Abstract
Estrogen inhibits osteoclastogenesis and induces osteoclastic apoptosis; however, the molecular mechanisms remain controversial. Recently, a group has demonstrated that osteoclasts are a direct target for estrogen because estrogen stimulates transcription of the Fas Ligand (FasL) gene in osteoclasts, which in turn causes cell death through an autocrine mechanism. In contrast, other groups have shown that the cells are an indirect target for estrogen because estrogen fails to stimulate the transcription of that in osteoclasts. Thus, two quite different molecular mechanisms have been suggested to explain the effects of estrogen in osteoclastic apoptosis. Here we show that the proapoptotic effect of estrogen during osteoclastogenesis is regulated by a posttranscriptional increase in FasL production by down-regulated microRNA-21 (miR-21) biogenesis. Previously, we reported that miR-21 is highly expressed in osteoclastogenesis. We found that estrogen down-regulates miR-21 biogenesis so that FasL, the targets of miR-21, protein levels are posttranscriptionally increased that induce osteoclastic apoptosis. Moreover, the gain-of-function of miR-21 rescued the apoptosis. In addition, we failed to detect estrogen-enhanced FasL levels at mRNA levels. Thus, osteoclastic survival is controlled by autocrine actions of FasL regulated by estrogen and miR-21 plays a central role during estrogen-controlled osteoclastogenesis.
Keywords: estrogen, miR-21, FasL, osteoclast
Introduction
MicroRNAs (miRs) are small non-coding RNAs that principally function in the spatiotemporal regulation of protein translation in animal cells [Bushati et al., 2007]. Emerging evidences suggests that miRs have critical roles in bone remodeling [Lian et al., 2012]. We have provided evidence that miR-223 [Sugatani et al., 2009] and miR-21 [Sugatani et al., 2011] play important roles during osteoclastogenesis. Osteoclasts, the exclusive bone resorptive cells, are derived from hematopoietic stem cells through the common myeloid progenitor to the colony-forming unit (CFU) for granulocytes and macrophages to the CFU for macrophages and into the osteoclast lineage [Suda et al., 1995]. Osteoclast differentiation, function and survival are modulated by several exogenous cytokines (macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor kappa-B ligand (RANKL), tumor necrosis factor-α, interleukin (IL)-1, and IL-6) and hormones (sex steroids, parathyroid hormone, vitamin D, insulin-like growth factor-1, calcitonin, and prostaglandins) through activity of several transcription factors identified via studies in genetically engineered mice [Tanaka et al., 2005]. Estrogen is the major hormonal regulator of bone metabolism not only in women but also in men [Khosla et al., 2012]. However, the molecular mechanisms by which osteoclasts are direct or indirect targets of estrogen remain controversial. Recently, Nakamura et al. [Nakamura et al., 2007] demonstrated that estrogen directly induces osteoclastic apoptosis. However, other groups have shown that estrogen does not stimulate FasL gene expression in osteoclasts [Martin-Millan et al., 2010] and that estrogen enhances gene expression in osteoblasts to induce osteoclastic apoptosis by a paracrine fashion [Krum et al., 2008]. To understand the molecular mechanism of estrogen action in osteoclastogenesis, we hypothesized that miR-21 may have a central role in the effects of estrogen. Firstly, estrogen-activated estrogen receptor α (ERα) directly suppresses conversion of primary miRs (pri-miRs) transcribed by RNA polymerase II into precursor-miRs (premiRs) through processing by the Drosha microprocessor complex [Fujiyama-Nakamura et al., 2010]. Thus the maturation of miRs is diminished [Fujiyama-Nakamura et al., 2010]. Secondly, miR-21 targets FasL [Sayed et al., 2010], and finally, miR-21 is highly expressed in osteoclastogenesis [Sugatani et al., 2011]. Our findings demonstrate that estrogen attenuates miR-21 biogenesis which results in an increase in FasL protein levels and caspase-3 activity during RANKL-induced osteoclastogenesis. The effects of estrogen function were blocked in ERα-deficient cells. Thus, our data support the conclusion that osteoclasts are direct targets of estrogen and the biological effects of estrogen are controlled by a nongenomic estrogen-activated ERα pathway through regulating miR-21 levels.
Materials & Methods
Mice
C57BL/6J, ERα deficient, and wild-type (wt) mice were purchased from the Jackson Laboratory. MiR-21 floxed (miR-21f/f) [Patrick et al., 2010] and CAG-Z-miR-21-EGFP transgenic [Hatley et al., 2010] mice were obtained from Dr. Eric N. Olson (University of Texas Southwestern Medical Center, Dallas, TX). All animals were housed under pathogen-free conditions according to the guidelines of the Division of Comparative Medicine at Washington University School of Medicine (WUSM). The animal ethics committee approved all experiments.
RNA Isolation
Primary mouse bone marrow-derived monocyte/macrophage precursors (BMMs) as osteoclast precursors were prepared from the femur and tibia of 4-to-6 week old mice and cultured with 50 ng/ml M-CSF (Peprotech) and 100 ng/ml RANKL (Peprotech) with or without 10−8 M β-estradiol (E2) (Sigma) for three days. Total RNA with small RNA (< 200 nucleotides) was isolated using the mirVana™ miRNA isolation kit (Life technologies) according to the manufacturer's instructions.
Reverse transcription
For end-point PCR and real-time PCR, cDNA was generated using the 1st strand cDNA synthesis system kit (OriGene) following the manufacturer's suggestion. For miR real-time PCR, cDNA was generated using the miScript reverse transcription kit (Qiagen) according to the manufacturer's instructions.
End-Point PCR
The PCR analysis was performed in 20 ul reaction volume using the GeneAmp fast PCR master mix (Life technologies) as per manufacturer's protocol. The primers for mouse ERα were sense (5’-GCGAAGGCTGCAAGGCTTTCTTTA-3’) and antisense (5’- GAAGCACCCATTTCATTTCGGCCT-3’) and mouse GAPDH were sense (5’-AACTTTGGCATTGTGGAAGGGCTC-3’) and antisense (5’- TGGAAGAGTGGGAGTTGCTGTTGA-3’). All primers were synthesized by Integrated DNA Technologies.
Real-time PCR (QRT-PCR)
QRT-PCR was performed using the OriGene qSTAR SYBR Green Kit on an Mx4000 multiplex quantitative PCR System (Stratagene). The relative expression for FasL mRNA was estimated from triplicate QRT-PCR reactions following normalization to the β2 microglobulin. All primers were purchased from the OriGene.
MiR Real-time PCR (miR QRT-PCR)
MiR QRT-PCR was performed using miScript SYBR Green PCR Kit (Qiagen) on an Mx4000 multiplex quantitative PCR System (Stratagene). The relative expression for each miR was estimated from triplicate QRT-PCR reactions following normalization to the small nucleolar RNA, snoRNA, using the delta-delta Ct method.
Construction of retroviral expression vector and infection
Cre inserted pCAGEN vectors were purchased from Addgene. Cre insert was shuttled into pMX-IRES-blasticidin retroviral vectors (Cell Biolabs) digested with EcoRΙ and NotΙ restriction enzymes (Thermo Scientific). The sequence of each construct was confirmed by the Protein and Nucleic Acid Chemistry Laboratories at WUSM. Subconfluent PLAT-E packaging cells (Cell Biolabs) were transfected with retroviral vectors using FuGENE (Roche). After 48 h, the supernatant was collected, filtered through a 0.45-μm syringe filter, and the cells were infected using 5 ml of αMEM (Sigma) media containing 10% FBS (Life technologies) and 5 ml of the supernatant including viruses with polybrene (8 ug/ml) (Santa Cruz). The cells were cultured for 6 h at 32°C, and after that, the cells were incubated at 37°C. Next day, the media was exchanged with the fresh 10 ml of αMEM media containing 10% FBS. Infected cells were cultured for an additional 2 days for the selection by blasticidin (4 ug/ml) (Life technologies), and the cells were harvested for assay.
Immunoblotting
To visualize FasL (Santa Cruz), α-tubulin (Santa Cruz), and caspase-3 (Cell Signaling), whole-cell lysates were prepared by the RIPA buffer (Thermo Scientific). Proteins were resolved by SDS-PAGE, electroblotted to polyvinylidene difluoride membrane (Millipore), blocked in 1 X TBST (Cell Signaling), and probed with primary antibodies (1:1000). Following incubation with anti-mouse IgG HRP-linked antibody (1:2000) (Cell Signaling) or anti-rabbit IgG HRP-linked antibody (1:2000) (Cell Signaling) was detected using enhanced chemiluminescence (Thermo Scientific).
Chromatin Immunoprecipitation (ChIP)
ChIP assay was performed with the imprint ChIP assay kit (Sigma) according to the manufacturer's suggestions using antibodies against c-Fos (Santa Cruz) and normal IgG (Santa Cruz). The purified DNA was analyzed by PCR using primers that detect sequences containing the mouse miR-21 promoter [Sugatani et al., 2011].
In Vitro osteoclastogenesis
BMMs were prepared as described previously [Sugatani et al., 2011]. BMMs were prepared from the femur and tibia of 4-6-week-old mice. To generate osteoclasts, 50 ng/ml M-CSF and 100 ng/ml RANKL were added to αMEM media containing 10% FBS for 3 days. The cells were stained for tartrate resistant acid phosphatase (TRAP) according to the manufacturer's suggestions (Sigma). TRAP-positive cells containing more than three nuclei were counted as osteoclasts under microscopic examination.
Caspase-3 activity
Caspase-3 activity was measured using the caspase-3 colorimetric assay kit (GeneScript) according to the manufacturer's instructions.
Results and Discussion
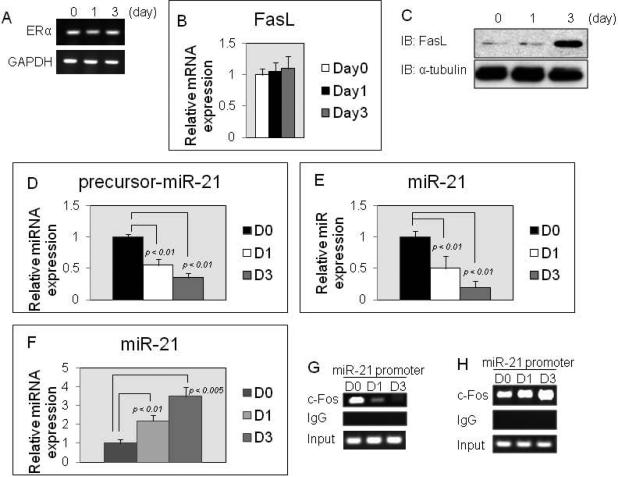
We confirmed the expression of ERα during RANKL-induced osteoclastogenesis by PCR analysis. ERα was expressed from M-CSF-dependent BMMs (day 0) through osteoclasts induced by RANKL (day 3) (Fig.1A). Recently, three groups have investigated whether osteoclasts are a direct target for estrogen [Nakamura et al., 2007; Martin-Millan et al., 2010; Krum et al., 2008]. One group demonstrated that osteoclasts are a direct target for estrogen because estrogen enhanced FasL mRNA and protein expression levels during RANKL-induced osteoclastogenesis [Nakamura et al., 2007]. In contrast, two other groups have shown that estrogen failed to enhance the expression of FasL mRNA and protein in RANKL-induced osteoclastogenesis [Martin-Millan et al., 2010; Krum et al., 2008]. Thus, the issue remains controversial. Therefore, we analyzed FasL gene expression levels during RANKL-induced osteoclastogenesis with E2 treatment. QRT-PCR analysis showed that E2 fails to stimulate FasL gene expression in RANKL-induced osteoclastogenesis (Fig.1B). However, immunoblotting analysis showed that FasL protein levels were strongly increased by day 3 in RANKL-induced osteoclastogenesis with E2 treatment (Fig.1C). On the other hand, the protein levels were not increased by day 3 in RANKL-induced ERα-deficient osteoclastogenesis with E2 treatment (data not shown). These data suggest that osteoclasts are a direct target for estrogen and estrogen enhances FasL protein levels, but not the transcriptional levels. However, our data raised the suggestion that the estrogen activated-ERα action is a non-genomic pathway in osteoclasts. Therefore, we hypothesized that miR-21 may be a critical intermediate in the pathway of ERα stimulation. We have reported that miR-21 is highly expressed in M-CSF-dependent BMMs as osteoclast precursors, and that miR-21 expression levels are up-regulated by RANKL stimulation during osteoclastogenesis [Sugatani et al., 2011]. Previous report demonstrates that p68/p72 binds to the microprocessor complex composed of Drosha and DiGeorge syndrome critical region gene 8 (DGCR8), and that the large Drosha complex produces pre-miRs from pri-miRs [Fujiyama-Nakamura., 2010]. Thereby, p68/p72 is a critical factor for biogenesis of miRs [Fujiyama-Nakamura., 2010]. Recently, it has been demonstrated that estrogen-activated ERα binds to p68/p72 and inhibits pri-miRs processing by the large Drosha complex [Fujiyama-Nakamura., 2010]. Therefore, we first asked whether estrogen down-regulates the expression of pre-miR-21 and miR-21 levels during RANKL-induced osteoclastogenesis. As predicted, the expression levels of pre-miR-21 and miR-21 were significantly decreased by E2 treatment during RANKL-induced osteoclastogenesis (Fig.1D and E). In contrast, the E2 effect was prevented in ERα-deficient cells (Fig.1F), suggesting that the estrogen-activated ERα pathway negatively regulates miR-21 biogenesis during RANKL-induced osteoclastogenesis so that FasL protein levels are enhanced in osteoclasts (Fig.1C). Thus, these data suggest that estrogen may directly control osteoclastic apoptosis by a non-genomic ERα-activated pathway. We have reported the binding of c-Fos, a critical transcriptional factor for osteoclast development [Grigoriadis et al., 1994], to the miR-21 promoter, and that the c-Fos association with the miR-21 promoter is stimulated by RANKL stimulation [Sugatani et al., 2011]. In addition, Estrogen inhibits c-Fos activity during RANKL-induced osteoclastogenesis [Srivastava et al., 2001]. We found that the binding of c-Fos to miR-21 promoter is strikingly diminished by E2 treatment in RANKL-induced osteoclastogenesis (Fig.1G) and the effect was blocked in ERα-deficient cells (Fig.1H), indicating that estrogen-activated ERα action is involved in osteoclast development, as well as in its apoptosis.
Figure 1. Estrogen inhibits miR-21 biogenesis.
(A) RT-PCR analysis of ERα expression in RANKL-induced osteoclastogenesis for the indicated days. (B) QRT-PCR analysis of FasL expression in RANKL-induced osteoclastogenesis for the indicated days. (C) Immunoblotting analysis of FasL expression during RANKL-induced osteoclastogenesis with E2 treatment for the indicated days. (D-F) MiR QRT-PCR analysis of pre-miR-21/miR-21 expressions in RANKL-induced osteoclastogenesis with E2 treatment for the indicated days. (D) and (E): ERα-wt cells, (F): ERα-null cells. (G and H) ChIP assay was performed to study the association of c-Fos with miR-21 promoter during RANKL-induced osteoclastogenesis with E2 treatment for the indicated days. (G): ERα-wt cells, (H): ERα-null cells.
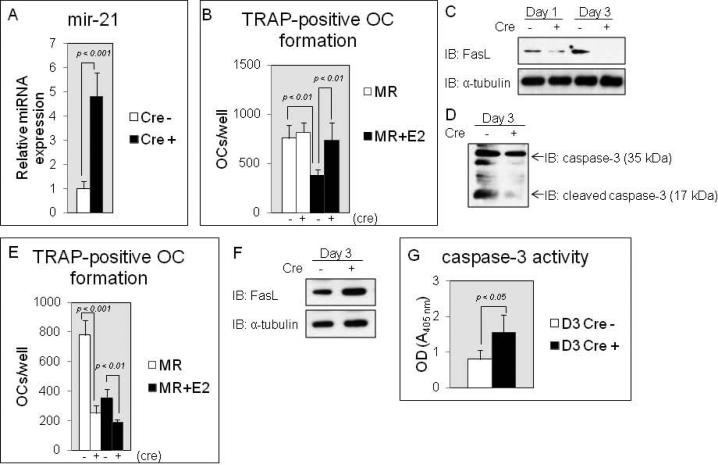
Next, we asked whether miR-21 plays a critical role for estrogen-controlled osteoclastic apoptosis. We isolated BMMs from the long bone tissues of the CAG-Z-miR-21-EGFP transgenic mice [Hatley et al., 2010] and the cells were transduced by Cre-containing retroviruses. The control cells were transduced by no Cre-containing pMX-IRES-blasticidin retroviruses. Mir-21 overexpression was achieved through the action of Cre recombinase from the CAG promoter and the Cre expressing cells overexpressed miR-21 4.8-fold over normal levels of the expression in control cells (Fig.2A). To study whether forced expression of miR-21 rescues the suppression of RANKL-induced osteoclastogenesis by estrogen treatment, the infected cells were induced into osteoclasts by M-CSF/RANKL stimulation with or without estrogen treatment, and after that, TRAP-positive osteoclast formation assay was performed to evaluate the numbers of osteoclasts. RANKL-induced osteoclast development was significantly inhibited by E2 treatment in the control cells (Fig.2B). Surprisingly, in culture without E2 treatment, miR-21 overexpression failed to enhance TRAP-positive osteoclast numbers compared to control cells (Fig.2B). The most important result was that with E2 treatment, forced expression of miR-21 blocked a decrease in RANKL-induced osteoclast development compared to control cells in culture (Fig.2B). Consistent with the results, in RANKL-induced osteoclastogenesis with E2 treatment, immunoblotting analysis showed that in control cells although FasL protein levels were already stimulated by day 1 and were strongly enhanced by day 3, they were strikingly attenuated by day 1 and were barely detectable by day 3 in the cells harboring up-regulated levels of miR-21 (Fig.2C). In addition, we measured cleaved caspase-3 protein levels because caspase-3 is activated in the apoptotic cells both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways [Budihardjo et al., 1999]. As shown in Fig.2D, the cleaved caspase-3 protein levels were remarkably suppressed by forced expression of miR-21 in osteoclasts compared to control cells in E2-treated culture. To further investigate our hypothesis, BMMs were isolated from the long bone tissues of miR-21 floxed mice. The cells were introduced by Cre-containing retroviruses and were induced into osteoclasts by M-CSF/RANKL stimulation with or without E2 treatment. Mir-21 expression levels were approximately 91.0 % inhibited in Cre expressing BMMs (data not shown) and TRAP-positive osteoclast numbers were significantly decreased in miR-21 down-regulated cells in E2 free culture (Fig.2E). Unlike the effects of miR-21 overexpression shown in Fig.2B, down-regulation of miR-21 levels failed to prevent the effect of estrogen in RANKL-induced osteoclastogenesis in culture with E2 treatment (Fig.2E). In fact, FasL protein levels and caspase-3 activity were significantly increased by day 3 in the cells harboring down-regulated levels of miR-21 with E2 treatment (Fig.2F and G), suggesting that the regulation of miR-21 expression levels by estrogen is closely related to the induction of osteoclastic apoptosis. In conclusion, we support the contention that osteoclast precursors and osteoclasts are direct targets of estrogen during RANKL-induced osteoclastogenesis and that the proapoptotic effect of estrogen is controlled by a posttranscriptional increase in FasL protein levels by down-regulated miR-21 biogenesis (Fig.3). Thus, an increase in miR-21 levels in osteoclasts may be associated with prolonged-osteoclastic survival that may causes osteopenia or osteoporosis in postmenopausal women.
Figure 2. Mir-21 overexpression rescues osteoclastic apoptosis induced by estrogen.
(A) MiR QRTPCR analysis of miR-21 expression in M-CSF-dependent BMMs. (B) TRAP-positive osteoclasts counted at 3 days after RANKL treatment, cultured in 24-well plates. Similar findings were obtained in 4 independent sets of experiments. (C and D) Immunoblotting analysis of FasL (C) and caspase-3/cleaved caspase-3 expression (D) in RANKL-induced osteoclastogenesis (C) and in osteoclasts (D) with E2 treatment. (E) TRAP-positive osteoclasts counted at 3 days after RANKL treatment, cultured in 24-well plates. Similar findings were obtained in 4 independent sets of experiments. (F) Immunoblotting analysis of FasL expression in osteoclasts with E2 treatment. (G) Caspase-3 activity assay in osteoclasts with E2 treatment.
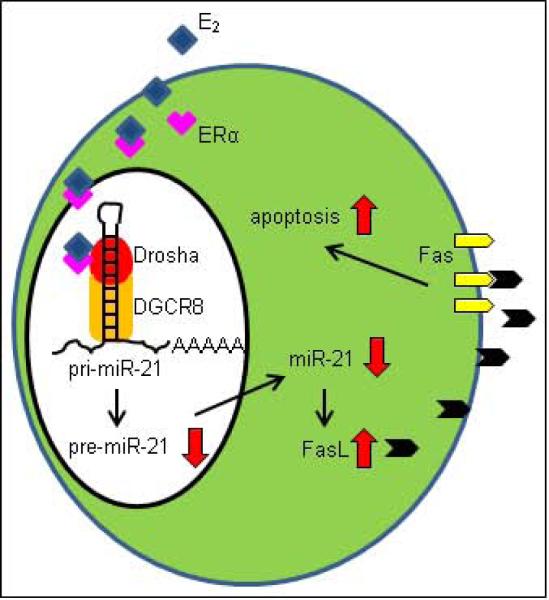
Figure 3. A model of a novel mechanism for controlling osteoclastic apoptosis by estrogen.
ERα pathway activated by estrogen inhibits miR-21 biogenesis so that enhanced-FasL proteins stimulate caspase-3 activity and that osteoclastic apoptosis is induced.
Acknowledgments
This work was supported by National Institutes of Health Grant DK070790 to Keith A. Hruska. We greatly appreciate the gift of miR-21 floxed and CAG-Z-miR-21-EGFP transgenic mice from Dr. Eric N. Olson (University of Texas Southwestern Medical Center, Dallas, TX). T.S. designed and performed the experiments, analyzed data, and contributed the primary draft of the manuscript. K.A.H. revised and produced the final manuscript.
References
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Fujiyama-Nakamura S, Yamagata K, Kato S. Hormonal repression of miRNA biosynthesis through a nuclear steroid hormone receptor. Adv Exp Med Biol. 2010;700:43–55. [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, Olson EN. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012 doi: 10.1016/j.tem.2012.03.008. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NFkappa B ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–40. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17:87S–91S. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem. 2009;284:4667–4678. doi: 10.1074/jbc.M805777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117:3648–3657. doi: 10.1182/blood-2010-10-311415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]