Abstract
Daily levels of physical activity vary greatly across individuals and are strongly influenced by genetic background. While moderate levels of physical activity are associated with improved physical and mental health, extremely high levels of physical activity are associated with behavioral disorders such as Attention Deficit Hyperactivity Disorder (ADHD). However, the genetic and neurobiological mechanisms relating hyperactivity to ADHD or other behavioral disorders remain unclear. Therefore, we conducted a selective breeding experiment for increased home cage activity starting with a highly genetically variable population of house mice and evaluated the line for correlated responses in other relevant phenotypes. Here we report results through Generation 10. Relative to the Control line, the High-Active line traveled approximately 4 times as far in the home cage (on days 5 and 6 of a 6-day test), displayed reduced body mass at maturity, reduced reproductive success, increased wheel running and open field behavior, decreased performance on the rotarod, decreased performance on the Morris water maze that was not rescued by acute administration of d-amphetamine, reduced hyperactivity from chronically administered low clinical doses of d-amphetamine, and increased numbers of new cells and neuronal activation of the dentate gyrus. Standardized phenotypic differences between the lines were compared to estimates expected from genetic drift to evaluate whether the line differences could have resulted from random effects as opposed to correlated responses to selection. Results indicated line differences in body mass and locomotor responses to low doses of amphetamine were more likely due to selection than drift. The efficacy of low doses of d-amphetamine in ameliorating hyperactivity support the High-Active line as a useful model for exploring the etiology of hyperactivity-associated comorbid behavioral disorders.
Keywords: ADHD, artificial selection, genetic drift, collaborative cross, home cage activity, heritability, amphetamine
Introduction
In human populations, individuals exhibit a wide variation in daily physical activity levels (e.g., distance traveled over the course of the day) (Trost et al., 2001). Significant deviations from healthy activity levels are associated with disease and behavioral disorders (Fox, 1999; Trost et al., 2001). Moreover, multiple studies show that levels of physical activity are heavily influenced by complex genetic factors that are inevitably entangled with other whole animal physiological and behavioral traits. For example, genetic predisposition for low levels of physical activity has been associated with increased adipose tissue mass (Choh et al., 2009; Demerath et al., 2013), while genetic predisposition for high levels of physical activity is associated with Attention Deficit Hyperactivity Disorder (ADHD) (Sharp et al., 2009; Elia et al., 2012). In the context of ADHD, hyperactivity refers to abnormally high levels of physical activity in the home or school environment, and difficulty remaining still for extended periods of time. The increasing prevalence of complex genetic disorders associated with hyperactivity, such as ADHD, necessitates further elucidation of the underlying genetic and neurobiological mechanisms which contribute specifically to increased physical activity and further how genetic-hyperactivity mechanisms influence other relevant traits such as inattention, impulsivity, or behavioral responses to psychostimulant drugs (McLoughlin et al., 2007; Froehlich et al., 2010; Greven et al., 2011; Thapar et al., 2013).
Previous research from our laboratory has successfully used a genetically variable starting population of Collaborative Cross G2:F1 (CC) mice (intercross of 8 divergent mouse strains; (Chesler et al., 2008) to found two lines of mice, High-Active and Control (Zombeck et al., 2011). The High-Active line is selectively bred each generation for high levels of home cage activity as measured by continuous video tracking over 6 days, while the Control line is bred randomly with respect to cage activity. Our previous paper indicated a successful response to selection in the High-Active lines across 5 generations (Zombeck et al., 2011). At Generation 5, on days 5 and 6 of a six day test, the Control line traveled an average of 0.35 km/day (± 0.026 SE), not statistically different from the level of activity in the starting population, whereas the High-Active line traveled an average of 1.0 km/day (± 0.076 SE), which was significantly different from the Control line and starting population. Further, the paper reported that selective breeding for home cage hyperactivity was not genetically correlated with other locomotor activity measures, including wheel running and open field behavior, suggesting specific genetic mechanisms for home cage activity. In a different study (Clark et al., 2011), we compared home cage activity in 9 standard inbred strains, 2 F1 hybrids, and one wild-derived mouse strain (CAST/EiJ) using the same 6-day video tracking procedure. Home cage activity ranged from approximately 0.1 km/day to 0.5 km/day among these strains. Hence, our High-Active line is hyperactive not only relative to our Control line, but also relative to all other mouse strains that have been measured in our laboratory, and twice as active as CAST/EiJ, the highest active strain we measured.
As our High-Active mice continue to demonstrate increased hyperactivity following selective breeding, we hypothesize that they may be useful as a model for genetic hyperactivity disorders, such as ADHD. In order to characterize these High-Active mice, we first wanted to establish that they continue to display hyperactivity, a clinical facet of common ADHD subtypes. Enhanced locomotor activity in the home cage was clearly demonstrated in High-Active mice through Generation 5 (Zombeck et al., 2011), and this paper provides an update through Generation 10. The model is also evaluated for predictive validity, in that these High-Active mice should demonstrate the appropriate behavioral responsiveness to therapeutic, chronic doses of d-amphetamine. Previous studies have demonstrated that mice selectively bred for increased physical activity, as indicated by levels of wheel running activity, exhibit differential responsivity to dopamine agonists and antagonists relative to control lines. Importantly, mice selectively bred for increased wheel running behavior diminished running levels following exposure to methylphenidate, while control lines increased wheel running following exposure to methylphenidate (Rhodes et al., 2001; Rhodes and Garland, 2003; Garland et al., 2011b). Therefore, we expected low doses of amphetamine to ameliorate hyperactivity in High-Active mice, whereas in the Control lines, we expected low doses of amphetamine to either have no effect or to stimulate activity (Gainetdinov, 2008; Napolitano et al., 2010).
Our amphetamine administration paradigm was based on previous research from our lab (Dabe et al., 2013), which reported that at these specific doses, and over the same extended period of administration, amphetamine enhances neurogenesis in the dentate gyrus of C57BL6/J mice without inducing activation of the region (as indicated by expression of the immediate early gene, c-fos). The C57BL/6J inbred strain is one of the 8 strains represented in the 8-way cross (CC) G2:F1 starting population. Therefore, an additional goal was to determine whether administration of these amphetamine doses would reduce hyperactivity while simultaneously enhancing the number of new cells in the dentate gyrus. Further, we wished to determine the acute effect of the amphetamine doses on a hippocampal dependent task, the Morris water maze test of spatial learning. Mice were exposed to either saline or amphetamine, and tested on the water maze precisely 15 minutes after injection. While some evidence suggests that high levels of physical activity-induced neurogenesis does not improve Morris water maze performance, other studies in less active strains exposed to running wheels suggest increased neurogenesis due to enhanced physical activity may be correlated with enhanced performance on the water maze (Rhodes et al., 2003; Clark et al., 2008). We hypothesize that the therapeutic doses of d-amphetamine could acutely improve deficient performance in High-Active lines (Brown et al., 2000).
Therefore, the purpose of the present paper is fourfold. The first goal is to characterize the progress of the selective breeding experiment to date and provide updated correlations with other physical activity, motor performance, and life history traits. Home cage activity levels collected during phenotyping are reported, in addition to a measure of reproductive success of the lines (number of successful pairs/generation) and body mass at the time of home cage activity phenotyping (approximately postnatal day 60). The second goal is to begin to characterize whether the model may be relevant for exploring etiology of genetic hyperactivity disorders, such as ADHD, by evaluating locomotor responses to psychostimulant administration, our prediction being that low doses would ameliorate hyperactivity. The third is to determine how chronic amphetamine affects neuronal activation and growth of new cells in the granule layer of the hippocampus of High-Active and Control lines, since previous studies from our lab indicated that chronic amphetamine increases adult hippocampal neurogenesis in C57BL/6J mice. The fourth aim is to characterize acute effects of amphetamine treatment on spatial learning in the water maze, a hippocampus-dependent task, in the High-Active versus Control lines.
MATERIALS AND METHODS
Animals
Mice from Generations 10 and 11 of a selective breeding experiment for increased distance traveled in the home cage were used (Zombeck et al., 2011). Our lab continues to maintain two lines of genetically variable (outbred) mice: a randomly bred, unselected (Control) line and a line selectively bred for increased distance traveled in the home cage (High-Active). Each line consists of 10–14 pairs of mice, and within-family selection is applied to minimize inbreeding. The starting population for each line was generated from a mix of 8 inbred strains that were specifically chosen to maximize genetic variation (collaborative cross mice G2:F1; Chesler et al. (2008). Each generation at weaning (21 days old), mice are housed in groups of 4 by sex. At approximately postnatal day 60, they are phenotyped individually for home cage activity. Mice are placed in custom-made acrylic home cages (18.5 cm x 33.5 cm x 16 cm) with clear plastic lids that allow continuous ceiling-mounted video tracking by Top Scan (Clever Systems, Reston, VA, USA). Each cage individually houses 4 mice, with an interaction zone constructed of wire mesh that allows for limited physical contact and interaction. The video coverage allows for continuous tracking of 64 individual mice over the span of 6 days. Well after an extended habituation period of 4 days, the average distance traveled during days 5 and 6 is used as the selection criterion. This criterion was chosen to represent behavioral patterns established in an acclimated, home cage environment, without any confounding effects of novelty. Moreover, this criterion of average activity over days 5 and 6 has been successfully implemented in other selective breeding experiments for heightened physical activity (Swallow et al., 1998).
General Husbandry
Rooms are kept controlled for temperature (21 ± 1°C) and photo-period (12:12 L:D; lights on at 10:00 PM and off at 10:00 AM). Food (Harlan Teklad, 7012) and water are provided ad libitum at all times. Corncob bedding (7097 Harlan Teklad, Madison, Wisconsin, USA) was provided in all cages. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines.
Experiment 1: Genetic differences in wheel running and distance traveled in the open field between High-Active and Control lines in Generation 10
High-Active mice (n=10 males and n=10 females) and Control mice (n=9 males and n=10 females) were randomly chosen from Generation 10 after excluding breeders for Generation 11. The mice were chosen from 10 different families per line, i.e., 1 male and 1 female was randomly selected per family. Mice were tested in the following order, first home cage activity as routinely performed starting at age 60 (± 2 days), then left undisturbed for 20 days, followed by 2 days of open field, 5 days of wheel running, and 3 days of rotarod testing.
Open Field
Testing occurred at approximately 11:00 AM during the animal’s normal dark cycle under diffuse white light. Mice were individually placed in a 67 cm x 67 cm x 30 cm (L x W x H) open field box for 5 minutes. The procedure was repeated the following day, and the distance traveled was averaged between the two days. TopScan was used to track distance traveled.
Wheel running
Mice were placed individually into cages with a running wheel for 5 days. Dimensions of running wheel cages were 36 cm × 20 cm × 14 cm (L × W × H) with a 23 cm diameter wheel mounted in the cage top (Respironics, Bend, OR). Wheel rotations were monitored continuously in 1 min increments.
Rotarod
Testing occurred at approximately 11:00 AM under red light during the animal’s normal dark cycle. Mice were placed on a stationary rotarod (AccuRotor Rota Rod Tall Unit, 63-cm fall height, 30-mm diameter rotating dowel; Accuscan, Columbus, OH). The dowel was then accelerated at 60 rpm/min, and the latency to fall (in seconds) was recorded. The procedure was repeated for 4 consecutive trials, which were averaged to give the daily latency to fall for each mouse. If an animal fell off the rotarod rapidly (e.g., due to inattention or slips), they were placed back on the rotarod for an additional trial, and the latency was not included in the average for the day. The entire procedure was repeated the following 2 days for a total of 3 days. In addition to the average latency across the 4 trials per day, the maximum latency to fall per day was also analyzed.
Experiment 2: Genetic differences in amphetamine-induced locomotor activity and hippocampal plasticity between High-Active and Control lines
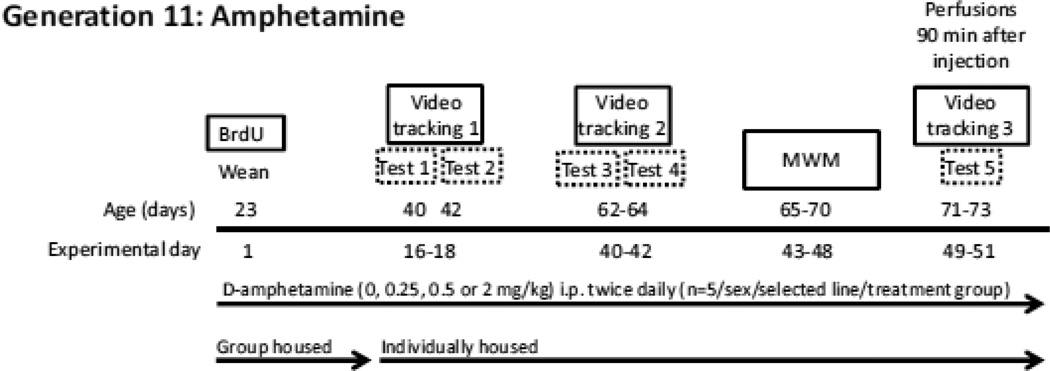
Mice from Generation 11 were used [n= 5 per sex (Male or Female)/line (High-Active or Control)/treatment (saline, 0.25, 0.5, or 2 mg/kg d-amphetamine) = 80 total]. Generation 10 breeders were allowed to produce an additional litter for continuing the lines (i.e., for selecting Generation 11 breeders). The litters used for this experiment were all born within 4 days of each other, which allowed all animals to be measured at the same time and at a similar age (Fig. 1). Mice from within a family were distributed across the 4 treatment groups to prevent possible family effects from confounding treatment differences. Mice were weaned into cages in groups of 4 until they were approximately 40 days old and underwent video tracking session 1, after which they were individually housed for the duration of the experiment.
Figure 1. Time-course for Experiment 2.
Animals were administered twice daily i.p. injections of saline, 0.25, 0.5, or 2 mg/kg d-amphetamine (n= 5 per sex/line/treatment = 80 total) for 49–51 days, starting from when the mice were 23 days old (Experimental day 1) and continuing until they were 73 days old (Experimental day 51). Mice also received 10 daily i.p. injections of BrdU (50 mg/kg) to label dividing cells on experimental days 1–10 (mouse age 23–32 days old). On Experimental days 16–18 and 40–42, 64 mice/80 total were placed in specialized cages which allowed for video tracking of home cage locomotor behavior (Tests 1–4). Prior to the first locomotor activity session, mice were separated from group housing conditions and individually housed to allow for individual locomotor tracking. Mice then remained singly housed throughout the duration of the experiment. On Experimental days 43–48, 40 mice/80 total were tested on the Morris water maze (MWM). On Experimental days 49–51, the same 64 mice were placed in the specialized home cages to allow for video tracking one final time (Test 5), then staggered over the course of the next two days for systemic perfusions with 4% paraformaldehyde exactly 90 min. following the final injection for each mouse.
Amphetamine injections
Beginning the second day after weaning at an age of 23 days old, all mice received twice daily intraperitoneal (i.p.) injections of saline, 0.25 mg/kg d-amphetamine, 0.5 mg/kg d-amphetamine, or 2 mg/kg d-amphetamine, approximately 3 hours apart (at 10:00 AM and 1:00 PM). These twice daily treatment injections continued throughout the entirety of the experiment until sacrifice, including all days of behavioral testing.
BrdU injections
Throughout the first 10 days, when mice were 23 through 32 days old, they received daily i.p. injections of 50 mg/kg 5’bromo-5’-deoxyuridine (BrdU) to label dividing cells. These injections occurred at approximately 12:00 PM.
Locomotor activity
A random sample of 64 mice (n=4/sex/line/treatment) of the 80 total were used to measure distance traveled in the home cage in response to d-amphetamine administration. The sample size of 64 was chosen because that is the limit for simultaneous video tracking in our laboratory. When the mice were 40–42 days old (video tracking session 1), 62–64 days old (video tracking session 2), and between 71–73 days old (video tracking session 3), they were placed individually in the custom cages with the clear lids for continuous 24 hour video tracking. The d-amphetamine/saline injections continued throughout the video tracking sessions. While TopScan continued to record activity for a 24 hour period, the analysis of locomotor response to amphetamine was restricted to only 90 minutes following the injection because after 90 min, brain concentrations of amphetamine would be expected to wane (Zombeck et al., 2010). In addition, locomotor responses to amphetamine at the doses that were given are known to last for approximately 90 min in mice (Dabe et al., 2013). Data are shown only for the second and third day of video tracking sessions 1 and 2, to allow for one day of habituation which was not analyzed. Locomotor response in video tracking session 3 represents only the 90 minutes between an animal’s final injection and sacrifice, after 1 to 2 days of habituation.
Morris Water Maze
A sample of 40 mice of the 80 total was tested on the Morris water maze. This sample included randomly selected: n=5 High-Active mice per amphetamine dose (3 males and 2 females), n=5 Control per dose (2 males and 3 females) times 4 doses equals 40 animals. Water maze training occurred exactly 15 minutes after their daily afternoon injection of amphetamine. The sample of 40 mice rather than all 80 were tested for feasibility and to keep the timing between amphetamine injections relatively constant. The maze consisted of a circular tub, 70 cm diameter and 30 cm deep. Extra-maze cues of varying geometric patterns, colors, and numbers were placed on the walls around the tub in order to provide spatial markers of location for the mice. A platform, made of a white plastic mesh measuring 8.5 cm x 8.5 cm, was placed in the middle of one maze quadrant and submerged 1 cm below the surface of the water. Crayola white tempera paint was added to make the water sufficiently opaque, thereby hiding the platform from sight. The whitened water provided contrast against the dark mice, so TopScan could clearly track the animal as it navigated the maze. Water temperature was maintained at 21–23°C. A trial lasted either 60 seconds or until the mouse reached the platform and remained on the platform for 10 seconds. If a mouse did not reach the platform in 60 seconds, it was gently guided there by hand. Mice were placed back in their cage and allowed to rest for approximately 10 minutes between trials. 24 hours after training Day 5, the platform was removed and mice were tested with a probe trial lasting 60 seconds. TopScan video tracking software was used to measure path length (mm) to platform, and time (seconds) spent in the target quadrant of the maze during probe trial.
Immunohistochemistry
Following Clark et al. (2008), animals were anesthetized with 100 mg/kg sodium pentobarbital via intraperitoneal injection, and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS). Brains were sectioned using a cryostat into 40 µm coronal sections and stored in tissue cryoprotectant at −20°C.
Fos-DAB
Following previous protocols from our lab (Zombeck et al., 2008),a 1-in-6 series of free-floating sections were pretreated with 0.6% hydrogen peroxide in PBS for 20 min at room temperature, then washed in PBS containing 0.2% Triton X-100 (PBS-X) and blocked with 6% goat serum for 1 h at room temperature. Sections were then incubated in monoclonal primary antibody against mouse Fos made in rabbit (Calbiochem, San Diego, CA) at a dilution of 1:20,000 in PBS-X containing 3% NGS for 48 h at 4 °C. Sections were subsequently washed in PBS-X and incubated in secondary biotinylated antibody against rabbit immunoglobulin made in goat (Vector Labs, Burlingame, CA) at a dilution of 1:200 in PBS-X with 3% NGS for 90 minutes at room temperature. Sections were then treated using the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine (DAB), enhanced with 0.008% nickel chloride (Sigma, St. Louis, MO).
BrdU-DAB
A 1-in-6 series of these sections (i.e., series of sections throughout the extent of the hippocampus with 240 µm increments separating each section) was stained to visualize BrdU to detect newly divided cells in the dentate gyrus. Free-floating sections were washed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide. To denature DNA, sections were treated with 50% deionized formamide, 10% 20xSSC buffer, 2N hydrochloric acid, and 0.1M boric acid. Sections were then treated with a solution of 0.1% Triton-X and 3% goat serum in TBS (TBS-X plus), and then incubated in primary antibody against BrdU made in rat (Accurate, Westbury, NY, USA) at a dilution of 1:200 in TBS-X plus for 72 h at 4°C. Sections were then washed in TBS, treated with TBS-X plus for 30 min, and then incubated in secondary antibody against rat made in goat at 1:250 in TBS-X plus for 100 min at room temperature. Sections were treated using the Vectastain Elite ABC Kit with DAB, enhanced with 0.008% nickel chloride.
Image Analysis
Fos-DAB and BrdU-DAB
The entire bilateral granule layer, represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope, and taking multiple photographs, via AxioCam interfaced to computer, under 10x (total 100x) magnification. These photographs were then analyzed using ImageJ software (NIH, Bethesda, MD, USA) to generate unbiased estimates of total number of BrdU- and Fos-positive cells. Specifically, in each image, the granule layer was traced and BrdU- or Fos-positive nuclei were counted within the traced region automatically by setting a fixed threshold to remove background. Following previous protocols from our lab (Clark et al., 2011), to obtain unbiased estimates of cell numbers, total counts were multiplied by 0.85, under the assumption that 15% of the nuclei counted would intersect with the plane of the section. This was estimated based on the observation that the average size of granule neuron nuclei was 6 µm, which is 15% of 40 µm, the thickness of the section.
Statistical Analysis
Data were analyzed with SAS (version 9.2) and R (3.0.2) statistical software. In all analyses, P≤0.05 was considered to be statistically significant. Two different statistical tests were performed to compare phenotypic differences between the lines. The first was a simple test using standard analysis of variance (ANOVA) methods and the Fisher exact test for the reproductive success count data. Home cage locomotor activity and body mass were analyzed per generation by 2-way ANOVA, with sex and line (High-Active and Control) as factors. To compare reproductive success between the lines, the total number of successful first litters out of the total number of pairs was compared between High-Active and Control lines using a Fisher exact test. Wheel running and Rotarod data were analyzed using a repeated-measure ANOVA, with line and sex as between-subjects factors and day as the within-subjects factor. Data were log transformed to improve homogeneity of variance between groups for Home cage activity and Rotarod. In Experiment 2, cumulative distance traveled 1.5 h after amphetamine or saline injections was analyzed by repeated-measures ANOVA with dose (saline, 0.25, 0.5, 2 mg/kg amphetamine), sex, and line as the between-subjects factors, and test day (Test 1–5) as the within-subjects factor. Two animals were removed (one from the female/Control/saline group, and the other from the female/High-Active/2 mg/kg amphetamine group) due to erroneous video tracking. The locomotor activity data were also analyzed collapsed across test day (i.e., the average over the 5 tests), which is presented in Table 2. In addition, the collapsed data from the amphetamine groups were expressed as a difference relative to the saline-treated controls (by line and sex), and analyzed a similar way with line, sex, and dose (3 levels, 0.25, 0.5 and 2 mg/kg) as factors. Distance to the hidden platform in the water maze was analyzed by repeated measures ANOVA with dose and line as the between-subjects factors, and day (1–5) as the within-subjects factor. Distance to the platform on days 2 and 3 were collapsed and further analyzed by post-hoc Tukey tests. Duration spent in the target quadrant for the probe trial was analyzed by 2-way ANOVA with dose and line as factors. To establish whether the average duration in the target quadrant was greater than expected by chance, the average was compared to 25% (for the 4 quadrants) using a t-test. Total numbers of BrdU+ and c-Fos+ cells were analyzed by ANOVAs using dose, sex, and line as factors; c-Fos data were log transformed in this analysis to improve homogeneity of variance. Pair-wise Pearson’s correlations were evaluated between distance traveled, total number of c-Fos cells, and number of new neurons. Total numbers of BrdU+ cells and c-Fos+ cells were also analyzed using a linear model that included distance traveled, line, dose, and sex in the model. Statistical power was estimated for the ANOVA analyses by simulating 10,000 data sets using the observed means and variances, and calculating the percentage of these data sets displaying statistically significant results for the main and interaction effects. For data sets with repeated measures, the measures were averaged to obtain one measurement for statistical power analysis.
Table II.
The magnitude and statistical significance of between-line differences in secondary traits expressed in standardized phenotypic SD units (Dy) and compared to 95% confidence intervals (95% CI) for Dy as computed from Equation 1 in the text
| Secondary Trait | Figure | Sex | High-Active line | Control line | p-value | h2 | F | n | 95% CI | Dy | h2Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body mass (g)a | 2C | Male | 23.48 (±0.44) | 27.53 (±0.87) | <0.0001 | 0.37 | 0.06217 | 24 | 1.00 | −1.08 |
Falconer, 1973 (Table 5) |
|
| Female | 18.58 (±0.29) | 22.32 (±0.77) | <0.0001 | 0.37 | 0.06217 | 24 | 1.00 | −1.35 | ||||
| Gen 10 Cohort Home Cage Activity (km/day) |
3A | Male | 1.10 (±0.20) | 0.31 (±0.05) | 0.002 | 0.33 | 0.06217 | 24 | 0.98 | 1.68 |
Zombeck et al., 2011 (Table 1) |
|
| Female | 1.83 (±0.28) | 0.34 (±0.05) | <0.0001 | 0.33 | 0.06217 | 24 | 0.98 | 2.35 | ||||
| Wheel running (km/day) | 3B | Male | 8.34 (±0.73) | 4.90 (±0.62) | 0.002 | 0.39 | 0.06217 | 24 | 1.01 | 0.57 |
Zombeck et al., 2011 (Figure 6C) |
|
| Female | 9.79 (±1.40) | 9.30 (±0.83) | NS | 0.39 | 0.06217 | 24 | 1.01 | 0.14 | ||||
| Open Field (m) | 3C | Male | 45.88 (±4.41) | 27.14 (±2.72) | 0.003 | 0.13 | 0.06217 | 24 | 0.87 | 0.55 | DeFries et al., 1970 | |
| Female | 37.65 (±2.60) | 29.12 (±4.01) | NS | 0.13 | 0.06217 | 24 | 0.87 | 0.80 | ||||
| Rotarod (s)b | 3D | Male | 49.06 (±4.09) | 50.54 (±3.55) | NS | 0.44 | 0.06217 | 24 | 1.03 | −0.04 |
Rustay et al., 2003 Belknap et al., 1993 |
|
| 3E | Female | 39.28 (±3.83) | 58.56 (±8.95) | 0.026 | 0.44 | 0.06217 | 24 | 1.03 | −0.89 | |||
| Response to 0.25 mg/kg Amphetamine (m/90 min)c Collapsed (test) |
4A+B | Male | −43.42 (±22.74) | 61.82 (±26.29) | 0.023 | 0.21 | 0.06217 | 22 | 0.95 | −2.18 | Alexander et al., 1996 | |
| 4C+D | Female | −84.55 (±31.02) | 27.84 (±17.47) | 0.02 | 0.21 | 0.06217 | 22 | 0.95 | −2.23 | |||
| Response to 0.5 mg/kg Amphetamine (m/90 min)c Collapsed (test) |
4A+B | Male | −1.20 (±28.57) | 16.24 (±7.50) | NS | 0.21 | 0.06217 | 22 | 0.95 | −1.64 | Alexander et al., 1996 | |
| 4C+D | Female | −53.78 (±17.46) | 27.42 (±8.89) | 0.006 | 0.21 | 0.06217 | 22 | 0.95 | −2.93 | |||
| Response to 2 mg/kg Amphetamine (m/90 min)c Collapsed (test) |
4A+B | Male | 70.43 (±41.36) | 64.94 (±21.31) | NS | 0.21 | 0.06217 | 22 | 0.95 | 0.09 | Alexander et al., 1996 | |
| 4C+D | Female | 58.13 (±58.16) | 59.33 (±17.73) | NS | 0.21 | 0.06217 | 22 | 0.95 | −0.02 | |||
| Morris water maze latency (m) |
6 |
Galsworthy et al., 2005 (Table 4) |
||||||||||
| Day 1 | Collapsed (sex, dose) |
10.64 (±0.64) | 11.74 (±1.05) | NS | 0.24 | 0.06217 | 0.96 | −0.28 | ||||
| Day 2 | Collapsed (sex, dose) |
9.36 (±0.92) | 5.09 (±0.63) | 0.001 | 0.24 | 0.06217 | 22 | 0.96 | 1.21 | |||
| Day 3 | Collapsed (sex, dose) |
6.21 (±0.92) | 3.50 (±0.53) | 0.015 | 0.24 | 0.06217 | 22 | 0.96 | 0.80 | |||
| Day 4 | Collapsed (sex, dose) |
5.48 (±0.81) | 4.26 (±0.71) | NS | 0.24 | 0.06217 | 22 | 0.96 | 0.36 | |||
| Day 5 | Collapsed (sex, dose) |
3.97 (±0.84) | 3.50 (±0.52) | NS | 0.24 | 0.06217 | 22 | 0.96 | 0.15 | |||
| Total number of new cells in dentate gyrus (# BrdU+ cells)d |
7B | Male | 5313.02 (±421.51) | 4173.35 (±588.72) | NS | 0.53 | 0.06217 | 22 | 1.10 | 0.27 | Clark et al., 2011 | |
| Female | 7592.58 (±758.05) | 5787.87 (±684.44) | 0.045 | 0.53 | 0.06127 | 22 | 1.10 | 0.59 | ||||
Body Mass refers to Generation 10.
Rotarod refers to average latency of the 4 trials per day over three days.
Locomotor response to amphetamine expressed as a difference score relative to the saline group of each sex and line.
Total number of new cells in dentate gyrus refers to average total number of BrdU cells in the granule layer of the dentate gyrus collapsed across dose.
Standard errors are shown in parentheses next to means for the secondary phenotypic traits under “High-Active line” and “Control line”. “P-value” refers to a pair-wise comparison of the High-Active line versus the Control line. “F” refers to the inbreeding coefficient estimated from the entire pedigree. “n” refers to the number of families represented for the specific phenotypic trait that was analyzed. “95% CI” refers to the 95% confidence interval for Dy expected by genetic drift as calculated from equation 1 in the text. “Dy” refers to the difference between lines in standardized phenotypic units.
Dy values larger than the 95% CI are shown in bold and provide evidence that the secondary trait has evolved as a correlated response to selection for home cage activity.
The second analysis was conducted to determine whether the observed phenotypic differences between the two lines (High-Active versus Control) were likely correlated responses to selection for home cage activity (e.g., pleiotropy or linkage disequilibrium) as opposed to a result of genetic drift or founder effects unrelated to selection. We acknowledge that it is preferable to include replicate selected and control lines to establish evidence for genetic correlations between primary traits under selection and secondary traits. However, in absence of replication, we followed the guidelines suggested previously (Henderson, 1989, 1997; Konarzewski et al., 2005). First, we calculated standardized estimates of phenotypic differences between the lines (Dy), as mean value of the trait in High-Active minus mean value of Control divided by the pooled estimate of the standard deviation of the trait within lines. We then used the following equation (Equation 1) to estimate the standard deviation for Dy as expected from genetic drift (σ(dy)):
| Equation 1 |
Here h2 refers to heritability and was estimated from the literature, F refers to the inbreeding coefficient which was estimated in our lines by entering the pedigree from Generations 1–10 in ASReml-R version 2.0 following Konarzewski et al. (2005). “n” refers to the number of families represented for the specific phenotypic trait that was analyzed.
The 95% confidence interval for Dy was then calculated by multiplying σ(dy) by 1.96 (from standard normal distribution). If the absolute value of Dy was greater than the 95% confidence interval value, then Dy was considered unlikely due to drift and more likely a correlated response to selection.
RESULTS
Response to selection, reproductive success, and body mass
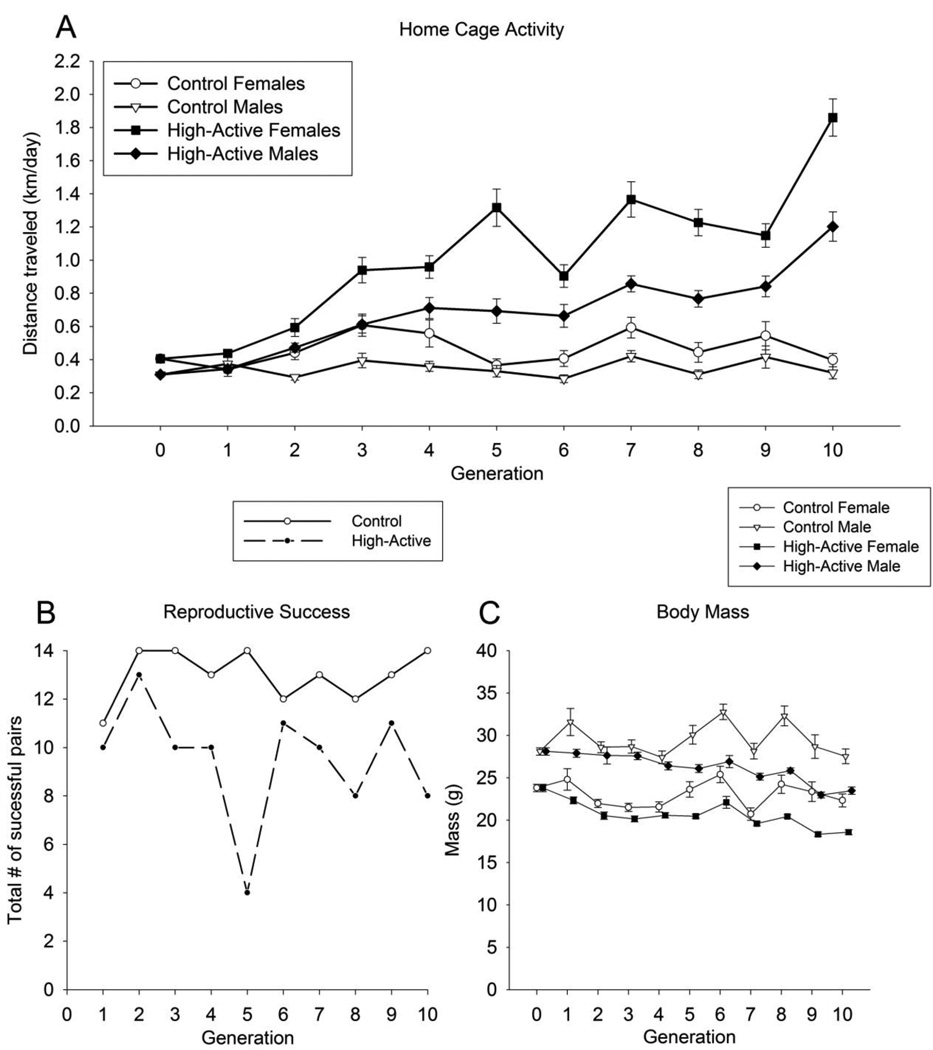
The High-Active line of mice continues to respond to selection through 10 generations with some exceptions at Generations 6 and 9 (Fig. 2A). No clear indication of a selection limit has been reached. Selection differentials (S), realized heritability estimates (h2realized) and responses to selection (R) for Generations 1–9 are shown in Table 1. At Generation 10, on days 5 and 6 of the six day test, the High-Active mice traveled an average of 1.51 ± 0.08 SE km/day whereas mice from the Control line traveled 0.36 ± 0.03 SE km/day (F1,171 = 101.5, P < 0.0001) in their cages, which is approximately a 4-fold increase. In the High-Active line, males traveled approximately 65% as far than females (F1,125 = 21.7, P < 0.0001). In the Control line, the sex difference was smaller and not significant, resulting in a significant interaction between sex and line (F1,171 = 6.2, P = 0.0135).
Figure 2. Home cage activity, reproductive success and body mass across generations.
A) Data points represent the population means (±SE) from each generation for home cage activity (distance traveled on days 5 and 6 in km/day). Males and females are plotted separately. B) Every generation, 14 pairs of High-Active mice and 14 pairs of Control mice were paired to produce the next generation. The total number of successful pairings from each line for generation are represented here (14 max). C) Average body mass (±SE) at the time of home cage phenotyping, PND 60–66. Males and females are plotted separately.
Table I.
Realized heritability (h2realized) estimates of home cage activity
| Generation | S (km/day) | R (km/day) | h2realized |
|---|---|---|---|
| 1 | 0.27 | 0.12 | 0.44 |
| 2 | 0.36 | 0.25 | 0.69 |
| 3 | 0.87 | 0.051 | 0.06 |
| 4 | 0.56 | 0.22 | 0.39 |
| 5 | 0.43 | 0a | 0 |
| 6 | 0.14 | 0.31 | 1b |
| 7 | 0.82 | 0a | 0 |
| 8 | 0.64 | 0.025 | 0.04 |
| 9 | 0.53 | 0.5 | 0.94 |
| Avg. | 0.51 | 0.16 | 0.40 |
The actual value was negative
The actual value was above 1.0
S = selection differential; R = response to selection
The High-Active line displayed significantly reduced fertility relative to the Control line as indicated by fewer successful pairs out of the 140 total (14 pairs per generation x 10 generations) that contributed progeny to the 2 lineages over the 10 generations (Fig. 2B). In the Control line, 130 pairs out of 140 total pairs successfully contributed offspring to the subsequent generation, while in the High-Active line only 95 pairs out of 140 successfully contributed (Fisher Exact test, P < 0.0001). The reason pairs failed to produce offspring included failure of the female to become pregnant, cannibalization of the pups, or death from poor mothering.
The High-Active mice display significantly reduced body mass as compared to Controls at the time of phenotyping (age approximately 60 days) (Fig. 2C). The difference was apparent starting at Generation 5 and continues through Generation 10. At Generation 10, High-Active mice were approximately 84% as heavy as Controls (F1,171 = 48.3, P < 0.0001) and Females were 80% as heavy as males (F1,171 = 81.2, P < 0.0001). No interaction between line and sex was detected. The difference in body mass between the lines in Generation 10 was more likely a correlated response to selection than a consequence of genetic drift (Table 2).
Experiment 1: Genetic differences in wheel running and distance traveled in the open field between High-Active and Control lines in Generation 10
Home Cage Activity
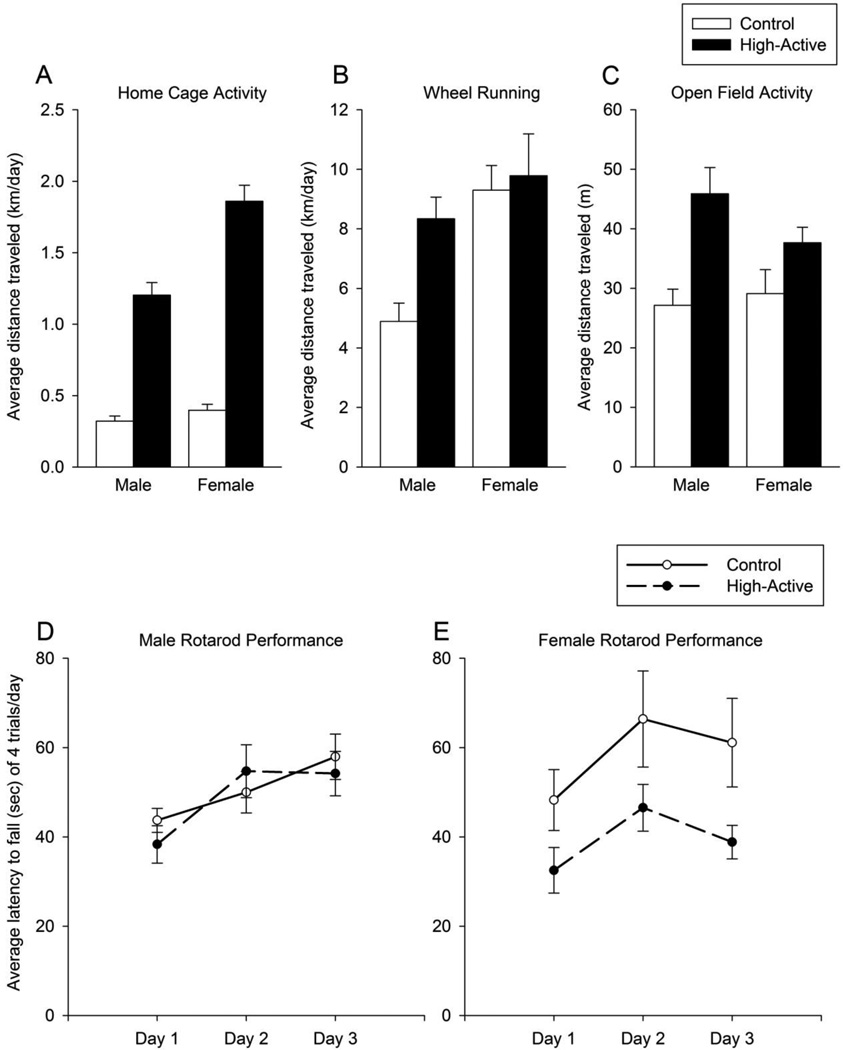
A sample of animals from Generation 10 (n=39 total) were used to examine wheel running and open field behavior in High-Active and Control Lines. Distance traveled in the home cage on days 5 and 6 during the routine screening in this sample, ranged from 0.153 km/day (Control male) up to 3.53 km/day (High-Active female). Within this sample, a significant effect of line (F1,35 = 40.3, P < 0.0001) and sex (F1,35 = 4.5, P = 0.041) was observed. No interaction between line and sex was detected (Fig. 3A).
Figure 3. Locomotor activity and motor performance in the High-Active and Control lines from Generation 10.
Males and females are plotted separately (n=10 High-Active males, n= 9 Control males, n= 10 High-Active females, n=10 Control females). A) Average distance traveled in the home cage (±SE) on days 5 and 6 (km/day) for the same animals shown in B-E. B) Average distance traveled on a running wheel (±SE) over 5 days (km/day). C) Average distance traveled in the open field (±SE), across 2 daily 5-minute open field tests (m/5 min). D and E) Average latency (±SE) to fall off the accelerating rotarod (sec) averaged across 4 sessions per day.
Wheel Running
Average distance traveled across a 5 day test was 7.2 km/day (± 0.73 S.E.) for Control mice, and 9.06 km/day (± 0.79 S.E.) for High-Active mice. Wheel running behavior showed main effects of line (F1,33 = 4.4, P = 0.044) and sex (F1,33 = 9.7, P = 0.0038), and a trend for an interaction between line and sex (F1,33 =2.5, P = 0.12; Fig. 3B). Statistical power was estimated to be 26% for detecting the interaction. The difference in wheel running between the lines is within the range expected by genetic drift and therefore we cannot conclude that it is necessarily a correlated response to selection (Table 2).
Open Field
Average distance traveled in the open field over 5 minutes was significantly greater in the High-Active line as compared to Control (F1,35 = 14.6, P = 0.0005; Fig. 3C). No main effect of sex was detected, but a trend for an interaction between sex and line was observed (F1,35 = 2.0, P = 0.16). Statistical power was estimated to be 29% for detecting the interaction. The difference in open field activity between the lines is within the range expected by genetic drift and therefore we cannot conclude that it is necessarily a correlated response to selection (Table 2).
Rotarod
All mice learned the rotarod task as indicated by increased latency to fall from the rotating beam from Day 1 to Days 2 and 3 (for average latency across 4 trials per day, F2,70 = 21.4, P < 0.0001; for max latency across 4 trials, F2,70 = 12.1, P < 0.0001). High-Active mice performed significantly worse on the rotarod as indicated by reduced latency to fall across days (for average latency, F1,35 = 5.0, P = 0.032; for maximum latency, F1,35 = 5.2, P = 0.029). No significant effect of sex was detected. However, a trend for an interaction between sex and line was observed (for average latency, F1,35 = 2.7, P = 0.10; for max latency, F1,35 = 3.9, P = 0.056; see Fig. 3D and E). Statistical power to detect the interaction was estimated to be 31% for average latency to fall collapsed across the 3 days. The difference in rotarod performance between the lines is within the range expected by genetic drift and therefore we cannot conclude that it is necessarily a correlated response to selection (Table 2).
Experiment 2: Genetic differences in amphetamine-induced locomotor activity and hippocampal plasticity between High-Active and Control lines
Locomotor Activity (Fig. 4 A-D, Fig. 5)
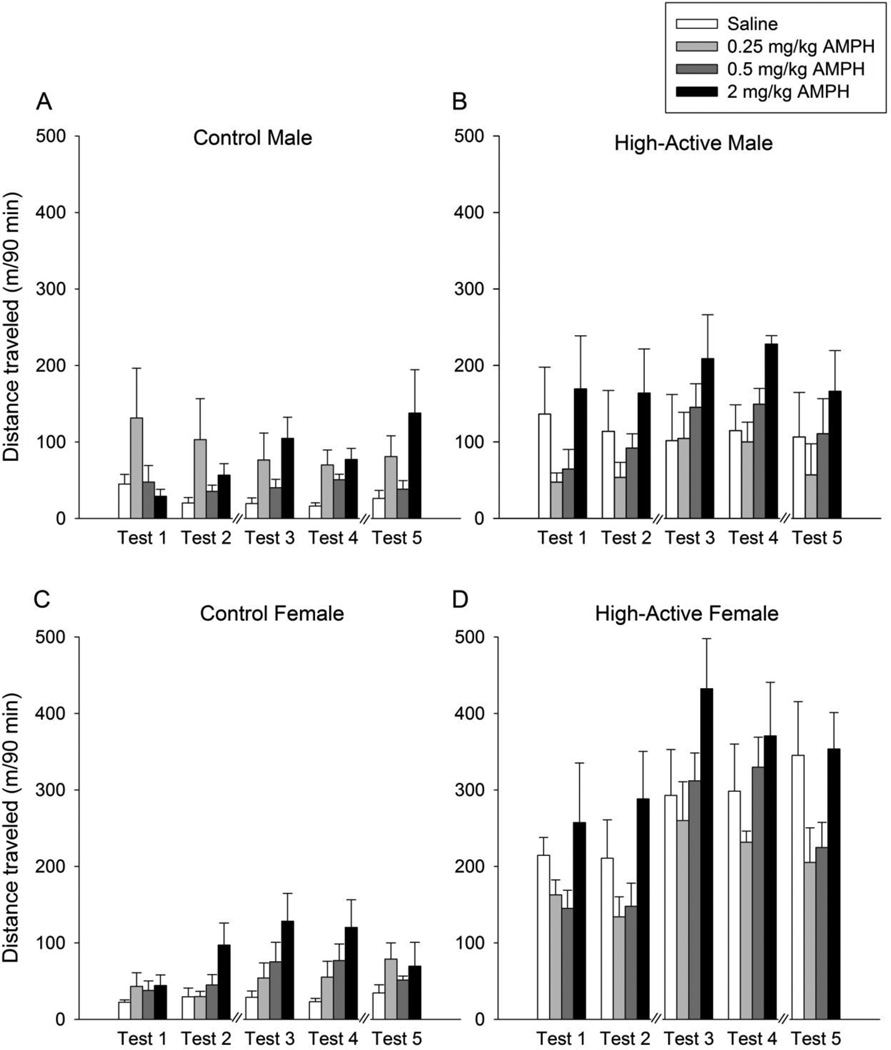
Figure 4. Locomotor activity following saline or amphetamine administration.
A) Average (±SE) distance traveled in the home cage by Control males within a 90 min period following an i.p. injection of saline, 0.25. 0.5, or 2 mg/kg d-amphetamine across 5 separate test days (Tests 1 and 2 occurred on postnatal days 41 and 42; Tests 3 and 4 occurred on postnatal days 63 and 64; Test 5 occurred between postnatal days 71 and 73 just prior to sacrifice). Each test day is an average of morning and afternoon injections. B), C), and D) Same as A) for cohorts of High-Active males, Control females, and High-Active females, respectively. The same 64 mice underwent video tracking on all Tests 1–5 (n=4/sex/line/treatment = 64 total).
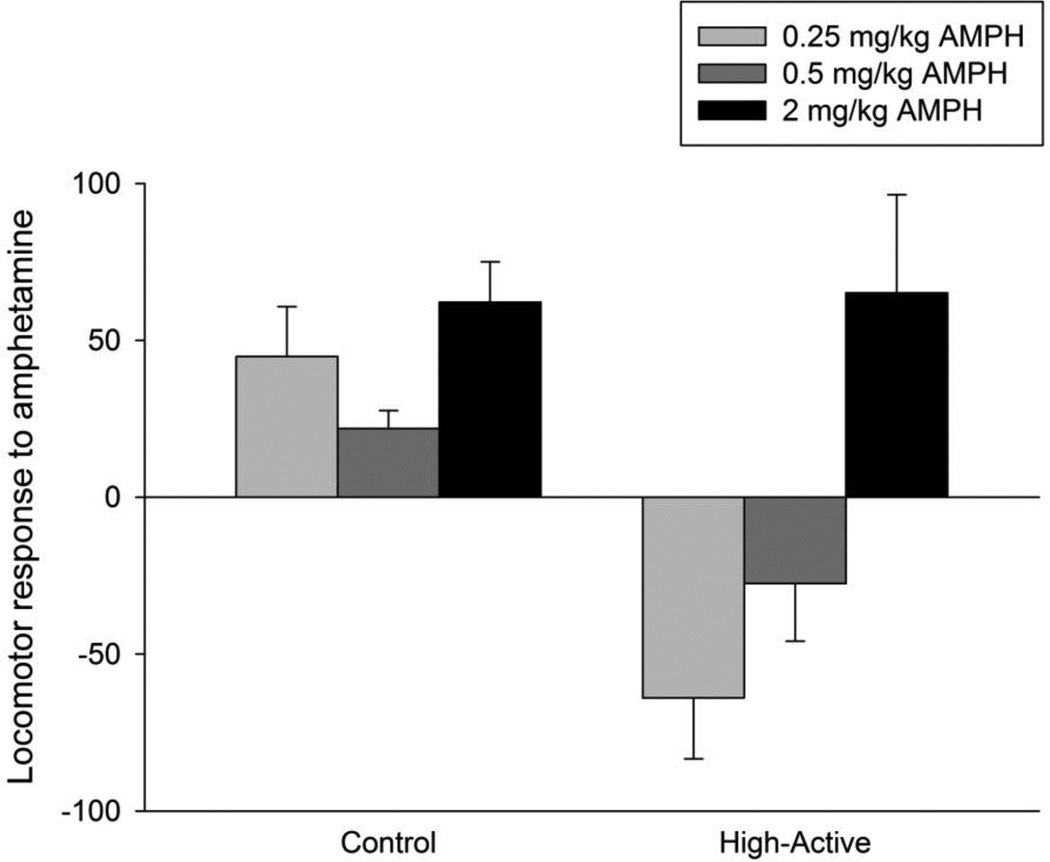
Figure 5. Locomotor response to amphetamine.
Average (±SE) locomotor response to 0.25, 0.5, and 2 mg/kg amphetamine in the Control and High-Active lines. Response was calculated for each individual receiving amphetamine as the distance traveled 90 minutes after administration of amphetamine minus the average distance traveled by the saline group from the same sex and line. Hence negative values indicate a suppression of locomotor activity from amphetamine whereas positive values indicate stimulation. Results are shown collapsed across sex (n=8/line/treatment = 64 total).
The overall ANOVA of distance traveled within the 90 min period after an injection of saline, 0.25, 0.5, or 2 mg/kg amphetamine in High-Active versus Control mice over the 5 testing days indicated a significant effect of day (F4,183 = 14.4, P<0.0001), line (F1,46 = 85.7, P<0.0001), dose (F3,46 = 5.4, P=0.003), sex (F1,46 = 22.8, P<0.0001), line*dose (F3,46 = 3.2, P=0.03), line*day (F4,183 = 7.3, P<0.0001), dose*day (F12,183 = 1.9, P=0.04), sex*day (F4,183 = 5.2, P=0.0005), line*sex (F1,46 = 24.4, P<0.0001), and line*sex*day (F4,183 = 2.4, P<0.05). Figure 4A-D shows the data separately by sex, line, day and dose.
When the locomotor data were analyzed collapsed across the test days, results showed a significant effect of line (F1,46 = 83.2, P<0.0001), dose (F3,46 = 5.3, P=003), sex (F1,46 = 23.5, P<0.0001), line*sex (F1,46 = 26.0, P<0.0001), and line*dose (F3,46 = 3.3, P=0.03) (Table 2). When the locomotor activity data for the amphetamine groups were expressed as a difference score relative to their respective saline controls (i.e., within each sex and line), results of the overall ANOVA showed a significant effect of line (F1,25 = 11.7, P=0.0016), dose (F2,35 = 9.1, P=0.0007), and line*dose (F2,35 = 4.4, P=0.02; Fig. 5). No other main effects or interactions were significant. The interaction between line and dose in these analyses is due to the low dose of amphetamine reducing locomotor activity in the High-Active line while increasing activity in the Control line (Fig. 5). This was indicated by a significant post-hoc Tukey test comparing the response to amphetamine at 0.25 mg/kg between Control and High-Active mice (P=0.0024). Tukey tests between High-Active and Control mice for 0.5 mg/kg and 2 mg/kg were not significant. The differential responses to low, clinically relevant doses of d-amphetamine between the lines in both sexes are likely correlated responses to selection as opposed to consequences of genetic drift (Table 2).
Morris Water Maze
Acquisition (Fig. 6 A-D)
Figure 6. Morris water maze acquisition in response to amphetamine.
A) Average (±SE) path length (m) to reach the submerged, hidden platform on each day, 15 minutes after receiving an afternoon i.p. injection of saline (n=3 High-Active males, n=3 Control females, n=2 Control males, n=2 High-Active females; n=10 total). B), C), and D), Same as A) except for the administration of 0.25 mg/kg d-amphetamine, 0.5 mg/kg d-amphetamine, or 2 mg/kg d-amphetamine, respectively.
On average, animals learned the Morris water maze as indicated by decreased path length with day (F4,128 = 34.7, P < 0.0001). In addition to a main effect of day, the overall analysis of variance indicated a significant effect of line (F1,32 = 5.6, P = 0.02), and interaction between day and line (F4,128 = 4.2, P = 0.003). No main effects or interactions with dose were detected. However, Figure 6 indicates that the learning curves for High-Active and Control mice were very similar following saline injections, but that the High-Active mice show impairment after amphetamine treatment, particularly on days 2 and 3. In an analysis that considered only days 2 and 3 (the days when most of the learning occurred), a trend for a significant interaction between line and dose was observed (F3,32 = 2.7, P = 0.06). Post-hoc Tukey tests indicated significant differences between High-Active and Control lines at the 0.5 mg/kg (P = 0.013) and 2 mg/kg (P = 0.008) doses of amphetamine. The impaired performance on the water maze in the High-Active line is likely a correlated response to selection as opposed to a consequence of genetic drift (see results for day 2; Table 2).
Probe test
On average, animals remembered the spatial location of the platform as indicated by significantly greater duration spent in the target quadrant than would be expected by chance (t38 = 2.8, P = 0.008). The average duration spent in the target quadrant across all animals was 28.3% ± 1.19 SE. No significant effects of line, dose or interaction were detected (data not shown).
Immunohistochemistry
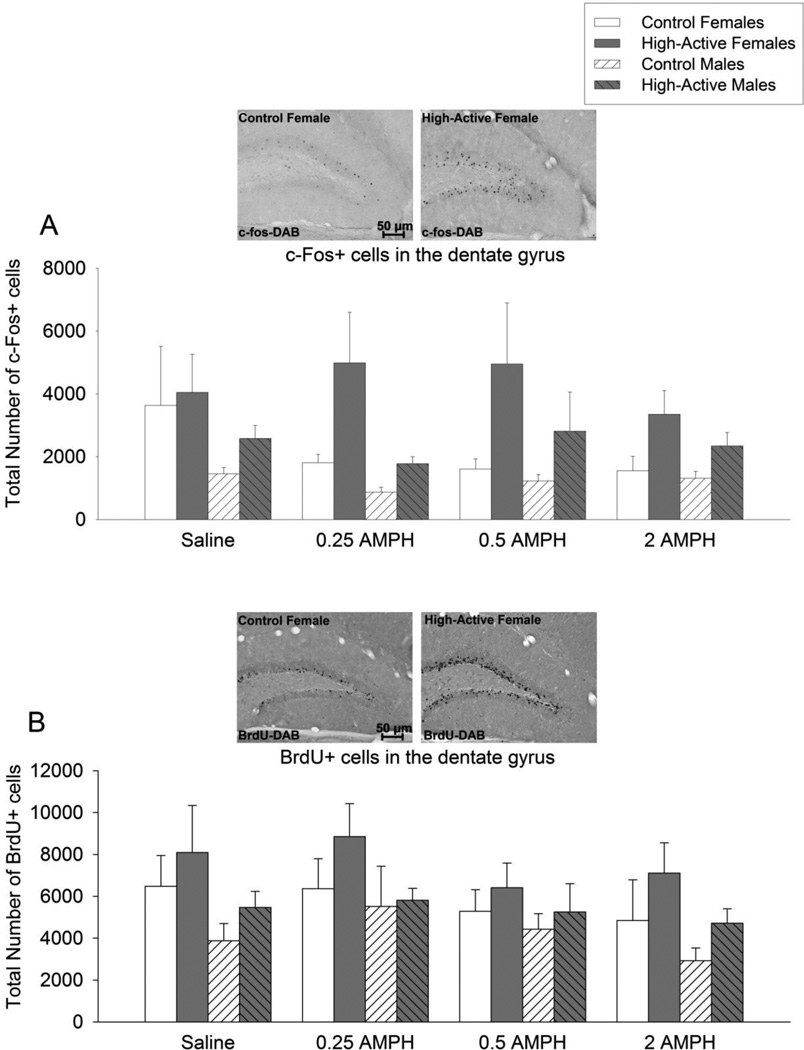
c-Fos-DAB (Fig. 7A)
Figure 7. Hippocampal activation and growth of new cells in response to amphetamine.
A) Average (±SE) total number of c-Fos-positive cells in the dentate gyrus granule layer of the hippocampus. B) Same as A for BrdU-positive cells.
The 3-way ANOVA indicated a significant main effect of line (F1,64 = 24.3, P < 0.0001) and sex (F1,64 = 11.5, P = 0.001). No main effect of dose or any interactions were detected. Control mice displayed approximately 50% the number of c-Fos cells as compared to High-Active mice and males displayed approximately 55% the number as females.
BrdU-DAB (Fig. 7B)
The 3-way ANOVA indicated a significant main effect of line (F1,61 = 5.3, P = 0.025) and sex (F1,61 = 8.7, P = 0.004). No main effect of dose or any interactions were detected. Control mice displayed approximately 77% the number of BrdU positive cells as compared to High-Active mice and males displayed approximately 71% the number as females.
Correlation between Locomotor Activity, BrdU-DAB, and c-Fos-DAB
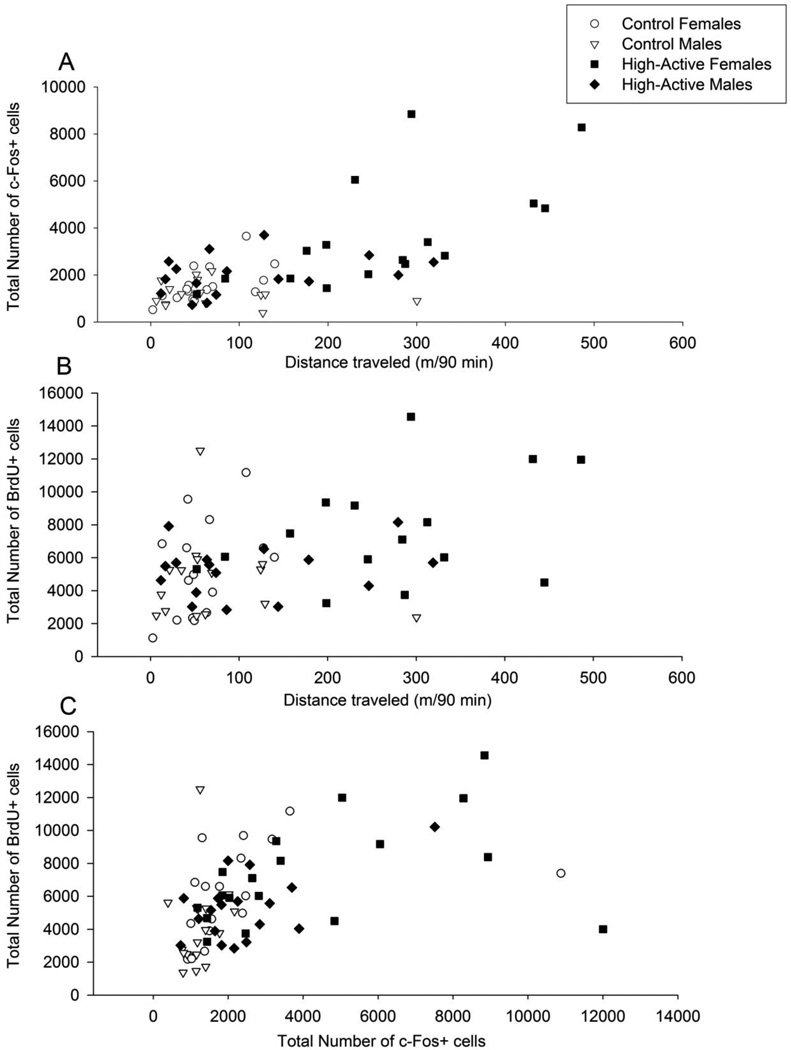
The distance traveled within 90 min preceding euthanasia (Test 5) was strongly correlated with total number of c-Fos positive cells in the granule layer of the dentate gyrus (Pearson’s r = 0.67; F1,62 = 51.7, P < 0.0001) (Fig. 8A). After including distance traveled as a covariate in a 3-way ANOVA with factors line, sex and dose, no other factor or interaction was significant, indicating that the differences in number of c-Fos cells shown in Fig. 7A can be explained by differences in locomotor activity.
Figure 8. Correlations between cellular activation, growth of new cells in the dentate gyrus, and locomotion.
A) Phenotypic correlation between total number of c-Fos+ cells and distance traveled (m) within the 90 minute period prior to euthanasia (n=4/sex/line/treatment = 64 total). B) Same as A for BrdU+ cells. C) Phenotypic correlation between total number of c-Fos+ cells and total number of BrdU+ cells for all mice, regardless of whether they underwent video tracking (n=5/sex/line/treatment = 80 total).
The distance traveled within 90 min preceding euthanasia (Test 5) was also correlated with total number of BrdU-positive cells in the granule layer (Pearson’s r = 0.40; F1,59 = 11.6, P = 0.001) (Fig. 8B). After including distance traveled as a covariate in the 3-way ANOVA, no other factor or interaction was significant except for dose (F3,44 = 3.3, P = 0.03). Comparison of least square means indicated that the 0.25 mg/kg dose increased BrdU-positive cells to 111% of saline levels, whereas 0.5 mg/kg reduced BrdU-positive cells to 87% of saline levels, and 2 mg/kg reduced BrdU-positive cells 65% of saline levels.
Numbers of c-Fos-positive cells were correlated with numbers of BrdU-positive cells in the granule layer of the dentate gyrus (Fig. 8C). The correlation without 2 outliers (with c-Fos numbers above 10,000) shown in Figure 8C was 0.67 (F1,73 = 58.6, P < 0.0001), and with the outliers present, the correlation was 0.51 (F1,75 = 26.8, P < 0.0001).
Table 2 indicates that the difference in BrdU cells between the lines is within the range expected by genetic drift. However, the phenotypic correlation between distance traveled and number of BrdU cells (Fig. 8B) supports the conclusion that the line difference is probably a correlated response to selection via the intermediate phenotype of physical activity.
Discussion
Here we report an update on the progress of a selective breeding experiment for increased distance traveled in the home cage through Generation 10, and initial evidence that the hyperactivity demonstrated by High-Active mice is ameliorated by the low (0.25 mg/kg) dose of d-amphetamine. The differential responses to the low doses of amphetamine were likely a correlated response to selection rather than a consequence of genetic drift, providing support for the suitability of the High-Active line to model genetic hyperactivity disorders. Importantly, the High-Active line continues to respond to selection without reaching a clear selection limit (Fig. 2A). Body mass at maturity significantly decreased in the High-Active line as compared to the Control line. The line difference is likely a correlated response to selection for home cage activity, and unlikely caused by random factors such as genetic drift (Fig. 2C, Table 2). Small differences between the lines were detected in wheel running, open field, rotarod performance, and water maze performance, but these differences may not be definitely ascribed to the effects of selection, and could have resulted from genetic drift rather than correlated responses to selection (Table 2). The increased number of BrdU cells in the in the dentate gyrus observed in High-Active mice is likely attributable to the neuronal activation (as measured by number of c-Fos positive cells) of the dentate gyrus associated acutely with increased levels or intensity of physical activity. Further work, including replicating the High-Active lines, will be necessary for greater statistical power to detect genetic correlations for these and other relevant traits.
The reduction in number of successful pairings of High-Active parents relative to unselected Controls potentially indicates less viable offspring or impaired maternal competence in High-Active mice (Fig. 2B). Future studies will determine whether instinctual maternal care is compromised by selection for hyperactivity, or whether pups are simply less viable. Some evidence suggests that maternal neglect has been linked to altered dopamine neurotransmission in key reward regions of the brain (Gammie et al., 2008), and selection for physical activity in other lines has itself been shown to alter those reward pathways (Rhodes et al., 2005; Garland et al., 2011b). Therefore, it is possible that over ten generations of selective breeding for hyperactivity has altered dopamine neurotransmission, which then produced secondary deficits in maternal care. Alternatively, as the High-Active mice exhibit lower body weights overall (Fig. 2C) and during weaning (unpublished observations) it is possible High-Active pups are less viable. Interestingly, clinical studies have associated lower body weight at birth with the development of ADHD later in life (Mick et al., 2002; Lahti et al., 2006; Strang-Karlsson et al., 2008). Regardless of the cause of reduced reproductive success, this phenomenon may become a significant contributor to the continued divergence of our High-Active populations. For example, diminished reproductive success at Generations 5 and 8 reduced the selection differential and response to selection in generations 6 and 9 (Fig. 2A and B; Table 1).
In addition to reduced reproductive success, the High-Active mice also display reduced body mass at maturity (Fig. 2C). The difference in body mass between the lines survives the test of whether these results could be due to genetic drift represented in Table 2, suggesting the differences in body mass between the lines are probably not caused by genetic drift alone, and that selection likely contributed to this effect. In a replicated selective breeding experiment for increased voluntary wheel running, a negative genetic correlation between levels of physical activity and body mass was also observed (Swallow et al., 1999). Taken together, these data provide strong support for the hypothesis that body mass at maturity and levels of physical activity in a habituated environment broadly speaking (e.g., as measured in cages without wheels or with wheels) are negatively genetically correlated. Further experiments are needed to determine whether the reduced body mass in our High-Active line results from a combination of reduced body fat and reduced skeletal length similar to mice selectively bred for increased voluntary wheel running behavior (Swallow et al., 1999, 2001; Houle-Leroy et al., 2003).
Another interesting comparison to clinical populations arises from the enhanced activity of females in our High-Active strain (Figs. 2A and 3A). While many other studies across different strains of mice and within other populations selectively bred for physical activity corroborate our observation of increased female activity relative to males (Clark et al., 2011; Garland et al., 2011a), our High-Active female hyperactivity contrasts clinical ADHD populations in which males tend to be diagnosed more frequently with ADHD Hyperactive Type, while females more frequently present as ADHD Inattentive Type (Gaub and Carlson, 1997; Gershon, 2002). Despite the preponderance of Hyperactive Type males in clinical literature, some females also exhibit a hyperactivity component to their ADHD diagnosis. The lack of clinical data on female children with ADHD Hyperactive Type contribute to an especially poor understanding of the neurobiological underpinnings in hyperactive female populations (Rucklidge, 2010), and our selectively bred population may be useful in elucidating these characteristics.
Our characterization of a subset of mice from Generation 10 (whose activity levels approximate the entirety of Generation 10, as shown in Fig. 3A) shows differences in wheel running and open field locomotor activity between the lines (Fig. 3B and C). However, Table 2 indicates that we cannot rule out the possibility that these differences are a result of genetic drift, rather than selection. Data from the original report in Zombeck et al. (2010) favor the drift hypothesis rather than selection because in that study we observed no significant genetic correlation between wheel running or open field activity and home cage physical activity, but significant heritability of wheel running and open field in the starting population. Although wheel running might be hypothesized to be correlated with home cage activity, open field is expected to have different genetic underpinnings. Behavior in the open field test is thought to indicate exploratory behavior, or anxiety in a novel environment (Choleris et al., 2001), as distinct from behavior in a habituated home environment.
The differential performance on the rotarod between the High-Active versus Control line did not survive the test for possible role of genetic drift (Fig. 3D and E, Table 2). Therefore the small reduction in performance (Fig. 3E) may not be related to selection, and could be caused by genetic drift. Replication of the lines would help resolve the selection versus drift issue. If the difference is related to selection, reduced rotarod performance in High-Active mice could have clinical relevance. Rotarod performance is generally considered to reflect cerebellar-mediated coordination (Goddyn et al., 2006), and the current data demonstrate impaired performance. The reason for the impairment could be one of two possibilities - that High-Active mice are less coordinated than Controls, or that they lack the attentional control necessary to successfully focus on the task. Regardless of the source of the impairment, it will be interesting to further characterize the High-Active model by directly correlating total cerebellar and vermis volume with rotarod performance, as this could corroborate mounting clinical evidence which implicates the cerebellum in the neuropathology underlying hyperactivity disorders such as ADHD. While some studies indicate cerebellar volume does not vary between mice selectively bred for increased wheel running behavior and control lines (Kolb et al., 2013), it may be that selection for home cage hyperactivity will produce different effects on brain volume measurements. Studies suggest that the cerebellum is important in not only motor coordination, but also mediating timing and attention functions through frontal associations (Allen et al., 1997), and children with ADHD have significantly smaller cerebellar hemispheres and smaller posterior-inferior vermal volumes (Mackie et al., 2007; Bledsoe et al., 2009; Cherkasova and Hechtman, 2009). Taken together, this evidence has resulted in the generation of the frontostriatal-cerebellar theory of ADHD (Krain and Castellanos, 2006), and provides another avenue by which we may characterize these High-Active mice.
The second aim of this paper was to determine whether chronic, therapeutic doses of d-amphetamine ameliorated hyperactivity in the High-Active lines. Toward this end, we observed that the low dose of 0.25 mg/kg amphetamine significantly reduced locomotor activity in our High-Active line whereas the same doses increased locomotor activity in the Control line (see Figure 5). The differential response to low dose amphetamine between our lines is most likely a correlated response to selection rather than a consequence of genetic drift (Table 2). Results establish the predictive validity of our model with respect to ameliorative effects of therapeutic doses of amphetamine on the hyperactive phenotype. Future studies will focus on evaluating the effect of daily 0.25 mg/kg amphetamine on other behavioral measures relevant to ADHD, such as impulsivity and inattention.
The third aim of this paper was to determine whether chronic amphetamine influences neuronal activation of the dentate gyrus and number of newly divided cells, as previous results from our lab using the exact dosing regimen over the same developmental period found dose-dependent increases in neurogenesis in C57BL/6J mice (Dabe et al., 2013). However, in the current study, no changes in the number of new cells in the dentate gyrus were observed in response to amphetamine without adjusting for the effects of distance traveled. After adjusting for distance traveled, the higher doses, 0.5 and 2 mg/kg, reduced numbers of BrdU+ cells. Although we did not perform a double label analysis to detect co-labeling between BrdU and a mature neuronal marker such as NeuN to confirm that the BrdU cells were in fact neurons, based on previous work in our lab, it is likely that a majority of these new cells were neurons (probably greater than 80%) (Clark et al., 2011). It is possible that the increase in neurogenesis from amphetamine observed in Dabe et al. (2013) is specific to the C57BL/6J genotype. The High-Active and the Control lines in this study were derived from a cross of 8 divergent strains (including 3 subspecies) and the individuals within the lines are expected to display a large degree of genetic variation as supported by the low inbreeding coefficient estimated in Table 2. Hence, if the influence of amphetamine on neurogenesis is highly genotype-specific, then large individual genetic variation in our lines would be expected to obscure detection of a response characteristic of a single genotype such as C57BL/6J.
Although group differences in neuroplasticity markers (c-Fos expression and BrdU positive cells) were not observed in response to amphetamine, significant line differences were detected. Mice from the High-Active lines displayed greater numbers of c-Fos and BrdU positive cells than mice from Control lines. The difference is likely due to the different levels of physical activity displayed by the animals and the strong phenotypic relationship between distance traveled and the outcome variables (Fig. 8A, B, C).
We endeavored to measure the Morris Water Maze (MWM), because we hypothesized that the High-Active mice might display poor performance relative to Control lines due to their inherent inattention, and that the amphetamine might ameliorate some of this decline. The MWM is also a hippocampal dependent task, and we hypothesized that changes in the number of new cells in the hippocampus or neuronal activation between the groups might relate to performance. While previous literature has reported that d-amphetamine facilitates learning in the MWM (Brown et al., 2000), in the present study all animals learned the task over 5 days without any overall significant effect of amphetamine. A significant effect of line was detected, with High-Active mice performing worse than Control. Inspection of Figure 6 indicates that the difference was most apparent at the higher doses of amphetamine. Results suggest impaired performance in High-Active mice relative to Controls on day 2 is likely a correlated response to selection rather than a consequence of genetic drift (Table 2). The difference might reflect inattention or inherently reduced hippocampal function in High-Active mice. Regardless of the cause, the water maze performance in High-Active mice was certainly not improved with amphetamine, and if anything, was impaired.
Results from this study provide support for using the High-Active mice as a model for ADHD. High-Active mice performed significantly worse on the water maze and rotarod which could be related to their inattention or inability to focus on the task at hand. However, attributing their performance to inattention or any other cognitive construct would have to be evaluated empirically using operant tests such as serial reaction time or Go/No go tasks (Bari and Robbins, 2013). Moreover, if the impaired performance was caused by inattention it certainly was not ameliorated with amphetamine as is typically observed in ADHD (see Fig. 6). On the other hand, amphetamine significantly ameliorated the hyperactivity in the High-Active line. Current studies are already in progress characterizing impulsive-like behavior in these High-Active mice, using the Go/No-go paradigm (Moschak and Mitchell, 2012). It will be interesting to observe whether impulsivity is affected by our continued selection for home cage hyperactivity, and the extent to which that impulsivity is ameliorated by d-amphetamine. Moreover, it will be important to evaluate whether the High-Active mice display any reduced dopamine function in prefrontal regions before continuing to evaluate this model in the context of ADHD (Arnsten, 2009).
In conclusion, our High-Active mouse line provides a unique tool which may be used to evaluate the genetic and neurobiological mechanisms underlying hyperactivity and its inevitable correlated responses such as reduced body mass, reduced reproductive success, and locomotor response to amphetamine administration. Results support the continued development of the High-Active line as a model for ADHD. Future studies will compare attention, impulsivity-like behavior, cerebellar volume and various measures of dopamine function between the lines.
Acknowledgments
Special thanks to Elissa Chesler for providing the original G2:F1 Collaborative Cross mice. This work was supported by grants from National Institutes of Health, MH083807 and DA027487.
Footnotes
Conflict of Interest Disclosures
Petra Majdak, Paula J. Bucko, Ashley L. Holloway, Tushar K. Bhattacharya, Erin K. DeYoung, Jonathan A. Zombeck, and Justin S. Rhodes declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- Alexander RC, Wright R, Freed W. Quantitative trait loci contributing to phencyclidine-induced and amphetamine-induced locomotor behavior in inbred mice. Neuropsychopharmacology. 1996;15:484–490. doi: 10.1016/S0893-133X(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;1(23 Suppl):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer PJ. D-amphetamine facilitation of morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behav Brain Res. 2000;114:135–143. doi: 10.1016/s0166-4328(00)00225-4. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatry. 2009;54:651–664. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, Voy BH, Culiat CT, Threadgill DW, Williams RW, Churchill GA, Johnson DK, Manly KF. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome. 2008;19:382–389. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choh AC, Demerath EW, Lee M, Williams KD, Towne B, Siervogel RM, Cole SA, Czerwinski SA. Genetic analysis of self-reported physical activity and adiposity: the Southwest Ohio Family Study. Public Health Nutr. 2009;12:1052–1060. doi: 10.1017/S1368980008003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011;10:345–353. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabe EC, Majdak P, Bhattacharya TK, Miller DS, Rhodes JS. Chronic D-amphetamine administered from childhood to adulthood dose-dependently increases the survival of new neurons in the hippocampus of male C57BL/6J mice. Neuroscience. 2013;231:125–135. doi: 10.1016/j.neuroscience.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFries JC, Wilson JR, McClearn GE. Open-field behavior in mice: selection response and situational generality. Behav Genet. 1970;1:195–211. doi: 10.1007/BF01074652. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Choh AC, Johnson W, Curran JE, Lee M, Bellis C, Dyer TD, Czerwinski SA, Blangero J, Towne B. The positive association of obesity variants with adulthood adiposity strengthens over an 80-year period: a gene-by-birth year interaction. Hum Hered. 2013;75:175–185. doi: 10.1159/000351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Sackett J, Turner T, Schardt M, Tang SC, Kurtz N, Dunfey M, McFarlane NA, Susi A, Danish D, Li A, Nissley-Tsiopinis J, Borgmann-Winter K. Attention-deficit/hyperactivity disorder genomics: update for clinicians. Curr Psychiatry Rep. 2012;14:579–589. doi: 10.1007/s11920-012-0309-4. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Replicated selection for body weight in mice. Genet Res. 1973;22:291–321. doi: 10.1017/s0016672300013094. [DOI] [PubMed] [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Health Nutr. 1999;2:411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, McGough JJ, Stein MA. Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS Drugs. 2010;24:99–117. doi: 10.2165/11530290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Liu L, Monleon S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav Genet. 2005;35:675–692. doi: 10.1007/s10519-005-3423-9. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Edelmann MN, Mandel-Brehm C, D’Anna KL, Auger AP, Stevenson SA. Altered dopamine signaling in naturally occurring maternal neglect. PLoS One. 2008;3:e1974. doi: 10.1371/journal.pone.0001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc Biol Sci. 2011a;278:574–581. doi: 10.1098/rspb.2010.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011b;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Goddyn H, Leo S, Meert T, D’Hooge R. Differences in behavioural test battery performance between mice with hippocampal and cerebellar lesions. Behav Brain Res. 2006;173:138–147. doi: 10.1016/j.bbr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Greven CU, Asherson P, Rijsdijk FV, Plomin R. A longitudinal twin study on the association between inattentive and hyperactive-impulsive ADHD symptoms. J Abnorm Child Psychol. 2011;39:623–632. doi: 10.1007/s10802-011-9513-7. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Interpreting studies that compare high- and low-selected lines on new characters. Behav Genet. 1989;19:473–502. doi: 10.1007/BF01066250. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Spurious associations in unreplicated selected lines. Behav Genet. 1997;27:145–154. doi: 10.1023/a:1025689425738. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy P, Guderley H, Swallow JG, Garland T., Jr Artificial selection for high activity favors mighty mini-muscles in house mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R433–R443. doi: 10.1152/ajpregu.00179.2002. [DOI] [PubMed] [Google Scholar]
- Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obenaus A, Garland T., Jr Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model of brain evolution. J Exp Biol. 2013;216:515–523. doi: 10.1242/jeb.076000. [DOI] [PubMed] [Google Scholar]
- Konarzewski M, Ksiazek A, Lapo IB. Artificial selection on metabolic rates and related traits in rodents. Integr Comp Biol. 2005;45:416–425. doi: 10.1093/icb/45.3.416. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Kajantie E, Heinonen K, Pesonen AK, Jarvenpaa AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47:1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. J Abnorm Child Psychol. 2007;35:999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23:16–22. doi: 10.1097/00004703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Mitchell SH. Acute ethanol administration and reinforcer magnitude reduction both reduce responding and increase response latency in a go/no-go task. Alcohol Clin Exp Res. 2012;36:1803–1810. doi: 10.1111/j.1530-0277.2012.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano F, Bonito-Oliva A, Federici M, Carta M, Errico F, Magara S, Martella G, Nistico R, Centonze D, Pisani A, Gu HH, Mercuri NB, Usiello A. Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine. J Neurosci. 2010;30:11043–11056. doi: 10.1523/JNEUROSCI.1682-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2003;167:242–250. doi: 10.1007/s00213-003-1399-9. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T., Jr Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2001;158:120–131. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33:357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Gurling HM. Genetics of attention-deficit hyperactivity disorder (ADHD) Neuropharmacology. 2009;57:590–600. doi: 10.1016/j.neuropharm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Strang-Karlsson S, Raikkonen K, Pesonen AK, Kajantie E, Paavonen EJ, Lahti J, Hovi P, Heinonen K, Jarvenpaa AL, Eriksson JG, Andersson S. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: the Helsinki study of very-low-birth-weight adults. Am J Psychiatry. 2008;165:1345–1353. doi: 10.1176/appi.ajp.2008.08010085. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T. Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J Exp Biol. 1999;202:2513–2520. doi: 10.1242/jeb.202.18.2513. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T., Jr Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol B. 2001;171:651–659. doi: 10.1007/s003600100216. [DOI] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54:3–16. doi: 10.1111/j.1469-7610.2012.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Kerr LM, Ward DS, Pate RR. Physical activity and determinants of physical activity in obese and non-obese children. Int J Obes Relat Metab Disord. 2001;25:822–829. doi: 10.1038/sj.ijo.0801621. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Deyoung EK, Brzezinska WJ, Rhodes JS. Selective breeding for increased home cage physical activity in collaborative cross and Hsd:ICR mice. Behav Genet. 2011;41:571–582. doi: 10.1007/s10519-010-9425-2. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]