilibrium [26] from which free drug in the blood stream can then accumulate in target tissues, antibody binding slows, but does not prevent all drug penetration into the brain. A critical feature of cocaine pharmacodynamics, however, is that the ‘rush’ aspect of cocaine abuse depends on the rapid occupation of dopamine transporters in the central nervous system, which quickly increases mesolimbic dopamine (or increase of striatal dopamine). This effect in turn induces a buildup of dopamine in the brain reward centers [27–30]. In animal studies, slowed cocaine delivery significantly reduces self-administration as compared with an equivalent dose given rapidly [31]. As drug self-administration in animal models correlates with the potential for substance abuse, slower brain accumulation in subjects with adequate antibody levels should reduce the effects of drug, and rate of administration has been clearly shown to correlate with subjective effects in humans [32,33]. Obviously, for antibodies to slow cocaine entry in the brain, they must be at least roughly equimolar with the serum drug concentration resulting from a typical recreational cocaine dose. Under such conditions, one could predict immediate binding of up to 90% of the drug molecules by antibodies with a mean binding affinity of < 100 nM [34]. Although brain entry is fast, with maximum physiological and psychological effects within minutes after administration [35], antibody binding is faster. Using isothermal titration calorimetry [26], we have proven that the antibody interactions with drug in free solution are on an appropriate time scale (seconds) for slowing and inhibiting cocaine accumulation in the brain. However, as free cocaine is removed from circulation into tissues, or by metabolism in plasma via serum butyrylcholinesterase (BChE), cocaine is gradually released from the antibody to continually reestablish equilibrium conditions at rates dependent on the antibodies’ dissociation constants. A thorough discussion of the issues of rate constants in this regard was recently published [26].
Anticocaine antibodies produced in mice and humans to the cholera toxin B succinylnorcocaine (CTB-SNC) conjugate vaccine have been shown to be specific for pharmacologically active cocaine molecules without any binding to benzoylecgonine [26], a prominent inactive metabolite of cocaine that persists longer in circulation than the intact drug [36]. Although antibody specificity is certainly critically important for an anticocaine vaccine, further improvement may be possible to enhance the affinity of the resulting antibodies by modifying the hapten [37,38]. Benzoylecgonine differs from cocaine only in that the methyl ester of the parent compound is hydrolyzed resulting into a zwitterion structure with an interactive positive charge on the nitrogen and a negative charge on the carboxyl group. This is important because the long half-life of benzoylecgonine causes it to accumulate over time and to exceed the concentration of native drug after repeated cocaine dosing. Binding to the inactive benzoylecgonine would reduce the number of antibody molecules available to bind any subsequent cocaine dose used. In addition, experiments using microscale thermophoresis have shown that effective antibody affinity for cocaine was significantly influenced by serum components that also bind cocaine, most likely α-1 acidic protein [26]. Hence, improved antibody affinity will be desirable to maximize beneficial effects on cocaine pharmacodynamics. Alternative linkers have been tested to determine whether it would be helpful to retain a relatively positive charge on the tropane nitrogen by attaching it to an aliphatic carbon chain terminal by a carboxylic acid, which could then conjugate to the carrier protein lysine sites. When this was done, however, the methyl ester moiety rapidly hydrolyzed [38], and the vaccine elicited antibodies to benzoylecgonine as efficiently as to cocaine. Further modifications of the linkage method to maintain the positive charge and stability as well are in development (Ramakrishnan, submitted).
3. Cocaine vaccine trials
Phase I, II and IIb clinical trials of a cocaine vaccine based on succinylnorcocaine (SNC) conjugated to the cholera toxin B subunit have been completed and published. The Phase I studies demonstrated that all CTB-SNC vaccinated subjects were able to produce detectable anticocaine antibodies [24,39] and that the presence of high concentrations of antibody diminished subjective measures of cocaine action (‘drug effect’ and ‘cocaine quality’) and objective measures (heart rate at the 50 mg dose) [39]. In the Phase II study of cocaine users with free recreational street access to this drug, a 24-week randomized double-blind placebo-controlled trial showed that 21 of the 55 subjects immunized with this cocaine CTB vaccine made 40 µg/ml or more IgG anticocaine antibody [11]. As significant reduction of brain cocaine uptake requires at least that concentration of anticocaine antibody with average affinity of 100 nM or better [40], the vaccine was immunologically effective in only the high responder subset of users in the study. The frequency of cocaine use, as measured by positive results in urine samples obtained three times per week, was significantly reduced in the group achieving the above criterion [11], but not in the group with lower levels. Thus, high antibody titers weakened cocaine activity enough to reduce or end cocaine use in this group of patients who were in a treatment program for co-dependence on heroin. This was promising as subjects were motivated enough about their substance abuse problems not to override the blockade by markedly increasing the dose and/or frequency of cocaine use. The results of a multisite Phase IIb follow-up trial recently completed (Kosten, submitted) and currently under review have not been as encouraging as the Phase IIa study, but they underscore the need for vaccine adjuvants better than alum alone and emphasize the potential for concurrent therapy with cholinesterase enzyme modified for enhanced cocaine hydrolysis, as will be discussed further on.
Several aspects of the clinical trials reveal features of the human immune responses that must be further improved before recipients of the cocaine conjugate vaccine can benefit substantially. First, too few patients respond to these conjugate vaccines with high titers, not only for conjugates with cocaine, but also for conjugates with nicotine [12]. It must be emphasized that this is not due to poor immunogenicity. In fact, most subjects respond at least as well to these vaccines as to other clinical vaccines. We demonstrated that the distribution of anticocaine responses in the Phase II trial was in fact quantitatively similar to those directed toward the carrier protein CTB itself, which is known to be a highly effective immunogen [41]. However, the nature and abundance of the vaccine target requires antibody levels much higher than are typically needed for the vaccines targeting microbe/toxin antigens to immunize against infectious diseases. The tetanus toxoid vaccine, for example, needs only 1 – 2 µg/ml of antitetanus toxin antibody to provide full protection against the disease [42]. Second, antibody titers among most high responders to the cocaine vaccine fall markedly within a few months after the last booster dose. As antibody levels below 40 µg/ml would likely fail to reduce cocaine euphoria after a cocaine encounter, even in high responders, the benefit in reducing the re-induction of craving by a dose of cocaine is too brief to be optimally effective. Finally, as discussed previously, any reasonably achievable antibody concentration can be overcome by a user determined to administer a high enough dose of cocaine. Thus, selecting appropriately motivated vaccine recipients, perhaps in conjunction with counseling or other management efforts, is essential to obtain a good therapeutic effect. Alternatively, antibody effectiveness may be markedly enhanced with complementary anticocaine therapies as discussed further below.
The low frequency of high responders among cocaine using vaccine recipients may have many causes. One could be prior immune recognition of cocaine protein adducts [43] resulting in T-cell-independent generation of anticocaine IgM antibody responses that prevent development of IgG responses to vaccination [41]. Another could be immunosuppression by cocaine itself [44] or the influence of contaminants such as levamisole [45,46] in drugs available on the street. We also recognize that many genes involved in regulating immune responses can influence the quantity and quality of antibody produced. An analysis of 1228 SNPs in the 3.6 Mb MHC region on chromosome 6 DNA from the 115 participants of the Phase II cocaine CTB vaccine study (Nielsen, Kosten, manuscript in preparation) showed several associations with reduced cocaine use. One of the most significant hits in the SNP study, a functional variant in the HLA-C 3′ untranslated region, correlated strongly with lower cocaine use and also with higher antibody responses and has been shown to influence HLA-C expression [47]. This striking association does not prove that HLA-C itself directly influences antibody responses to the cocaine hapten, but it strongly suggests involvement of a common extended haplotype associated with this polymorphism or possibly another nearby gene or set of genes.
Several genes in the HLA region are known to affect antibody responses. One of the best characterized is a complement gene encoded in the MHC region C4, also known as HLA IIIC4. Polymorphism in this gene is highly associated with failure to respond to HBV vaccination [48]. It is located in an extended haplotype (HLA-C4AQ0,DRB1*0301, DQB1*02) whose genes are in linkage disequilibrium. Family studies have shown that this haplotype is often transmitted in block and that vaccine recipients are either total nonresponders or slow responders to routine vaccination [49]. Of course, many other signaling and activation response genes are involved in vaccine responses, and polymorphisms in some of these may well be involved in the relatively low responses of some drug conjugate vaccine recipients. Studies of HBV vaccines and other standard clinical vaccines have implicated numerous additional gene polymorphisms, as reported by Chen et al. [19] and others [21,50]. In the HBV response analysis, SNPs for IL4, IL13, IL4R, IL1B and TLR2 were shown to independently influence responses or interact with each other in that regard. Although this finding strongly supports the notion that immune response genes regulate antibody responses to the HBV vaccine, it provides little guidance on what to do about the low responder and nonresponder genotypes. These studies have sometimes provided conflicting results [51], perhaps due to differences in environment, health conditions or racial background, among other variables. Although those aspects are also important, they may dilute or mask some gene effects in other individuals. Furthermore, despite the intense interest and numerous gene associations demonstrated, there has been little progress toward novel vaccine formulations to address the low-response problem in individuals with these genotypes. As discussed in the next section, the humanized mouse model may enable us to study such gene associations directly and evaluate interventions and alternative formulations to improve responses within a reasonable time frame and at reasonable cost.
3.1 Animal models
With vaccines in general use, subsets of vaccinated individuals respond unusually poorly or well to otherwise highly immunogenic formulations [18,21,49–51]. For cocaine and nicotine conjugate vaccines, only ~ 30% of the patients produce sufficient antibody to prevent drug entry into the brain [11,12]. Studying different vaccine formulations in humans is not possible because of ethical concerns, costs, feasibility and time concerns. Neither primate nor nonhuman primate studies are attractive alternatives, being both slow and expensive, and they also do not directly address the issue of polymorphism among humans on an individual basis, although identification of specific gene influences would be relevant. Therefore, rodent models are essential to evaluate novel vaccines and formulations for such issues as toxicities and general properties. Immunization of experimental animals with cocaine conjugate vaccines has been reviewed extensively [40,52–54], but the limitations of rodent immune responses have become more apparent in recent years with increasing understanding of the innate immune system, particularly the TLR system and other activation pathways that help regulate the interactions of the adaptive and innate immune systems. As a result, current animal models cannot address the problem of the wide range in individual human responses. Although inbred strains of mice show different vaccine responses, for example, it is well known that BALB/c tend to produce high antibody levels dominated by Th2 cells (helper T cell type 2), whereas C57BL/6 mice tend to produce lower antibody responses but better Th1-dominated responses [55]. Most important, there are significant differences in immune response genes between humans and rodents, from the different roles of specific TLR and other activation and suppression genes, to the absence of some genes in either species [56,57]. As a result, the genetic influences on immune responses in rodents are unreliable indicators of human responses, especially at the level of individual gene polymorphisms.
To address this problem, we have begun to study immunodeficient mice engrafted with human hematopoietic stem cells that may allow for the characterization of responses to multiple vaccine formulations by immune cells from a single person. Although such humanized immune system mice (HIS-mice) have been used for other purposes for many years [58,59], the early models of this system were inadequate because of the short-lived nature of the mice and/or the human cell engraftment [60]. Recent improvements, however, have resulted in mice that are not only relatively long-lived, but also remain stably engrafted for months with the essential functional human immune cells including dendritic cells [61–63]. The NOD-Rag1nullIL2rγnull mouse is a strain of relatively long-lived immunodeficient radioresistant nonobese diabetic mice that contain targeted dominant negative mutations in the recombination activating gene-1 (Rag1null) and the interleukin (IL)-2 receptor common γ chain (IL2rγnull). These mice lack a functional adaptive immune system. The loss of RAG prevents the development of T and B cells, and the lack of the common γ chain prevents the development of mouse NK cells that would otherwise destroy engrafted human stem cells. Human antigen presenting cells (APC) are required to present antigen effectively to human lymphocytes in these mice [64], and so murine APC do not play a role in this model. Additionally, most murine cytokines tested do not signal human cells effectively [65] (other than IL12 [66], which does not enhance cocaine conjugate vaccines; Singh, manuscript in preparation). Therefore, chimerism is not a significant problem in this model. As a result, engraftment with human hematopoietic stem cells allows these mice to acquire and maintain a durable and functional human immune system. Humanized mice produce specific human antibodies when they are immunized, under control of activation and maturation signals from human dendritic cells and human T cells.
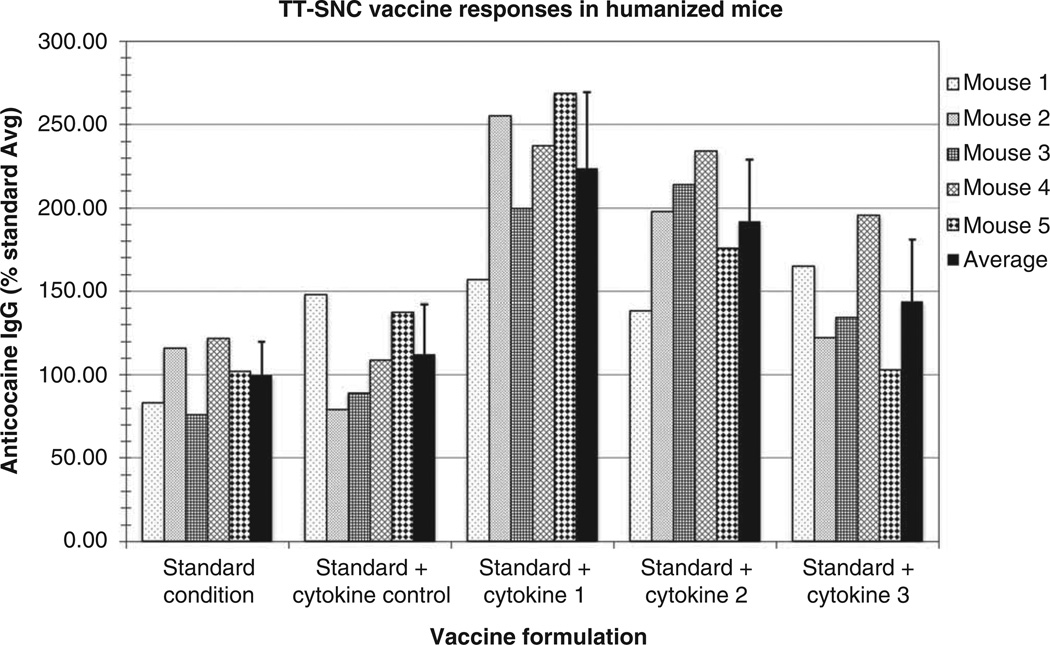
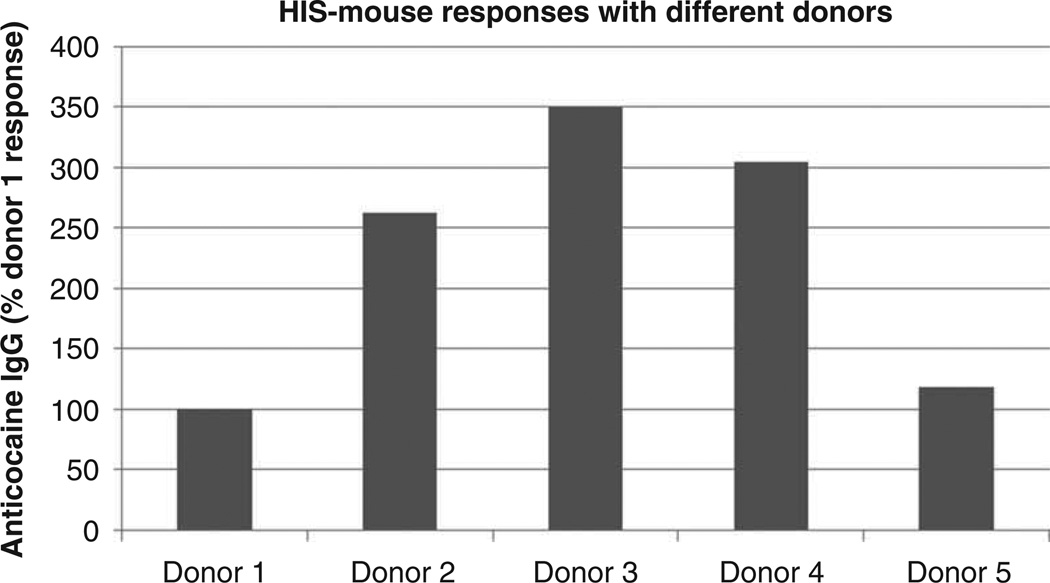
In order to apply this model to the problem of individual human vaccine responses, HIS-mice will have to meet several functional criteria. First, it is essential to produce a sufficient number of mice from single donors. In our preliminary studies, we have shown that purified stem cells from buffy coat cells of single unit blood donors are able to stably engraft an average of 15 – 25 mice. With five mice per group, therefore, up to five different vaccine conditions or formulations can potentially be tested for each stem cell donor. Of course, it may not be feasible to collect full units of blood from many subjects. To obtain a large number of cells from a limited number of specific individuals, leukapheresis after mobilization of peripheral blood stem cells by filgrastim is the preferred methodology [67]. With several groups of mice from a single donor, the second criterion to meet for the results to be properly predictive of vaccine responses is for each mouse within a group of mice stimulated with a specific formulation to show a relatively similar response, that is, a single stimulatory condition should elicit a predictable response from the putatively identical mice. Third, it is essential that distinct formulations in the individual donor show distinguishable differences from other formulations that provide different activation signals (adjuvants, cytokines, carrier proteins and/or hapten differences, etc.). Pilot experiments with cocaine conjugate vaccines in our lab have examined groups of human hematopoietic stem cell engrafted immunodeficient mice created from a single human donor. Their responses to each of several vaccine formulations were appropriately uniform (Figure 1), having a within-group standard deviation of 20 – 25%, consistent with criteria 2 and 3. The fourth criterion is that with a sufficient number of different donors, there must be a wide range of responses, roughly reflecting the range seen in the normal human population. Consistent with this criterion, additional pilot experiments demonstrated that HIS-mice engrafted with stem cells from five different donors did respond with different antibody levels to the same standard vaccine formulation (Figure 2), showing response differences up to 3.5-fold. Finally, it would be ideal if the HIS-mice engrafted with a given donor’s stem cells responded to a specific vaccination formulation in the same relative way as the donor. To meet this criterion and establish the full validity of the model, however, will require more donors in experiments using standard vaccines as well as drug conjugate vaccines. The essential goals of HIS-mice vaccine studies are to determine the influence of the individual’s immune response genes upon quantitative and qualitative antibody responses. Obviously, many environmental factors can influence an immune response in a human patient that would not be easily modeled in HIS-mice. These would include donor health (e.g., nutritional status or concurrent illness), certain medications, ongoing immune responses to other stimuli and chemical exposures (e.g., in drug users, adulterants in street cocaine such as levamisol [68,69]). Nonetheless, variations in immune response genes have a large role in determining the range of human antibody responses to vaccines, as has been demonstrated for some clinical vaccines [15,18–21,49–51].
Figure 1. Individual responses in groups of HIS-mice engrafted with single-donor stem cells.
In this experiment, actively immunized groups of HIS-mice prepared from a single donor’s stem cells were vaccinated with 100 µg of succinylnorcocaine conjugated to tetanus toxoid (TT-SNC) with alum adjuvant at the beginning of week 1 with or without supplemental active cytokines (or cytokine control). Animals were boosted with the same vaccine conditions at week 3, and serum from each animal was collected at week 6 and tested by ELISA for anticocaine IgG antibody. The individual results are shown for each vaccine condition, expressed as the percent response compared with the standard condition group average (± standard deviation) defined as 100%.
Figure 2. Group responses of HIS-mice engrafted with different donor stem cells.
In this experiment, actively immunized groups of HIS-mice prepared from different donors were vaccinated with 100 µg of TT-SNC with alum adjuvant at the beginning of week 1 and a booster dose of the same vaccine at week 3. Serum from each animal was collected at week 6 and pooled serum from each group was tested by ELISA for anticocaine IgG antibody. The group results are shown for each vaccine condition, expressed as the percent response compared to the donor 1 response arbitrarily defined as 100%. HIS-mice: Humanized immune system mice.
4. New formulations
The simplest modification of anticocaine vaccines would be to select alternative carriers and/or adjuvants to enhance antibody responses. In our laboratory, the responses in mice to keyhole limpet hemocyanin-SNC and tetanus toxoid-SNC using the alum adjuvant approved for human use are substantially higher than that to CTB-SNC in both initial and later response periods (Ramakrishnan, submitted). This is fortunate for development of clinically useful drug conjugates for vaccination as tetanus toxoid conjugates with other antigens have been approved for clinical use by the FDA. The differences in these carriers, however, are still modest, and by no means is it clear that such changes will carry over from results in mice into a large proportion of the human subjects who may benefit from anticocaine vaccines.
Regarding new adjuvants, several reviews have been published on the general properties of these agents [70–72], but there is limited information about their influence on small-hapten conjugate vaccines. Although only alum is approved as a general adjuvant, monophosphoryl lipid A and squalene have been approved in the context of specific vaccines, and certain carriers in clinical use for conjugate vaccines have notable adjuvant properties, like the outer membrane protein complex (OMPC) of Neisseria meningitidis [73] and diphtheria toxoid [74]. An alternative carrier construct, adenovirus capsid proteins, was recently developed and has also shown adjuvant-like properties [75], and shown promise in cocaine conjugate vaccination in both rodents [76] and nonhuman primates [77]. We have evaluated several agents, specifically cytokines (e.g., IL4, IL12 and GM-CSF) and direct TLR agonists (for TLR2 – diacyl lipopeptides, TLR4 – monophosphoryl lipid A and E6020 and TLR9 – CpG oligonucleotides) with or without alum as an additional adjuvant. In many cases using predominantly mice as the model to evaluate these conditions, a doubling of the quantitative response could be achieved over the already quite robust responses seen using standard alum vaccine formulations (Orson, unpublished). However, this improvement alone is not sufficiently robust to greatly impact the problem of generally low antibody levels (relative to need) in many human subjects, even if it did impact everyone equally, which is unlikely. When evaluating new formulations like these, it is always important to use a legitimate maximum standard stimulation condition currently approved for human studies as the goal is to achieve the highest possible antibody responses [78], not merely reducing the dose of antigen needed for a given response. An adjuvant that only works well compared with a suboptimal vaccine formulation does not add much value here. In any event, our tests with various adjuvants have not yielded any large improvements in binding affinity as measured via competitive inhibition ELISAs, using free cocaine as the antagonist (Ramakrishnan, submitted).
In order to surmount these obstacles, we have begun investigating an alternative strategy: manipulation of regulatory T cells (Treg) that control the level of antibody responses to immune stimulation. Transient suppression of Treg at the time of vaccination may boost the maximal antibody level achievable from any vaccine formulation. In addition, combining co-stimulant molecules may provide better activation of strong immune responses than do single adjuvants alone. If either strategy proves fruitful, significant advances in antidrug vaccines might come within reach.
Immune responses to antigenic stimuli require a balance of inhibitory regulation as well as stimulatory support to achieve appropriately balanced levels of effector cells and molecules to meet the microbial and oncogenic challenges confronted in life [79]. To maintain a healthy population under natural conditions, variability in immune responses is essential so that some individuals are able to withstand dangerous microbial challenges that may arise, whereas the burden of high-level adaptive immune responses does not need to exist in every individual, as the costs of such high responsiveness may be increased risk for autoimmune disease [80]. The waves of regulation that occur after stimulation of natural antibody responses to vaccines include a programmed tamping down of the production of specific antibody over time for all individuals, but, as suggested above, with a considerable range of variation in both the positive and the negative aspects of regulation. Inhibition of Treg at an appropriate time point could thus be useful in amplifying the level of immune responses to vaccination. Complete depletion of Treg via antibody depletion [80] or genetic manipulation [81] results in a dramatic increase in antibody responses to vaccination, but also overwhelming autoimmunity [82]. Nonetheless, transient inhibition of Treg cells can enhance vaccine responses in both T effector cells and antibodies to protein antigens, as has been studied especially with cancer vaccines [13,83,84]. No studies have been published regarding antismall molecule vaccines, such as drug conjugates. We recently did pilot studies in mice genetically engineered to express the diphtheria toxin receptor (DTR) on Treg cells (Foxp3-DTR mice). These mice showed much higher titers of antibody production against cocaine after cocaine conjugate vaccination when the mice were completely depleted of Treg cells by treatment with diphtheria toxin (Wang, unpublished), but they also developed the expected autoimmunity problems.
The signaling pathways that recruit immune responses against foreign or altered self-antigens are complex, activating NF-kB, type I interferon (IFN) and the inflammasome, through families of innate immune system receptors designed to recognize particular structural patterns. This signaling leads to the production of inflammatory cytokines, which in turn promote dendritic cell maturation programs for the induction of both cellular and antibody responses [85,86]. We have identified several negative regulators (NLRC5, NLRX1 and NLRP4) that inhibit NF-κB and type I interferon signaling [87–89], and furthermore, we have also found that TLR8 ligands (ssRNA40 and Poly-G10 oligonucleotides) can directly reverse the suppressive function of human (but not murine) Treg cells [90]. More importantly, the suppressive function of other subsets of Treg cells, including CD8+ Treg cells and gammadelta T cell receptor Treg cells, can be reversed by Poly-G oligonucleotide treatment [91,92], suggesting that these cells share a common signaling pathway for modulating Treg cell suppressive function. Studies of Treg cell pathways have recently revealed subsets of these cells that may be more specific for inhibiting antibody production. A small subset of BCL6+ CXCR5+ Treg cells, so-called follicular regulatory T cells (TFR), localizes to the germinal center reaction in lymph nodes and inhibits affinity maturation of antibody and the differentiation of plasma cells [93–95], suggesting that TFR cells and different signaling pathways control antibody production in humoral immunity. Therefore, depletion of this subset of Treg cells or selectively blocking their suppressive function may increase antibody production without autoimmune complications.
5. Complementary therapy
Although anticocaine vaccines alone may be beneficial to those who are motivated to stop abusing cocaine and are also able to produce high levels of specific anticocaine antibodies, it is clear that they are ineffective for those who produce small amounts of antibody [11], and also for those addicts who continue frequently using large quantities of the drug to override the modest antibody-induced blockade. Beyond psychological counseling and contingency management, which can be helpful to some users [96], enhancing cocaine degradation, serving the same purpose as antibody (to prevent cocaine from reaching the receptors in the brain), shows real promise for protection against both the psychological and the toxic effects of cocaine. BChE is a plasma enzyme that appears to have the general function of hydrolyzing toxic or bioactive esters that may enter the body through the diet or other routes. High doses of this enzyme in purified recombinant form have been tested in animal models and humans for prophylaxis against chemical warfare agents with no ill effects [97]. Although native BChE does hydrolyze cocaine to the inactive metabolites benzoic acid and ecgonine, its natural form is quite inefficient with that drug, and it acts too slowly to prevent cocaine from reaching the brain. As has been reviewed recently, however, selected modifications in the active site of BChE have converted it into a more specific cocaine hydrolase (CocH) that dramatically increased the efficiency against that substrate [52]. These advances make therapeutic applications very attractive. Unfortunately, the half-life of passively administered enzyme is too short to make it useful for sustained therapy [98], resulting in a need for an alternative delivery system to achieve optimal benefits. One modification to address this problem has been the fusion of the enzyme with albumin [99], and a version of this construct has advanced to a current clinical trial using weekly injections of the recombinant product [100]. Another attractive alternative is viral gene transfer technology, which, although not yet approved, now has a new pathway for FDA evaluation to speed the process [101]. In rats and mice, a single treatment with a viral vector for CocH can result in production of this enzyme for > 2 years without apparent ill effect [102]. The resulting enzyme levels counteract or abolish the effects of high (or even lethal) cocaine doses [103]. However, despite the success of gene therapy in experimental animals and selected human deficiency conditions [104], achieving high concentrations of desired gene products in man remains challenging, in part due to safety issues [105] involving the inflammatory response to viral antigens [106]. Despite much work to diminish such reactions [107–110], including transient immunosuppression [111], it is not clear when this technology will be ready for routine clinical applications. Nonviral methods of gene delivery may be safer, but tend to provide less than optimal protein expression [112]. On the other hand, a combination therapy, using the enzyme at lower levels to complement antibodies in intercepting and degrading cocaine in the blood stream, is particularly attractive, and these treatments should be both compatible and mutually reinforcing.
We have recently shown that at lower CocH doses the protection against cocaine toxicity can be remarkably enhanced when the enzyme (whether administered passively or through virally mediated expression) is combined with anticocaine antibodies, whether passively administered or elicited by vaccine in vivo [113–115]. The mechanisms of action are reinforcing because the binding rate of antibody is extremely rapid, thus acting as a ‘sponge’ for much of the initial pulse of administered cocaine in a typical dose, whereas the lower dose of the CocH enzyme has fewer drug binding sites and a lower affinity, but catalyzes hydrolysis of free molecules extremely rapidly. The enzyme is then able to capture and hydrolyze additional cocaine molecules in real time as they are released from antibody into plasma. These functions have been demonstrated in vitro, showing that 1 µM cocaine could be bound 90% by anticocaine IgG, and then be 98% hydrolyzed by CocH within 90 s [116]. The mechanisms of action may thus provide an additive or even synergistic blockade against the reinforcing effect of cocaine. However, the complex interactions of antibody concentrations, enzyme levels and the metabolic fates of all these components remain to be delineated. The HIS-mouse model provides the opportunity to investigate the details of these parameters using the human enzyme as well as human antibodies. A further alternative approach to combine antibody with enzyme using gene therapy could be to use viral vectors expressing anticocaine monoclonal antibodies [117]. The limitations currently applying to gene therapy regarding high-level expression would be similar to that with the enzyme alone, but with progress in gene therapy, this may become another viable option. As a result, combining therapy with anticocaine antibodies and cocaine metabolizing esterase deserves serious consideration as a viable approach toward assisting addicts to discontinue cocaine abuse and remain drug free.
6. Expert opinion
Therapeutic trials of an alum adjuvanted cocaine vaccine have demonstrated that some patients can achieve levels of antibody sufficient to block the rapid effects of cocaine, as calculated from the amount of drug needed to achieve psychological effects in the laboratory setting [39], and thereby reduce drug use in subjects motivated to discontinue use [11]. However, it is also apparent that these minimum effective antibody levels, and in fact any realistically achievable level from vaccination, can be overcome by sufficiently high or repeated doses of cocaine if the subject chooses to do so. Nonetheless, high levels of high affinity antibody would clearly be useful in appropriate patients that could be complemented by motivational therapy [118,119] to reduce the needed height of the drug exposure barrier so that lapses do not become relapses. Nonetheless, new means of achieving these levels are urgently needed in a large proportion of the addicts seeking help. Advances in understanding immunoregulation, as well as the use of humanized mouse models to evaluate individual immune responses to different vaccine formulations will provide the opportunity to achieve this technological goal. Although we recognize that no animal model can perfectly reflect a human’s response, because no model can address the myriad environmental issues that affect any human donor, we believe that the HIS-mouse model will enable better evaluation of human responses to specific vaccine formulations. Furthermore, this model should also allow selection of optimized formulations for individuals or related groups of individuals based on their genetic profile. As a result, the individual donor with poor responses to standard vaccine formulations may be evaluated for different formulations that elicit high responses in his or her specific genetic context. We expect that future results will confirm the hypothesis that these results reflect gene polymorphisms associated with an individual’s innate ability to produce antibodies after an antigen challenge.
To raise the bar of blockade to higher and more frequent cocaine dosing, however, complementary therapy with enzyme-mediated enhanced degradation of cocaine shows great potential [116]. Although very high level CocH alone from high-dose virus transfection in rodents is sufficient to prevent cocaine-specific reinstatement of self-administration and even to stop ongoing cocaine self-administration [102,103,120], safely achieving such levels of gene expression in humans with current technologies is not yet a realistic possibility. Nonetheless, prolonged lower levels of CocH expression in humans should be achievable as indicated by gene therapy for other proteins [104,110], and these levels of enzyme may well be sufficient when combined with adequate antibody levels produced from optimized vaccines. Determination of the minimum antibody and enzyme combinations will be able to be studied directly using the HIS-mouse model system in which both human antibodies and the modified human BChE are present. As a result, we believe anticocaine vaccines, especially in combination with anticocaine enzyme therapy, will have a large role in future treatment for this often devastating addiction.
Article highlights.
High concentrations of anticocaine antibodies can help addicts motivated to stop cocaine abuse reduce their drug use.
Current vaccine formulations must be improved because they elicit clinically effective antibody levels in only a minority of immunized patients.
Using new adjuvants and other molecules to modify both activation and suppression limbs of immunoregulatory responses to vaccination will permit improvement of both the quantity and persistence of anticocaine antibodies.
Use of humanized mouse models may help optimize vaccine designs over the next several years by defining the influence of genetic polymorphisms on immune responses, thereby allowing selection of specific vaccine formulations for individual patients.
Treatment of addicts with a combination of anticocaine vaccines and the cocaine hydrolase enzyme to hydrolyze cocaine has the most promise for most effectively blocking the physiological and psychological effects of the drug.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA) grants R01 DA030338, R01 DA025223, R01 DA023979, DP1 DA031340, R21 DA035591 and the Michael E. DeBakey Veterans Affairs Medical Center Research Program, and the Department of Veterans Affairs Merit Review Program.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Degenhardt L, Baxter AJ, Lee YY, et al. The global epidemiology and burden of psychostimulant dependence: findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Robles G, Calderon G, Magaloni B. [Cited 31 January 2014];The economic consequences of drug trafficking violence in Mexico. 2013 Available from: http://iisdb.stanford.edu/pubs/24014/RoblesCalderonMagaloni_EconCosts5.pdf.
- 3.Wyler LS. [Cited 31 January 2014];International drug control policy: background and U.S. responses. August 13, 2013 crime economics implications for US and other countries. 2013 Available from: http://www.fas.org/sgp/crs/row/RL34543.pdf.
- 4.Results from the 2012 National Survey on Drug Use and Health: summary of national findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Available from: http://oas.samhsa.gov. [Google Scholar]
- 5.UNODC, World Drug Report 2013 (United Nations publication, Sales No. E.13.XI.6) [Cited 31 January 2014];2013 Available from: http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
- 6.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States 2000–2001. Neuropsychopharmacology. 2005;30(5):1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 7.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 8.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orson FM, Kinsey BM, Singh RA, et al. Vaccines for cocaine abuse. Hum Vaccin. 2009;5(4):194–199. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paula S, Tabet MR, Farr CD, et al. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47(1):133–142. doi: 10.1021/jm030351z. • Thorough demonstration of antibody binding properties and structures.
- 11. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. •• Demonstrates that high cocaine antibody concentrations help addicts reduce cocaine use.
- 12. Hatsukami DK, Jorenby DE, Gonzales D, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–399. doi: 10.1038/clpt.2010.317. •• Demonstrates that high nicotine antibody concentrations help addicts reduce smoking.
- 13.Rolla S, Ria F, Occhipinti S, et al. Erbb2 DNA vaccine combined with regulatory T cell deletion enhances antibody response and reveals latent low-avidity T cells: potential and limits of its therapeutic efficacy. J Immunol. 2010;184(11):6124–6132. doi: 10.4049/jimmunol.0901215. [DOI] [PubMed] [Google Scholar]
- 14.Buonaguro L, Pulendran B. Immunogenomics and systems biology of vaccines. Immunol Rev. 2011;239(1):197–208. doi: 10.1111/j.1600-065X.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinetti M, De Silvestri A, Belloni C, et al. Humoral response to recombinant hepatitis B virus vaccine at birth: role of HLA and beyond. Clin Immunol. 2000;97(3):234–240. doi: 10.1006/clim.2000.4933. [DOI] [PubMed] [Google Scholar]
- 16.Ovsyannikova IG, Poland GA. Vaccinomics: current findings, challenges and novel approaches for vaccine development. Apps J. 2011;13(3):438–444. doi: 10.1208/s12248-011-9281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonds MH, Rohani P. Herd immunity acquired indirectly from interactions between the ecology of infectious diseases, demography and economics. J R Soc Interface. 2010;7(44):541–547. doi: 10.1098/rsif.2009.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhiman N, Ovsyannikova IG, Vierkant RA, et al. Associations between cytokine/cytokine receptor single nucleotide polymorphisms and humoral immunity to measles, mumps and rubella in a Somali population. Tissue Antigens. 2008;72(3):211–220. doi: 10.1111/j.1399-0039.2008.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Liang Z, Lu F, et al. Toll-like receptors and cytokines/cytokine receptors polymorphisms associate with non-response to hepatitis B vaccine. Vaccine. 2011;29(4):706–711. doi: 10.1016/j.vaccine.2010.11.023. • Very clear demonstration of the influence of human genetic polymorphisms in immune activation pathways on vaccine-elicited antibody responses.
- 20.Dhiman N, Ovsyannikova IG, Vierkant RA, et al. Associations between SNPs in toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: preliminary results. Vaccine. 2008;26(14):1731–1736. doi: 10.1016/j.vaccine.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiertsema SP, Baynam G, Khoo SK, et al. Impact of genetic variants in IL-4, IL-4 RA and IL-13 on the anti-pneumococcal antibody response. Vaccine. 2007;25(2):306–313. doi: 10.1016/j.vaccine.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Saveliev A, Tybulewicz VL. Lymphocyte signaling: beyond knockouts. Nat Immunol. 2009;10(4):361–364. doi: 10.1038/ni.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterston RH, Lindblad-Toh K, Birney E, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 24.Martell BA, Mitchell E, Poling J, et al. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58(2):158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 25. Jenkins AJ, Keenan RM, Henningfield JE, Cone EJ. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anal Toxicol. 2002;26(7):382–392. doi: 10.1093/jat/26.7.382. • Classic paper on the pharmacodynamics of cocaine in humans.
- 26. Ramakrishnan M, Alves De Melo F, Kinsey BM, et al. Probing cocaine-antibody interactions in buffer and human serum. PLoS One. 2012;7(7):e40518. doi: 10.1371/journal.pone.0040518. •• Thorough analysis on antibody binding of cocaine and the influences of physiological conditions on binding.
- 27.Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22(8):3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samaha AN, Mallet N, Ferguson SM, et al. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24(28):6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith BJ, Jones HE, Griffiths RR. Physiological, subjective and reinforcing effects of oral and intravenous cocaine in humans. Psychopharmacology (Berl) 2001;156(4):435–444. doi: 10.1007/s002130100740. [DOI] [PubMed] [Google Scholar]
- 31. Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486(3):251–257. doi: 10.1016/j.ejphar.2004.01.003. • Illustrates the importance of cocaine dose rates on pharmacodynamics.
- 32.Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154(1):76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- 33.Nelson RA, Boyd SJ, Ziegelstein RC, et al. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82(1):19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Orson FM, Kinsey BM, Ramakrishnan M, et al. Immunotherapeutic vaccines for substance abuse. In: Canales JJ, editor. Emerging targets for drug addiction treatment. New York, USA: NOVA Publishers; 2012. [Google Scholar]
- 35.Newton TF, De La Garza R, II, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82(1):90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Jufer RA, Wstadik A, Walsh SL, et al. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24(7):467–477. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 37.Cai X, Whitfield T, Hixon MS, et al. Probing active cocaine vaccination performance through catalytic and noncatalytic hapten design. J Med Chem. 2013;56(9):3701–3709. doi: 10.1021/jm400228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai X, Whitfield T, Moreno AY, et al. Probing the effects of hapten stability on cocaine vaccine immunogenicity. Mol Pharm. 2013;10(11):4176–4184. doi: 10.1021/mp400214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haney M, Gunderson EW, Jiang H. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67(1(59)):65. doi: 10.1016/j.biopsych.2009.08.031. • Demonstration of anticocaine antibody effects on cocaine pharmacodynamics in humans.
- 40.Orson FM, Kinsey BM, Singh RA, et al. The future of vaccines in the management of addictive disorders. Curr Psychiatry Rep. 2007;9(5):381–387. doi: 10.1007/s11920-007-0049-z. [DOI] [PubMed] [Google Scholar]
- 41.Orson FM, Rossen RD, Shen X, et al. Spontaneous development of IgM anti-cocaine antibodies in habitual cocaine users: effect on IgG antibody responses to a cocaine cholera toxin B conjugate vaccine. Am J Addict. 2013;22(2):169–174. doi: 10.1111/j.1521-0391.2013.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens RH, Saxon A. Reduced in vitro production of anti-tetanus toxoid antibody after repeated in vivo immunization with tetanus toxoid. J Immunol. 1979;122(2):592–598. [PubMed] [Google Scholar]
- 43.Deng SX, Bharat N, Fischman MC, Landry DW. Covalent modification of proteins by cocaine. Proc Natl Acad Sci USA. 2002;99(6):3412–3416. doi: 10.1073/pnas.042700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrino TC, Dunn KL, Bayer BM. Mechanisms of cocaine-induced decreases in immune cell function. Int Immunopharmacol. 2001;1(4):665–675. doi: 10.1016/s1567-5769(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 45.Ananthan D, Shah S, Koya HH, et al. Levamisole tainted cocaine: an emerging health issue. QJM. 2014 doi: 10.1093/qjmed/hcu028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Tallarida CS, Egan E, Alejo GD, et al. Levamisole and cocaine synergism: a prevalent adulterant enhances cocaine’s action in vivo. Neuropharmacology. 2014;79:590–595. doi: 10.1016/j.neuropharm.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni S, Savan R, Qi Y, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472(7344):495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohler T, Stradmann-Bellinghausen B, Starke R, et al. C4A deficiency and nonresponse to hepatitis B vaccination. J Hepatol. 2002;37(3):387–392. doi: 10.1016/s0168-8278(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 49.De Silvestri A, Pasi A, Martinetti M, et al. Family study of non-responsiveness to hepatitis B vaccine confirms the importance of HLA class III C4A locus. Genes Immun. 2001;2(7):367–372. doi: 10.1038/sj.gene.6363792. [DOI] [PubMed] [Google Scholar]
- 50.St Sauver JL, Schaid DJ, Vierkant RA, et al. Associations between measles antibody levels and the protein structure of class II human leukocyte antigens. Hum Immunol. 2003;64(7):696–707. doi: 10.1016/s0198-8859(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 51.Ovsyannikova IG, Haralambieva IH, Vierkant RA, et al. The role of polymorphisms in Toll-like receptors and their associated intracellular signaling genes in measles vaccine immunity. Hum Genet. 2011;130(4):547–561. doi: 10.1007/s00439-011-0977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brimijoin S, Shen X, Orson F, Kosten T. Prospects, promise and problems on the road to effective vaccines and related therapies for substance abuse. Expert Rev Vaccines. 2013;12(3):323–332. doi: 10.1586/erv.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosten T, Domingo C, Orson F, Kinsey B. Vaccines against stimulants: cocaine and methamphetamine. Br J Clin Pharmacol. 2014;77(2):368–374. doi: 10.1111/bcp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno AY, Janda KD. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav. 2009;92(2):199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ariffin JK, Sweet MJ. Differences in the repertoire, regulation and function of Toll-like receptors and inflammasome-forming nod-like receptors between human and mouse. Curr Opin Microbiol. 2013;16(3):303–310. doi: 10.1016/j.mib.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Moesta AK, Graef T, Abi-Rached L, et al. Humans differ from other hominids in lacking an activating NK cell receptor that recognizes the C1 epitope of MHC class I. J Immunol. 2008;185(7):4233–4237. doi: 10.4049/jimmunol.1001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shultz LD, Brehm MA, Bavari S, Greiner DL. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann NY Acad Sci. 2011;1245:50–54. doi: 10.1111/j.1749-6632.2011.06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu CI, Gallegos M, Marches F, et al. Broad influenza-specific CD8+ T-cell responses in humanized mice vaccinated with influenza virus vaccines. Blood. 2008;112(9):3671–3678. doi: 10.1182/blood-2008-05-157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brehm MA, Cuthbert A, Yang C, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lang J, Weiss N, Freed BM, et al. Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rgammanull mouse model: a multivariable optimization approach. Clin Immunol. 2011;140(1):102–116. doi: 10.1016/j.clim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013;25(3):403–409. doi: 10.1016/j.coi.2013.03.009. • Introduction of idea for humanized mouse as a vaccine assessment tool beyond modeling cancer and infectious diseases.
- 63.Spranger S, Frankenberger B, Schendel DJ. NOD/scid IL-2Rg(null) mice: a preclinical model system to evaluate human dendritic cell-based vaccine strategies in vivo. J Transl Med. 2012;10:30. doi: 10.1186/1479-5876-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramer PC, Chijioke O, Meixlsperger S, et al. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol. 2011;89(3):408–416. doi: 10.1038/icb.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosmann TR, Yokota T, Kastelein R, et al. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4) J Immunol. 1987;138(6):1813–1816. [PubMed] [Google Scholar]
- 66.Zou JJ, Schoenhaut DS, Carvajal DM, et al. Structure-function analysis of the p35 subunit of mouse interleukin 12. J Biol Chem. 1995;270(11):5864–5871. doi: 10.1074/jbc.270.11.5864. [DOI] [PubMed] [Google Scholar]
- 67.Shpall EJ, Jones RB, Bearman SI, et al. Peripheral blood stem cell transplantation in breast cancer. Baillieres Best Pract Res Clin Haematol. 1999;12(1–2):219–232. doi: 10.1053/beha.1999.0019. [DOI] [PubMed] [Google Scholar]
- 68.Larocque A, Hoffman RS. Levamisole in cocaine: unexpected news from an old acquaintance. Clin Toxicol (Phila) 2004;50(4):231–241. doi: 10.3109/15563650.2012.665455. [DOI] [PubMed] [Google Scholar]
- 69.Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63(12):3998–4001. doi: 10.1002/art.30590. [DOI] [PubMed] [Google Scholar]
- 70.Buonaguro FM, Tornesello ML, Buonaguro L. New adjuvants in evolving vaccine strategies. Expert Opin Biol Ther. 2011;11(7):827–832. doi: 10.1517/14712598.2011.587802. [DOI] [PubMed] [Google Scholar]
- 71.Mosca F, Tritto E, Muzzi A, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105(30):10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson-Welder JH, Torres MP, Kipper MJ, et al. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98(4):1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai Z, Schreiber JR. Outer membrane protein complex of Meningococcus enhances the antipolysaccharide antibody response to pneumococcal polysaccharide-CRM(1)(9)(7) conjugate vaccine. Clin Vaccine Immunol. 2011;18(5):724–729. doi: 10.1128/CVI.00053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlin G, Viitanen E. In vitro pyrogenicity of the diphtheria, tetanus and acellular pertussis components of a trivalent vaccine. Vaccine. 2005;23(28):3709–3715. doi: 10.1016/j.vaccine.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Hicks MJ, De BP, Rosenberg JB, et al. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther. 2011;19(3):612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wee S, Hicks MJ, De BP, et al. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology. 2011;37(5):1083–1091. doi: 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hicks MJ, Kaminsky SM, De BP, et al. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anti-cocaine vaccine. Hum Gene Ther Clin Dev. 2014 doi: 10.1089/humc.2013.231. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Orson FM, Kinsey BM, Singh RA, et al. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–269. doi: 10.1196/annals.1441.027. • Detailed discussion of antidrug antibody binding kinetics.
- 79.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14(3):154–65. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 80.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4(6):351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 82.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 83.Elia L, Aurisicchio L, Facciabene A, et al. CD4+CD25+ regulatory T-cell-inactivation in combination with adenovirus vaccines enhances T-cell responses and protects mice from tumor challenge. Cancer Gene Ther. 2007;14(2):201–210. doi: 10.1038/sj.cgt.7701004. [DOI] [PubMed] [Google Scholar]
- 84.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann NY Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 85.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 86. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. • Classic review of TLR interactions with antigen-specific immune responses.
- 87.Cui J, Li Y, Zhu L, et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol. 2012;13(4):387–395. doi: 10.1038/ni.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui J, Zhu L, Xia X, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141(3):483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia X, Cui J, Wang HY, et al. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34(6):843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309(5739):1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 91.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13(23):6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 92.Peng G, Wang HY, Peng W, et al. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 93.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim HJ, Verbinnen B, Tang X, et al. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Secades-Villa R, Garcia-Fernandez G, Pena-Suarez E, et al. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat. 2013;44(3):349–354. doi: 10.1016/j.jsat.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 97. Brimijoin S, Gao Y. Cocaine hydrolase gene therapy for cocaine abuse. Future Med Chem. 2012;4(2):151–162. doi: 10.4155/fmc.11.183. •• Thorough detailing of cocaine hydrolase aspects of treatment for cocaine addiction.
- 98.Gao Y, LaFleur D, Shah R, et al. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact. 2008;175(1–3):83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brimijoin S, Gao Y, Anker JJ, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33(11):2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teva PI. Efficacy and safety of TV-1380 as treatment for facilitation of abstinence in cocaine-dependent subjects. 2014 Available from: http://clinicaltrials.gov/ct2/show/NCT01887366.
- 101.Byrne BJ. Pathway for approval of a gene therapy orphan product: treading new ground. Mol Ther. 2013;21(8):1465–1466. doi: 10.1038/mt.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geng L, Gao Y, Chen X, et al. Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One. 2013;8(6):e67446. doi: 10.1371/journal.pone.0067446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zlebnik NE, Brimijoin S, Gao Y, et al. Long-term reduction of cocaine self-administration in rats treated with adenoviral vector-delivered cocaine hydrolase: evidence for enzymatic activity. Neuropsychopharmacology. 2014;39(6):1538–1546. doi: 10.1038/npp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukherjee S, Thrasher AJ. Gene therapy for PIDs: progress, pitfalls and prospects. Gene. 2013;525(2):174–181. doi: 10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80(1–2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 106.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 107.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brunetti-Pierri N, Ng P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum Mol Genet. 2011;20(R1):R7–R13. doi: 10.1093/hmg/ddr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morral N, O’Neal W, Rice K, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96(22):12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vetrini F, Ng P. Liver-directed gene therapy with helper-dependent adenoviral vectors: current state of the art and future challenges. Curr Pharm Des. 2011;17(24):2488–2499. doi: 10.2174/138161211797247532. [DOI] [PubMed] [Google Scholar]
- 111.Seregin SS, Appledorn DM, McBride AJ, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17(4):685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lufino MM, Edser PA, Wade-Martins R. Advances in high-capacity extrachromosomal vector technology: episomal maintenance, vector delivery, and transgene expression. Mol Ther. 2008;16(9):1525–1538. doi: 10.1038/mt.2008.156. [DOI] [PubMed] [Google Scholar]
- 113.Brimijoin S, Orson F, Kosten TR, et al. Anti-cocaine antibody and butyrylcholinesterase-derived cocaine hydrolase exert cooperative effects on cocaine pharmacokinetics and cocaine-induced locomotor activity in mice. Chem Biol Interact. 2012;203(1):212–216. doi: 10.1016/j.cbi.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao Y, Geng L, Orson F, et al. Effects of anti-cocaine vaccine and viral gene transfer of cocaine hydrolase in mice on cocaine toxicity including motor strength and liver damage. Chem Biol Interact. 2012;203(1):208–211. doi: 10.1016/j.cbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carroll ME, Zlebnik NE, Anker JJ, et al. Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion. PLoS One. 2012;7(8):e43536. doi: 10.1371/journal.pone.0043536.• Inhibition of cocaine effects with combined antibody and enzyme therapy.
- 116.Gao Y, Orson FM, Kinsey B, et al. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem Biol Interact. 2010;187(1–3):421–424. doi: 10.1016/j.cbi.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rosenberg JB, Hicks MJ, De BP, et al. AAVrh.10-mediated expression of an anti-cocaine antibody mediates persistent passive immunization that suppresses cocaine-induced behavior. Hum Gene Ther. 2012;23(5):451–459. doi: 10.1089/hum.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia-Fernandez G, Secades-Villa R, Garcia-Rodriguez O, et al. Long-term benefits of adding incentives to the community reinforcement approach for cocaine dependence. Eur Addict Res. 2011;17(3):139–145. doi: 10.1159/000324848. [DOI] [PubMed] [Google Scholar]
- 119.Stein MD, Herman DS, Anderson BJ. A motivational intervention trial to reduce cocaine use. J Subst Abuse Treat. 2009;36(1):118–125. doi: 10.1016/j.jsat.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 120.Anker JJ, Brimijoin S, Gao Y, et al. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry. 2012;71(8):700–705. doi: 10.1016/j.biopsych.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]