Abstract
Background
Endoscopic screening for esophageal neoplasia can identify patients eligible for early intervention for pre-cancerous lesions. Unsedated transnasal esophagoscopy may provide an efficient and accurate endoscopic assessment with fewer risks and less cost compared to conventional upper endoscopy.
Objective
To assess the feasibility, safety, acceptability and yield of unsedated transnasal esophagoscopy in a primary care population.
Design
Multi-center, prospective, cross-sectional study.
Setting
Two outpatient tertiary centers.
Patients
General medical clinic population between the ages of 40 and 85.
Interventions
Unsedated, office-based transnasal esophagoscopy.
Main outcomes measurements
1) Procedure yield, 2) Completeness of examination, 3) Procedure length, 4) Adverse events and complications, 5) Choking, gagging, pain or anxiety during the examination, and 6) Overall tolerability
Results
Four hundred and twenty-six participants (mean age 55.8 ± 9.5, 43% male) enrolled in the study, and 422 (99%) completed the examination. Mean examination time was 3.7 ± 1.8 minutes. There were no serious adverse events and 12 participants (2.8%) reported minor complications. Participants reported minimal choking, gagging, pain or anxiety. The examination was well tolerated by most participants. Overall, 38% of subjects had an esophageal finding that changed management (34% erosive esophagitis, 4% Barrett’s esophagus).
Limitations
Nonrandomized study; tertiary centers only; self-selected population with a large proportion reporting esophageal symptoms.
Conclusions
Unsedated transnasal esophagoscopy is a feasible, safe, and well-tolerated method to screen for esophageal disease in a primary care population. Endoscopic findings are common in this patient population.
Keywords: Barrett’s esophagus, endoscopy, screening, transnasal, esophageal cancer
Esophageal adenocarcinoma has undergone the most rapid increase in incidence of any neoplasia in the United States in the last three decades.[1, 2] Although standard upper endoscopy allows the identification of precancerous lesions or cancer of the esophagus, this examination is commonly performed with conscious sedation and occasionally under general anesthesia.[3] Conscious sedation is associated with substantial cost. The use of sedation with endoscopy requires additional monitoring, nursing support, and a recovery area. Conventional upper endoscopy with biopsies costs an estimated $866 based on Center for Medicare and Medicaid Services data.[4] Beyond the direct costs associated with the examination, sedation requires a temporary guardian to accompany the patient home, which results in substantial indirect costs and sometimes a reluctance to undergo the procedure for both the patient and guardian. Sedation is also associated with some risk. Among patients undergoing upper endoscopy with conscious sedation, the incidence of cardiopulmonary events is estimated at 0.6%.[3] Unsurprisingly, given the substantial cost burden and inconvenience associated with standard sedated upper endoscopy, most subjects who develop esophageal adenocarcinoma have not previously been screened, nor had a precancerous lesion identified.[5]
Unsedated small-caliber transnasal esophagoscopy offers the possibility of efficient and accurate endoscopic assessment of the esophagus with less cost and fewer risks compared to sedated upper endoscopy.[6–16] The sensitivity of detecting esophageal abnormalities with unsedated small-caliber esophagoscopy is comparable to conventional upper endoscopy.[7–11] Although unsedated transnasal esophagoscopy is well tolerated in cultures less accustomed to conscious sedation,[11, 17–20] studies in North America have been limited by small sample sizes and have been performed in specialized populations selected for either esophageal symptoms or Barrett’s esophagus.[9, 10] Moreover, most studies in the U.S. have been limited to selected populations in referral settings.
The aim of this study was to assess the feasibility, safety, acceptability and yield of unsedated transnasal esophagoscopy in the general medical outpatient setting as a screening method for esophageal disease.
Methods
Study Design and Subjects
We performed a prospective, cross-sectional study of unsedated, office-based transnasal esophagoscopy in two outpatient centers between 2009–2010. Participants were recruited from the general medical clinic populations using flyers and e-mail announcements. We included participants between the ages of 40 and 85, regardless of GERD symptomatology. We excluded those with a history of anti-reflux surgery, esophageal diverticula or varices, cirrhosis, head and neck or esophageal malignancy, recurrent epistaxis, previously diagnosed Barrett’s esophagus, or New York Heath Association Class IV congestive heart failure. Additionally, we excluded anyone with an active pulmonary or sinus infection, recent ENT surgery, participants currently pregnant, on anticoagulation or with an oxygen requirement. All participants gave informed consent. The Institutional Review Boards of both centers approved the study.
Endoscopic Examination
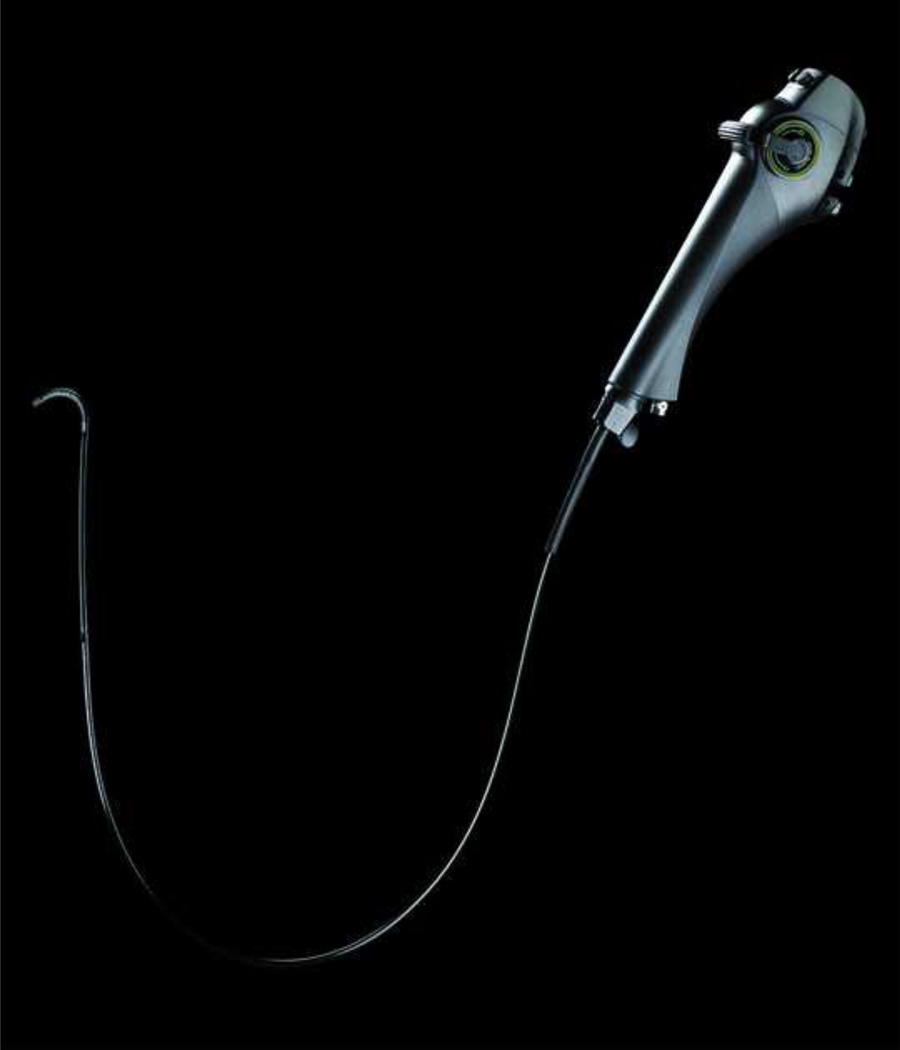
Examinations were performed using the one-wheel TNE-5000 endoscope system, a 650 mm video endoscope with a 120 degree field of view, encased in a disposable sheath of 4.7 mm diameter (Vision Sciences, Orangeburg, NY) (Figure 1). Two endoscopists (BAJ, NJS) experienced in transnasal upper esophagoscopy (>100 examinations before the study) performed the examinations. A combination of a topical decongestant (oxymetazoline hydrochloride, 0.05%, Afrin, Schering-Plough Healthcare Products, Inc., Memphis, Tennessee) and topical anesthesia with 4% lidocaine was used to anesthetize the nares and posterior pharynx. Topical anesthetic was delivered to the nares with a disposable atomizer (Wolfe Tory Medical, Salt Lake City, UT) over a 5–10 minute period. Examinations were performed with the participant in the sitting position. The posterior pharynx, esophagus and proximal stomach were examined and biopsies were taken with forceps through a coaxial channel in the sheath as noted below. Upper esophageal sphincter intubation was facilitated by asking the subject to ingest water through a straw with scope advancement. The examination was performed to the mid-stomach, and scope retroflexion was performed to examine the cardia. After removal of the endoscope, the disposable sheath was stripped, and the endoscope was wiped with an alcohol swab. The endoscope was then re-sheathed for the next patient (for more details, see the video in the Supplementary Appendix).
Figure 1.
One-wheel TNE-5000 video endoscope encased in 4.7 mm diameter disposable sheath (Vision Sciences, Orangeburg, NY
When identified, the length of Barrett’s esophagus was measured in centimeters from the most proximal extent of the gastric folds to the most proximal squamocolumnar junction (SCJ). The presence of a hiatal hernia was documented and measured at the nares in centimeters beginning at the crural pinch distally to the most proximal extent of the gastric folds. Additionally, we evaluated the SCJ using the z-line appearance (ZAP) classification scheme.[21] A sharp and circular SCJ without tongues of endoscopically visible columnar epithelium denoted grade 0. Patients with Grade 0 ZAP classification were considered negative for BE. Grade I ZAP meant the Z line was irregular with tongue-like protrusions or islands; grade II was present when obvious tongues of columnar epithelium <3 cm were noted, and grade III, distinct tongues of columnar epithelium >3 cm, or a cephalad displacement of the squamocolumnar junction > 3 cm. Four-quadrant esophageal biopsies with a 1.8 mm needle forceps (Olympus, PA) were obtained beginning immediately proximal to the top of the gastric folds and extending every 2 cm to the level of the squamocolumnar junction for all patients with ZAP Grades I–III. Biopsies were fixed in formalin, routinely processed, stained with hematoxylin and eosin (H&E), and interpreted by an expert pathologist using standardized criteria.[22] The diagnosis of Barrett’s esophagus required a ZAP score >0, as well as histological confirmation of the presence of specialized intestinal metaplasia. The presence of esophagitis was documented and graded using the Los Angeles Classification.[23]
A research assistant observed each esophagoscopy . The research assistant documented completion of the examination and if not completed, the reason for discontinuation, the length of the procedure, endoscopic findings as dictated by the endoscopist, and any adverse events or complications. After the examination, participants completed a 10-question assessment to measure procedure acceptability (Figure 2).
Figure 2.
10-question assessment to measure procedure acceptability
Data Collection and Analysis
For each subject, the following variables were collected: age, sex, race, height, weight, education, current alcohol use (yes/no), history of tobacco use (yes/no), a gastroesophageal reflux disease health related quality of life instrument,[24] the reflux symptom index score,[25] medication use, co-morbidities, family history of Barrett’s esophagus, family history of esophageal cancer, esophageal symptoms, completeness of the endoscopic examination, and if not, why, procedure time, adverse events or complications, endoscopic findings, biopsy results (if obtained), and patient reported acceptability. Potential GERD signs and symptoms assessed included acid reflux, heartburn, chest pain, hoarseness, chronic sore throat, excess mucus in nose or throat, chronic sinusitis, chronic bronchitis, chronic throat clearing, difficulty swallowing, pain with swallowing, vomiting blood, and globus.
Our primary outcome variables were: 1) procedure yield, defined as the proportion of subjects noted to have esophageal disease, including erosive esophagitis, Barrett’s esophagus, esophageal nodules or mass, or esophageal cancer; 2) completeness of the examination (treated as a binary variable, with a complete examination considered one where visualization was carried through to the mid-stomach); 3) Procedure length, measured in minutes from scope insertion into the nare to complete withdrawal; 4) adverse events and complications, such as epistaxis, dizziness, syncope or near-syncope, and gastrointestinal bleeding; 5) choking, gagging, pain and anxiety during the examination, using a standardized questionnaire (figure 2); and overall tolerability, measured on a 1–10 scale, with 1 being well-tolerated and 10 being poorly tolerated. Exploratory data analysis was performed to examine the distribution of continuous and categorical values, and to determine missing or implausible values. Bivariate analysis was then performed to identify variables associated with 1) risk factors for poor tolerability of the endoscopic examination, 2) risk factors for erosive esophagitis and 3) risk factors for the combined outcome of erosive esophagitis and Barrett’s esophagus. Bivariate analysis was performed using Student’s t-tests for continuous variables, and Pearson’s Chi-squared tests or Fisher’s exact tests for categorical variables. Means and standard deviations were reported for continuous variables. Medians and interquartile ranges were reported for continuous variables with skewed distributions. Proportions were reported for categorical data.
Multiple linear regression using maximum likelihood ratio techniques was used to assess risk factors for lower overall tolerability of the endoscopic examination. To construct these models, we used non-collinear variables significantly related to the outcome in bivariate analyses using p value of ≤0.1, and limiting the model to M/10 components (beta coefficients + intercept), where M=[3*(n1)(n2)]/n (where n1 and n2 are the number of subjects in each group, and n is the total number of subjects). Lower overall tolerability was defined as a score of 3 or higher on the 1–10 Likert scale (>75th % percentile). Potential predictor variables assessed in this model included: sex, age, race, BMI, alcohol use, tobacco use, gastroesophageal reflux disease health related quality of life score, reflux symptom index score, acid reflux, heartburn, dysphagia, odynophagia, chest pain, hoarseness, chronic sore throat, excess mucous in the nose or throat, chronic sinusitis, hematemesis, globus, unintentional weight loss, and feeling of something in the back of the throat. The initial model was created with variables that were significantly associated with lower overall tolerability in a bivariate analysis. The model was reduced using likelihood ratio tests on non-significant variables.
Multiple linear regression was also used to assess risk factors for erosive esophagitis, as well as the combined outcome of erosive esophagitis and Barrett’s esophagus. We considered all of the variables detailed in the prior model in addition to NSAID, aspirin, proton pump inhibitor and H2 receptor antagonist use. The model was created and reduced using the methodology detailed above.
All tests of significance were two-tailed and p-values <0.05 were considered significant. All data were entered into and analyzed using Stata 11.0 statistical software (StataCorp, College Station, TX).
Results
A total of 426 participants were enrolled in the study. The mean age was 55.8 ± 9.5 years and 43% were male (Table 1). The mean BMI was 28.0 ± 6.1. Other characteristics of the cohort are reported in table 1.
Table 1.
Participant Characteristics
| Characteristics (n = 426) | Mean ± standard deviation or n (%) |
|---|---|
| Age, years | 55.8 ± 9.5 |
| Male | 182(43) |
| White | 359 (85) |
| BMI, kg/m2 | 28.0 ± 6.1 |
| >16 years education | 280 (66) |
| Alcohol use | 298 (70) |
| History of tobacco use | 217 1) (5 |
| Reflux Symptom Index*† | 5 |
| GERD Health Related Quality of Life*‡ | 3.5 |
| Medication Use: | |
| Proton pump inhibitor | 185 (44) |
| H2 receptor antagonist | 105 (25) |
| NSAID use | 148 (35) |
| ASA use | 163 (38) |
| Co-Morbidities: | |
| Asthma | 27 (6) |
| Allergies | 154 (36) |
| Diabetes | 28 (7) |
| Coronaryartery disease | 6 (1) |
| COPD | 9 (2) |
| Hypertension | 113 (27) |
| GERD | 128 (30) |
| Familyhistory of Barrett’s esophagus | 16 (5) |
| Familyhistory of esophageal cancer | 45 (12) |
Medians reported for skewed distributions
The reflux symptom index is graded on a 0 –45 scale with a score > 13 suggestive of laryngopharyngeal reflux
GERD Health Related Quality of Life is graded ona 0–51 scale with higher scores suggestive of worse GERD symptom severity
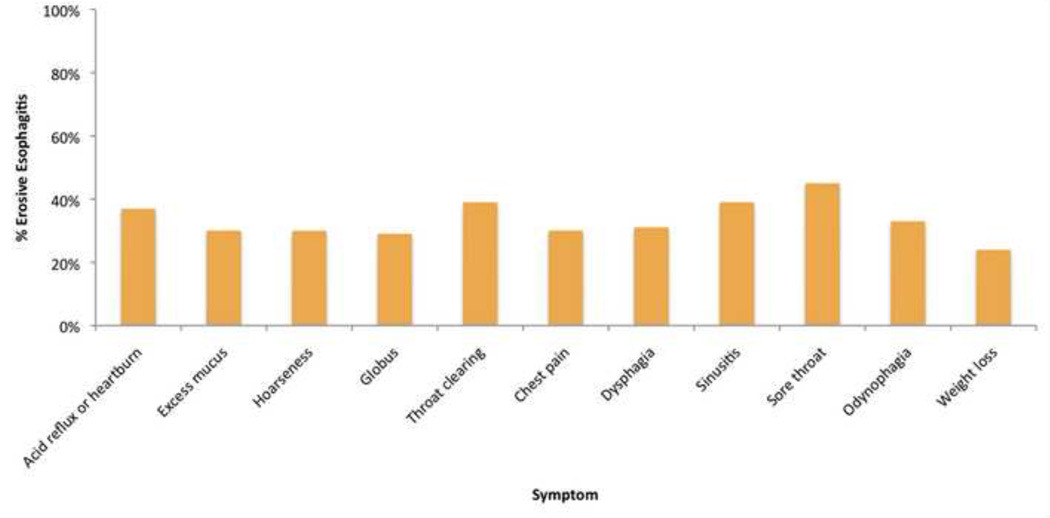
The majority (70%) of participants reported reflux or heartburn symptoms. Almost a third (29%) reported excess mucus in the nose or throat and a quarter reported (25%) hoarseness. Approximately, a fifth of participants reported globus (20%), chronic throat clearing (20%), chest pain (19%), or dysphagia (18%). Finally, 13% reported chronic sinusitis, 7% chronic sore throat, and 5% odynophagia.
A large proportion of our population had significant endoscopic findings on examination (Table 2). A third (34%) of participants had erosive esophagitis (51% LA Grade A, 32% LA Grade B, 13% LA Grade C, 2% LA Grade D). Bivariate analysis demonstrated that erosive esophagitis was associated with male sex, heartburn symptoms and not taking a PPI or H2RA (Table 3). Eighteen participants (4%) were diagnosed with Barrett’s esophagus. Almost half (n=180, 43%) of participants had a hiatal hernia. Twelve participants (3%) were found to have an esophageal nodule. All were referred for further evaluation with conventional upper endoscopy. Of the twelve participants, four have had an upper endoscopy, five have an upper endoscopy pending and three have yet to schedule follow up. Of the four participants with an esophageal nodule who underwent upper endoscopy, one was found to have intestinal metaplasia with high-grade dysplasia and the rest had nonneoplastic lesions.
Table 2.
Endoscopic Findings
| Findings | n (%) |
|---|---|
| Erosive Esophagitis | 143 (34) |
| LAGradeA | 73 (51) |
| LAGradeB | 46 (32) |
| LAGradeC | 18 (13) |
| LA Grade D | 3 (2) |
| Hiatal Hernia | 180 (43) |
| Barrett’s Esophagus | 18 (4) |
| Esophageal Mass/Nodularity Requiring Subsequent Endoscopic Resection | 12 (3) |
| Gastritis | 15 (4) |
Table 3.
Participant Reported Acceptability
| Characteristic | Median (interquartile range) |
|---|---|
|
Pain
during insertion of the endoscope [0= No pain,10= Severepain] |
2 (2) |
|
Pain
during the procedure [0= No pain,10= Sever pain] |
2 (2) |
|
Gagging or wretching
during insertion of the endoscope [0= No gagging, 10= Worst gagging] |
2 (2) |
|
Gagging or wretching
during the procedure [0= No gagging, 10= Worst gagging] |
2 (2) |
|
Choking
during insertion of the endoscope [0= No choking, 10= Worst choking] |
1 (1) |
|
Choking
during the procedure [0= No choking, 10= Worst choking] |
1 (1) |
|
Anxiety, nervousness or worried
during insertion of the endoscope [0 = No worries, 10= I was terrified] |
3 (3) |
|
Anxiety, nervousness or worried
during the procedure [0 = No worries, 10= I was terrified] |
2 (3) |
|
Anxiety, nervousness or worried
before having the endoscopy [0 = No worries, 10= I was terrified] |
2 (3) |
|
Overall, procedure tolerability [0 = Well-tolerated, 10= Poorly tolerated] |
1 (2) |
In multivariate analysis, predictors of erosive esophagitis included male sex (OR 2.7, 95% CI 1.7–4.2), heartburn symptoms (OR 1.5, 95% CI 0.97–2.4), the presence of a hiatal hernia (OR 3.7, 95% CI 2.4–5.8), and non-use of a PPI or H2RA (OR 0.57, 95% CI 0.37–0.90) (Supplementary Table 1). The combined outcome of erosive esophagitis and Barrett’s esophagus was similarly predicted by male sex (OR 2.9, 95% CI 1.8–4.5), heartburn symptoms (OR 1.6, 95% CI 1.0–2.5), the presence of a hiatal hernia (OR 3.8, 95% CI 2.4–6.0), and non-use of a PPI or H2RA (OR 0.57, 95% CI 0.36–0.89).
Feasibility and Safety
Of the 426 subjects who enrolled in the study, 422 (99%) completed the examination. Four participants discontinued the examination secondary to nasal discomfort with insertion of the endoscope. The mean examination time was 3.7 ± 1.8 minutes (3.3 ± 1.6 minutes without esophageal biopsies and 4.8 ± 1.7 minutes with esophageal biopsies (p<0.001)).
No subject experienced a serious adverse event. Twelve participants (2.8%) developed a minor complication. Minor complications included lightheadedness (n=7, 1.6%), self-limited epistaxis (n=3, 0.7%), nausea (n=2, 0.4%), headache (n=1, 0.2%) and nasal irritation (n=1, 0.2%).
Acceptability Analysis
Participants reported minimal anxiety, pain, gagging or choking with insertion of the endoscope or during the procedure (Table 3). Participants reported minimal anxiety before the endoscopy. Overall, most participants reported that the procedure was well tolerated. Lower overall tolerability was associated with female sex and younger age in a bivariate analysis (Supplementary Table 2). In multivariate analysis, lower overall tolerability was predicted by female sex (OR 1.7, 95% CI 1.1–2.8) and younger age (age < 50, OR 2.0, 95% CI 1.2–3.2).
Discussion
Our study demonstrates that unsedated trans-nasal esophagoscopy is both feasible and safe in a primary care population, achieving short procedure times, a high diagnostic yield and minimal anxiety. Patients reported good acceptability and minimal discomfort. Male sex, heartburn symptoms, a hiatal hernia, and non-use of PPI’s were predictors of erosive esophagitis in this patient population.
Our study is the largest reported experience with transnasal esophagoscopy in the United States. Four studies have assessed unsedated small-caliber transnasal esophagoscopy in North America.[7, 9, 10, 20] In general, these studies demonstrate that transnasal esophagoscopy is well tolerated. However, these studies are limited by small sample sizes and/or specialized populations with a history of esophageal symptoms or Barrett’s esophagus. Studies assessing concordance rates between transnasal esophagoscopy and standard endoscopy demonstrate moderate to good agreement in findings between the two techniques. [7–11]
Unlike prior studies, our research was a prospective evaluation of a large group of general medical clinic patients. , We began with well-defined study outcomes, including a complete assessment of potential complications and a detailed questionnaire to assess procedure acceptability. Standardized methods were used to assess symptom burden and endoscopic findings. Study limitations included a self-selected populations recruited in two centers. A large proportion of these subjects had symptoms of heartburn or reflux and may therefore have had a lower threshold for procedure acceptability. Subjects were recruited in academic centers and may differ in important ways from the general community. Because two endoscopists with extensive experience in transnasal esophagoscopy performed the examinations, it is possible that patient tolerability would be lower with less experienced endoscopists.
Although the primary goal of these examinations was to screen for esophageal disease, it is important to note that distal stomach or proximal duodenum pathology might not be detected with the examination described here.. For subjects with a concern for distal gastric or duodenal disorders, this particular endoscopic approach would not be appropriate.
Although transnasal esophagoscopy has been available for more than a decade, the technique has not been widely used in practice. Physician concerns may include safety, patient tolerance, and uncertainty regarding the time and facilities required to complete the examination. Training in the technique is not widely available, and criteria for training and exam quality are not standardized. For endoscopists already performing standard upper endoscopy, transnasal esophagoscopy may be viewed as a competing technology, with the potential to decrease income by shifting full examinations performed in ambulatory care centers to brief, office-based examinations.
Although these problems are substantial, significant data suggest that alternative screening modalities for esophageal pathology are needed. Considerable concern regarding the cost-effectiveness of upper endoscopy as a screening tool [26] has been further heightened by recent data demonstrating that the risk of esophageal adenocarcinoma in the setting of Barrett’s esophagus may be lower than previously estimated. [27, 28] Endoscopic screening programs were long considered important because of their yield of newly-diagnosed cases of Barrett’s esophagus, but a lower risk of cancer in Barrett’s raises the natural question of how much we should value a Barrett’s screening test. Given the marked rise in the incidence of adenocarcinoma of the esophagus in the last four decades, it seems unwise and illogical to abandon upper endoscopic screening examinations entirely. Therefore, a technology with which to screen large numbers of subjects quickly and at a fraction of the cost of standard endoscopy is attractive from a public health perspective.
Transnasal esophagoscopy might be a suitable candidate for this technology. Examinations are short in duration, suggesting that the modality might be appropriate for wide scale programs in primary care populations. This technique avoids many of the costs associated with sedation, endoscope sterilization, and GI suite nursing. Because of the low cost of the endoscope sheath used in this study ($40), and because the costs of the endoscope itself could be amortized over many examinations, the overall cost profile of a screening program using this technology would be expected to be much less than the current standard of care, and would likely be sensitive to the program’s case volume. Further, if applied in a primary care clinic population, transnasal esophagoscopy could be used in a population that might otherwise go unexamined.
To date, no data are available to ascertain which healthcare providers should implement transnasal esophagoscopy screening programs. Gastroenterologist and surgeon endoscopists might be expected to require the least training, given their experience. However, a large body of data documents the successful performance of other endoscopic procedures by generalist physicians [29, 30] and even physician extenders [31] in other clinical settings. Whether high quality, cost-effective screening programs might be implemented by these providers remains to be demonstrated. By better defining quality metrics for such examinations, rational training strategies might be developed for these practitioners.
In our study, both erosive esophagitis and the combined outcome of erosive esophagitis and Barrett’s esophagus were predicted by male sex, the presence of a hiatal hernia, heartburn symptoms, and absence of PPI or H2A therapy. These risk factors for acid-peptic esophageal disease are consistent with studies using standard per oral endoscopy. Similarly, GERD symptoms, hiatal hernia and male sex have been previously associated with Barrett’s esophagus risk.[32–35] Although we did not assess concordance rates of transnasal esophagoscopy and standard endoscopy in the current study, the presence of similar risk factors for erosive esophagitis and Barrett’s esophagus suggests that transnasal esophagoscopy is identifying patients with upper gastrointestinal pathology similar to those identified by standard endoscopy in previous studies.
In conclusion, unsedated transnasal esophagoscopy is a safe and well-tolerated method to screen for esophageal disease in a primary care population. In our population, screening identified a significant number of abnormalities that might have otherwise gone undetected until further progression of disease. Unsedated transnasal esophagoscopy may provide an accurate, lower-cost alternative to standard endoscopy, and may allow for affordable screening for general medical patients at risk for esophageal neoplasia or other esophageal diseases.
Supplementary Material
This video demonstrates the equipment and technique for performing unsedated transnasal endoscopy. For this procedure, a one-wheel ultrathin endoscope is encased in a disposable sheath 4.7 mm in diameter. The sheath also has a channel that allows passage of the biopsy forceps for tissue sampling. Prior to starting the exam, the patient’s nares are anesthetized with a lidocaine spray. After this takes effect, the ultrathin endoscope is inserted and maneuvered past the turbinates. Upon reaching the hypopharynx, the laryngeal anatomy is assessed by having the patient vocalize. The patient takes a sip of water through a straw, which facilitates esophageal intubation while suppressing the gag reflex. The esophagus is then examined using routine endoscopic technique.
Figure 3.
Reported esophageal symptoms and percentage found to have erosive esophagitis on transnasal examination
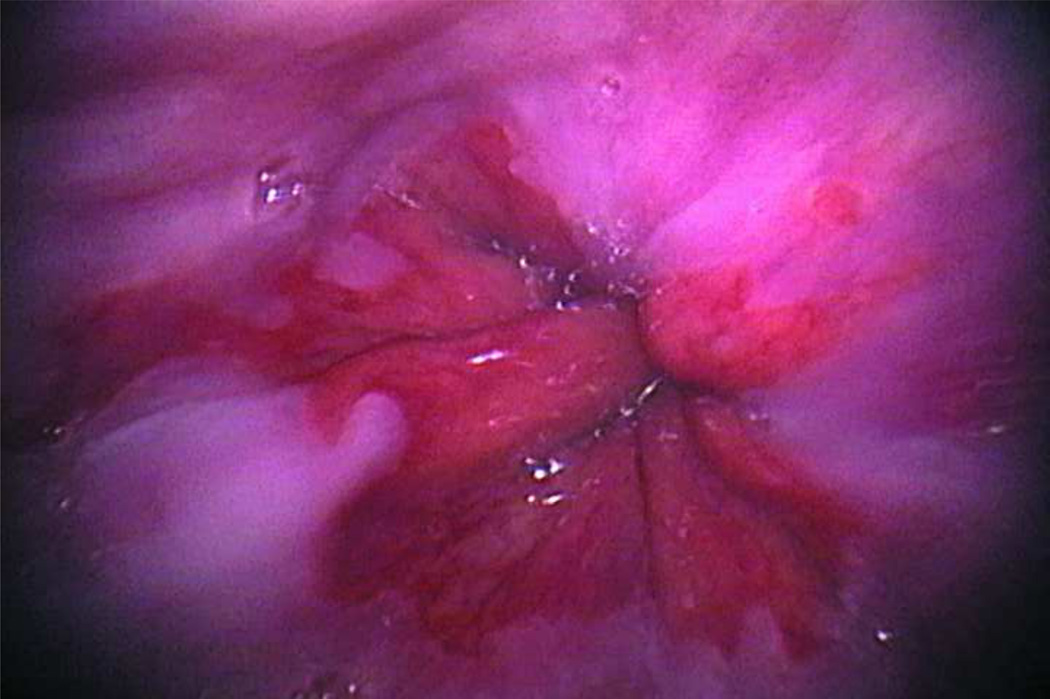
Figure 4.
An image captured from a participant with intestinal metaplasia
Acknowledgments
Sponsor/Funding: This study was funded in part with grants from the National Institutes of Health K23 DK066165, T32 DK 07634, and R21 DK076827. Equipment for the trial was donated by Vision Sciences (Orangeburg, NY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentations: Oral presentation, Digestive Diseases Week, Chicago, Illinois, May 2011
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology. 2009;136:2101–14. e1–e6. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 6.Garcia RT, Cello JP, Nguyen MH, Rogers SJ, Rodas A, Trinh HN, et al. Unsedated ultrathin EGD is well accepted when compared with conventional sedated EGD: a multicenter randomized trial. Gastroenterology. 2003;125:1606–1612. doi: 10.1053/j.gastro.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett's esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693–2703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 8.Saeian K, Staff DM, Vasilopoulos S, Townsend WF, Almagro UA, Komorowski RA, et al. Unsedated transnasal endoscopy accurately detects Barrett's metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472–478. doi: 10.1067/mge.2002.128131. [DOI] [PubMed] [Google Scholar]
- 9.Mokhashi MS, Wildi SM, Glenn TF, Wallace MB, Jost C, Gumustop B, et al. A prospective, blinded study of diagnostic esophagoscopy with a superthin, stand-alone, battery-powered esophagoscope. Am J Gastroenterol. 2003;98:2383–2389. doi: 10.1111/j.1572-0241.2003.08701.x. [DOI] [PubMed] [Google Scholar]
- 10.Dean R, Dua K, Massey B, Berger W, Hogan WJ, Shaker R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc. 1996;44:422–424. doi: 10.1016/s0016-5107(96)70092-5. [DOI] [PubMed] [Google Scholar]
- 11.Mori A, Ohashi N, Yoshida A, Nozaki M, Tatebe H, Okuno M, et al. Unsedated transnasal ultrathin esophagogastroduodenoscopy may provide better diagnostic performance in gastroesophageal reflux disease. Dis Esophagus. 2011;24:92–98. doi: 10.1111/j.1442-2050.2010.01098.x. [DOI] [PubMed] [Google Scholar]
- 12.Catanzaro A, Faulx A, Pfau PR, Cooper G, Isenberg G, Wong RC, et al. Accuracy of a narrow-diameter battery-powered endoscope in sedated and unsedated patients. Gastrointest Endosc. 2002;55:484–487. doi: 10.1067/mge.2002.122576. [DOI] [PubMed] [Google Scholar]
- 13.Faulx AL, Catanzaro A, Zyzanski S, Cooper GS, Pfau PR, Isenberg G, et al. Patient tolerance and acceptance of unsedated ultrathin esophagoscopy. Gastrointest Endosc. 2002;55:620–623. doi: 10.1067/mge.2002.123274. [DOI] [PubMed] [Google Scholar]
- 14.Catanzaro A, Faulx A, Isenberg GA, Wong RC, Cooper G, Sivak MV, Jr, et al. Prospective evaluation of 4-mm diameter endoscopes for esophagoscopy in sedated and unsedated patients. Gastrointest Endosc. 2003;57:300–304. doi: 10.1067/mge.2003.113. [DOI] [PubMed] [Google Scholar]
- 15.Faulx AL, Vela S, Das A, Cooper G, Sivak MV, Isenberg G, et al. The changing landscape of practice patterns regarding unsedated endoscopy and propofol use: a national Web survey. Gastrointest Endosc. 2005;62:9–15. doi: 10.1016/s0016-5107(05)00518-3. [DOI] [PubMed] [Google Scholar]
- 16.Boolchand V, Faulx A, Das A, Zyzanski S, Isenberg G, Cooper G, et al. Primary care physician attitudes toward endoscopic screening for GERD symptoms and unsedated esophagoscopy. Gastrointest Endosc. 2006;63:228–233. doi: 10.1016/j.gie.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Dumortier J, Ponchon T, Scoazec JY, Moulinier B, Zarka F, Paliard P, et al. Prospective evaluation of transnasal esophagogastroduodenoscopy: feasibility and study on performance and tolerance. Gastrointest Endosc. 1999;49:285–291. doi: 10.1016/s0016-5107(99)70002-7. [DOI] [PubMed] [Google Scholar]
- 18.Preiss C, Charton JP, Schumacher B, Neuhaus H. A randomized trial of unsedated transnasal small-caliber esophagogastroduodenoscopy (EGD) versus peroral small-caliber EGD versus conventional EGD. Endoscopy. 2003;35:641–646. doi: 10.1055/s-2003-41513. [DOI] [PubMed] [Google Scholar]
- 19.Murata A, Akahoshi K, Sumida Y, Yamamoto H, Nakamura K, Nawata H. Prospective randomized trial of transnasal versus peroral endoscopy using an ultrathin videoendoscope in unsedated patients. J Gastroenterol Hepatol. 2007;22:482–485. doi: 10.1111/j.1440-1746.2006.04730.x. [DOI] [PubMed] [Google Scholar]
- 20.Cho S, Arya N, Swan K, Cirocco M, Kandel G, Kortan P, et al. Unsedated transnasal endoscopy: a Canadian experience in daily practice. Can J Gastroenterol. 2008;22:243–246. doi: 10.1155/2008/514297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallner B, Sylvan A, Janunger KG. Endoscopic assessment of the "Z-line" (squamocolumnar junction) appearance: reproducibility of the ZAP classification among endoscopists. Gastrointestinal endoscopy. 2002;55:65–69. doi: 10.1067/mge.2002.119876. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 23.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130–134. doi: 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 26.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 28.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xirasagar S, Hurley TG, Sros L, Hebert JR. Quality and safety of screening colonoscopies performed by primary care physicians with standby specialist support. Med Care. 2010;48:703–709. doi: 10.1097/MLR.0b013e3181e358a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins T, LeClair B, Smolkin M, Davies K, Thomas A, Taylor ML, et al. Screening colonoscopies by primary care physicians: a meta-analysis. Ann Fam Med. 2009;7:56–62. doi: 10.1370/afm.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton K, Reffel A, Rosen K, Farraye FA. Training of nurse practitioners and physician assistants to perform screening flexible sigmoidoscopy. J Am Acad Nurse Pract. 2001;13:455–459. doi: 10.1111/j.1745-7599.2001.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett's esophagus. Am J Gastroenterol. 2010;105(1729):30–37. doi: 10.1038/ajg.2010.194. quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conio M, Filiberti R, Blanchi S, Ferraris R, Marchi S, Ravelli P, et al. Risk factors for Barrett's esophagus: a case-control study. Int J Cancer. 2002;97:225–229. doi: 10.1002/ijc.1583. [DOI] [PubMed] [Google Scholar]
- 34.Gerson LB, Edson R, Lavori PW, Triadafilopoulos G. Use of a simple symptom questionnaire to predict Barrett's esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol. 2001;96:2005–2012. doi: 10.1111/j.1572-0241.2001.03933.x. [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein JH, Mattek N, Eisen G. Age- and sex-specific yield of Barrett's esophagus by endoscopy indication. Gastrointest Endosc. 2010;71:21–27. doi: 10.1016/j.gie.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates the equipment and technique for performing unsedated transnasal endoscopy. For this procedure, a one-wheel ultrathin endoscope is encased in a disposable sheath 4.7 mm in diameter. The sheath also has a channel that allows passage of the biopsy forceps for tissue sampling. Prior to starting the exam, the patient’s nares are anesthetized with a lidocaine spray. After this takes effect, the ultrathin endoscope is inserted and maneuvered past the turbinates. Upon reaching the hypopharynx, the laryngeal anatomy is assessed by having the patient vocalize. The patient takes a sip of water through a straw, which facilitates esophageal intubation while suppressing the gag reflex. The esophagus is then examined using routine endoscopic technique.