Background and perspectives
Keynote address: Frederick S. vom Saal
(University of Missouri-Columbia)
Fetal programming is an enormously complex process that relies on numerous environmental inputs from uterine tissue, the placenta, the maternal blood supply, and other sources. Recent evidence has made clear that the process is not based entirely on genetics, but rather on a delicate series of interactions between genes and the environment. It is likely that epigenetic ("above the genome") changes are responsible for modifying gene expression in the developing fetus, and these modifications can have long-lasting health impacts. Determining which epigenetic regulators are most vital in embryonic development will improve pregnancy outcomes and our ability to treat and prevent disorders that emerge later in life.
"Fetal Programming and Environmental Exposures: Implications for Prenatal Care and Preterm Birth" began with a keynote address by Frederick vom Saal, who explained that low-level exposure to endocrine disrupting chemicals (EDCs) perturbs hormone systems in utero and can have negative effects on fetal development. vom Saal presented data on the EDC bisphenol A (BPA), an estrogen-mimicking compound found in many plastics. He suggested that low-dose exposure to EDCs can alter the development process and enhance chances of acquiring adult diseases, such as breast cancer, diabetes, and even developmental disorders such as attention deficit disorder (ADHD).1 vom Saal noted that conventional risk-assessment strategies used for most chemicals cannot be used to assess toxicity of endocrine-active compounds, which often display nonmonotonic dose responses (Fig. 1).2
Figure 1.
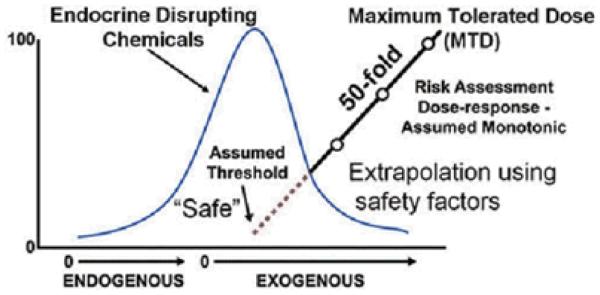
Chemical risk assessment assumes a monotonic dose-response relationship and makes assumptions about low-dose effects.2
Session I: Genetic/epigenetic programming of preimplantation development
Chair: Marco Conti (University of California, San Francisco)
Marco Conti spoke about networks of RNA-binding proteins that regulate oocyte and embryonic development. Conti focused on the mechanism by which newly synthesized proteins "set the stage" for the developing embryo between the stages of oocyte maturation and activation of the embryonic genome. Conti discussed the discovery of the gene Dazl (deleted in azoospermia-like), which encodes a maternal protein that positively regulates RNA translation in the maturing oocyte before embryogenesis occurs.3 These effects are due to epigenetic effects on DNA and chromatin structure. Conti's research showed that the absence of DAZl prevents meiosis by disrupting microtubule organization and spindle formation.
Kelle H. Moley (Washington University School of Medicine) presented her work in a mouse model of type 1 diabetes, in which oocytes are smaller and show impaired mitochondrial function and aberrant morphology, as well as abnormal meiotic spindle formation and aneuploidy.4 Transfer of these fertilized oocytes into nondiabetic mice results in poor reproductive outcomes, including growth restriction and congenital anomalies,5 suggesting that mitochondrial dysfunction leads to either aneuploidy and embryo arrest or fetal growth abnormalities in the next generation.
Similarly in mouse models of obesity, exposure to an abnormal endocrine environment affects the oocyte, the embryo, and pregnancy outcomes. Moley noted that oocytes from mice maintained on a high-fat diet are significantly smaller, show delayed meiotic maturation, and show impaired mitochondrial function and aberrant morphology, as well as abnormal meiotic spindle formation and aneuploidy. Transfer of these fertilized oocytes into nondiabetic mice results in poor reproductive outcomes, including growth restriction and brain anomalies.6 These findings suggest that metabolic changes in the mother can affect development as early as the unfertilized oocyte, and that these changes may have long-term effects on offspring.6 In humans, as well, abnormal levels of free fatty acids in follicular fluid, as well as sera, have been associated with poor oocyte quality and decreased chance of pregnancy in patients undergoing in vitro fertilization (IVF), respectively.7,8
The session concluded with a presentation from James Charles Cross (University of Calgary). Cross examined cells of the placenta-fetus interface and found that several types of trophoblast cells line the canal leading to the umbilical cord. The fetus relies on these cells for transportation of maternal blood and nutrients. Specialized placental cells express a family of genes that encode for prolactins, hormones required for insulin production during pregnancy.9 Cross noted that prolactin hormones protect female mice from developing gestational diabetes during pregnancy, further illustrating the importance of the specialized cells that bridge the gap between mother and fetus.
Session II: Embryo-uterine crosstalk
Chair: Adrian Erlebacher (New York University Langone Medical Center)
The second session began with a presentation by Francesco DeMayo (Baylor College of Medicine). DeMayo spoke on the role progesterone receptors play in embryo implantation and decidualization. DeMayo noted that complex signaling events occur between the epithelium and the stroma before implantation.10 His group discovered that activation of the progesterone receptor in the epithelium sets off this cascade by regulating expression of the morphogen Indian Hedghog (Ihh). His group is also seeking to understand the mechanism by which progesterone transforms the endometrium during pregnancy.
DeMayo also spoke about the effects of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases on decidualization.11 His group found that progesterone action is regulated by EGFR family members. DeMayo explained that pharmacological inhibitors of EGFR and HER2 prevent decidualization and may be used as a therapeutic to treat endometriosis. DeMayo's group is undertaking genome-wide studies to identify which genes are targets of the progesterone receptor and to further define how uterine biology is dependent on this hormone.12 Preliminary findings indicate the importance of genes involved in the circadian cycle and of the GATA2 gene, which encodes a transcription factor that tunes progesterone sensitivity in the epithelium.
Bruce Murphy (University of Montreal) spoke next about a family of genes called orphan nuclear receptors, which are similar to estrogen and progesterone receptors, except that no activating hormones have yet been identified (hence the term orphan receptor). The NR5A2 gene encodes an orphan receptor that is expressed in granulosa and luteal cells in the ovary and the uterine stroma.13 Murphy and colleagues are investigating this orphan receptor using tissue-specific knockout mice with the NR5A2 gene deleted in progesterone-receptive tissues only. His group discovered that NR5A2 deletion causes problems early in gestation by inhibiting progesterone synthesis. Murphy noted that knockout mice treated with progesterone exhibited gestational failure later, due to defects in the uterus. Gene-expression experiments suggest that the NR5A2 gene may play an important role in decidualization by regulating networks of signaling molecules and transcription factors that are required for fertility. While much attention has been directed toward understanding the action of well-established hormone receptors, these results indicate the importance of orphan nuclear receptors in regulating reproductive processes.
Adrian Erlebacher (NYU Langone Medical Center) ended the session venturing into the field of immunology. Erlebacher began by explaining why the immune system fails to reject a fetus. To investigate this, Erlebacher reactivated memory T cells in a transgenic mouse model and asked whether these cells could migrate to the maternal-fetal interface. His team found that T cells cannot accumulate within the decidua, suggesting that there is a molecular barrier preventing infiltration; they discovered that an innate epigenetic mechanism represses certain chemokine genes that are required for T cell migration.14 Erlebacher speculated that insufficient epigenetic silencing of chemokine genes would result in fetal/placental "rejection" or preterm birth secondary to deciduitis. Conversely, excessive silencing would result in an inability to combat decidual infection and lead to preterm birth secondary to chorioamnionitis or to stillbirth.
Session III: Presentations from young investigators
Chair: Sudhansu K. Dey
(Cincinnati Children's Hospital)
In the next session, graduate students and postdoctoral fellows presented their research projects in fetal programming in a series of presentations. The first talk was given by Jenny Sones (Cornell University), who presented work on a mouse model called BPH/5, an inbred strain that spontaneously develops symptoms similar to human preeclampsia, including late-gestational hypertension, proteinuria, renal glomerular lesions, and endothelial dysfunction.15 She discovered that the model mice had inappropriate hormone dynamics, which led to abnormal expression of two important genes that regulate implantation, LIF and COX2.
Jeeyeon Cha from S. K. Dey's group (Cincinnati Children's Hospital Medical Center) presented work that showed induction of preterm birth in mice conditionally deleted of uterine Trp53 than encodes p53. Cha and colleagues showed that the important enzymatic complex mTORC1 is upregulated in decidual cells and induces their senescence—premature exit from the cell cycle, resulting in heightened Cox2 activity in the decidua. Hypothesizing that preterm birth is characterized by senescent decidual cells that have undergone premature terminal differentiation (Fig. 2), they found that preterm birth is reversed by a selective Cox2 inhibitor, celecoxib, or by a low dose of the anti-inflammatory and anti-immunosuppressive drug rapamycin, which is commonly used to inhibit mTORC1 activity. This work is clinically relevant and could one day facilitate novel preventative treatment strategies for women at risk for preterm birth.
Figure 2.

Cellular senescence and premature differentiation of decidual cells may lead to defective decidualization and preterm birth. The above scheme is adapted from Ref. 46.
Alicia B´cena (University of California, San Francisco) presented her work on hematopoietic cells in the placenta and the chorion.16 She discovered that a certain type of hematopoietic stem cell exhibiting high expression of a primitive cell surface marker is capable of repopulating the extraembryonic niche with diverse blood cells; these stem cells also communicate with endothelial cells in the vasculature, as well as with trophoblasts in the chorion. This work will further our understanding of how primitive blood development occurs early in embryogenesis.
Finally, Martha Susiarjo (University of Pennsylvania) presented her work on the effects of BPA in the mouse placenta. Susiarjo's talk revisited the role of BPA in altering epigenetic regulation and causing developmental defects and other diseases. She hypothesized that BPA alters DNA methylation of imprinted genes, leading to aberrant expression. She found that low-dose exposure leads to loss of imprinting of several genes at multiple domains, including Snrpn and Kcnq1. These gene-expression changes were associated with DNA hypomethylation, an epigenetic mechanism linked to gene silencing.17,18 Future studies will determine the molecular mechanisms involved in BPA-induced DNA methylation changes, as well as in the developmental effects of exposure in the placenta.
Session IV: Decidualization and placentation
Chair: R. Michael Roberts (University of Missouri-Columbia)
Susan J. Fisher (the University of California, San Francisco) began the next session describing how trophoblast development and invasion are essential steps in placentation, a process that establishes a fetal connection to maternal blood flow. Fisher noted that species differences in this developmental process have highlighted the need to establish better model systems to study human trophoblast invasion. Fisher described a new method to derive human embryonic stem cell (hESC) lines from single blastomeres from eight-cell embryos rather than from whole blastocysts.19 The human embryonic stem cell is a powerful model for understanding human development, but lines that are currently available have been established after weeks of in vitro development and thus exhibit heterogeneity with reference to gene expression and differentiation capacity. Fisher's new hESC lines appear to be more primitive and display a slightly different gene-expression profile, with fewer genes expressed than previously established hESC lines (these new cell lines are awaiting National Institutes of Health (NIH) registration). Experiments are underway to differentiate these lines into trophoblast cells for studying human trophoblast development.
Michael J. Soares (Kansas University Medical Center) described several models for studying trophoblast invasion. Soares investigated the molecular pathways that regulate trophoblast invasion using both transgenic rats, a model system with an invasive trophoblast lineage similar to that of humans, and blastocyst-derived trophoblast stem cell lines.20 Fosl1 and hypoxia inducible factor (HIF)-signaling pathways regulate trophoblast invasion in rats.21,22 Fosl1 encodes a transcription factor that is a component of the PI3K/Akt signaling pathway, which is important in promoting cell growth and survival and in trophoblast cells to regulate invasiveness and the expression of matrix metalloproteinase 9 (MMP9).22 Activated HIF induces the expression of several genes, including the histone demethylase Kdm3A, which regulates trophoblast invasion and the expression of another matrix metalloprotease, MMP12.21 Members of the MMP family are key regulators of cell adhesion, extracellular matrix remodeling, and invasion.
R. Michael Roberts (University of Missouri- Columbia) continued the discussion of trophoblast invasion and development. His results differed from those reported showing that hESCs treated with the growth factor BMP4 differentiate along the mesodermal lineage.23 Roberts' data show, instead, that treating hESCs with BMP4 under standard culture conditions switches on expression of trophoblast markers and minimal upregulation of mesoderm markers. The MatrigelTM invasion assay revealed that cells became increasingly invasive over time, especially in an environment high in oxygen (20%). Roberts suggested that the different results could be explained by the use of different media and substrata by the two groups to drive differentiation. His group has now derived 14 human-induced pluripotent stem cell (hiPSC) lines from fetal umbilical cord tissue (obtained from cases of preeclampsia, a condition that can develop during pregnancy and can result in the death of the baby and/or mother from seizures and organ failure). Roberts' team plans to use the hiPSC lines to investigate possible genetic and cellular defects related to preeclampsia.
Session V: Environmental contributors: drugs, diet, and consumer products
Chair: Margaret Ann Miller (FDA/National Center for Toxicological Research)
Keynote Speaker: Sarah Leibowitz (The Rockefeller University)
The second day of the conference highlighted the impact of diet and drugs on fetal programming and presented current research into the causes and prevention of preterm birth. It is clear that parental diet can affect fetal development: the challenge is to understand its effects and to pinpoint periods of gestation during which it is most influential on health outcomes. John R. G. Challis (University of Toronto) discussed a prenatal study of individuals whom experienced the Dutch winter famine.24 The study found, compared to baseline, that prenatal malnutrition caused numerous changes that influence disease, including decreased glucose tolerance and elevated insulin concentrations. Further analyses revealed epigenetic alterations including decreased DNA methylation of the IGF2 gene. These findings highlight the significant effect that maternal nutrition during gestation has on health later in life.25
Data showing that paternal diet also affects offspring metabolism were presented by Oliver J. Rando (University of Massachusetts Medical School). His group is developing a mouse epigenetic pipeline to determine whether dietary information is carried by sperm.26 Using sperm from males with varied diets to fertilize eggs in vitro, researchers derive embryonic stem cells from developing blastocysts for further in vitro studies or to implant these blastocysts into pseudo-pregnant females for in vivo studies. This strategy eliminates other factors that could potentially affect offspring metabolism, such as seminal fluid peptides, and, in theory, enables researchers to study only the epigenetic impact of the sperm in an otherwise genetically homogenous population. Thus far, analysis of the genetic and epigenetic landscape of sperm has revealed modest cytosine methylation changes at putative gene enhancer regions, which could influence gene expression. Although methylation patterns generally clustered better with the generation of mice than with their diet, the interferon zeta cluster displayed a 10 to 15% decrease in methylation with a low protein diet. Research is ongoing to discover the implications of these results and to further refine the technique used.
Pat Levitt (University of Southern California) continued the discussion of metabolic pathways and fetal development, focusing on the role played by the placenta. There is a great deal of evidence supporting the hypothesis that maternal serotonin (5HT) affects fetal brain development by directly influencing serotonin levels in the developing brain.27 Levitt showed that 5HT produced by the placenta contributes to serotonin levels in the developing brain: in Pet-1 knockout mice 5HT levels in the forebrain remained normal until embryonic day (E) 16.5, despite the lack of 5HT neurons. Demonstrating that the placenta is capable of producing 5HT until E18.5, Levitt's data shed light on the importance of this extraembryonic source of a neurotransmitter for fetal development.
John Schimenti (Cornell University) presented work using forward and reverse genetic approaches to identify genes and pathways that influence germ cell survival.28 Germline mutations, if not corrected or eliminated by the DNA damage response (DDR) pathway, can result in birth defects, reduced fitness, and sterility. Intact cell-death machinery facilitates the elimination of germ cells that fail to be corrected by the DDR. Targeted deletion of either Mcm9 or Fancm, two DDR pathway genes that are needed for correcting mutations, resulted in germ-cell depletion in the ovaries and testes of mice.29 Deletion of the p53 gene, which triggers cell death in response to DNA damage, resulted in germ-cell depletion in Mcm9-knockout mice but not in Fancm knockout mice. Analysis of a number of other knockouts revealed that germ cell depletion in response to DNA damage can occur via p53-dependent, as well as p53-independent and Chek2-independent mechanisms. This provides evidence that several molecular pathways are at work to cull germ cells carrying mutations that could result in embryos at risk for birth defects and sterility.
Sarah Leibowitz focused on the positive feedback loop between maternal diet during gestation and the dietary habits of offspring over their lifetime.
In one study in rats, a high-fat diet and high alcohol consumption were both correlated with increased production of hypothalamic peptides in offspring, which drive an appetite for a high-fat diet. Prenatal exposure to a high-fat diet increased the genesis of peptide-expressing neurons in the hypothalamus of developing rats at embryonic day (E) 11.5, and offspring exhibited a higher density of these neurons after birth.30 This higher density persisted even in the absence of a high-fat diet over their lifetime and resulted in a range of behavioral and metabolic effects, such as a higher food intake and a preference for fatty foods. This study underscores the significant impact of maternal diet during pregnancy on the dietary habits of offspring.
Session VI: Pregnancy disorders and prematurity: gene-environment interactions
Chair: S. Ananth Karumanchi (Beth Israel Deaconess Medical Center and Harvard Medical School)
Louis J. Muglia (Cincinnati Children's Hospital Medical Center) began the next session discussing factors influencing preterm delivery. Inactivation of cyclooxygenase 1 (Cox1)—a key regulator of prostaglandin biosynthesis—is implicated in human parturition-delayed birth in mice; however, implantation of Cox1-deficient blastocysts into the uterus of normal females resulted in normal duration of labor, suggesting that maternal Cox1 expressed in the uterine wall is both necessary and sufficient for normal labor.31 Muglia uses comparative and whole-genome studies to identify gene variants in humans that may contribute to preterm labor;32,33 this could allow for prenatal identification of at-risk individuals and help researchers to develop new therapies for treatment.
Roberto Romero (Eunice Kennedy Shriver National Institute of Child Health and Human Development /NIH) reviewed the concept of preterm parturition as a syndrome and the importance of progesterone for pregnancy maintenance. The administration of progesterone receptor antagonists induces cervical ripening in animals and in pregnant women in the first, second, and third trimesters. He described the relationship between a sonographic short cervix, detected by ultrasound in the mid trimester of pregnancy, and the risk for spontaneous preterm delivery. For example, an asymptomatic patient with a cervix of 15 mm or less has a 50% risk of preterm delivery of <32 weeks.
Two randomized clinical trials in which vaginal progesterone was used to prevent preterm delivery in women with a short cervix were presented. The results of the PREGNANT Trial (international trial)34 showed that the administration of 90 mg of progesterone/day (vaginal route) reduced the rate of preterm delivery of <33 weeks by 45% and the rate of respiratory distress syndrome by 61%. The results of an individual patient meta-analysis35 were presented in which vaginal progesterone administration reduced the rate of preterm delivery at <28, <33, and <35 weeks of gestation. This approach was also associated with a reduction in the number of admissions to newborn intensive care units, respiratory distress syndrome, the need for mechanical ventilation, and a composite score of neonatal morbidity and mortality. It is estimated that universal screening to identify a short cervix and treatment of this condition with vaginal progesterone would save $19 million per 100,000 women screened, and the total estimate of savings of the U.S. healthcare system would be $500-$700 million per year.36 This effect is not achieved by using 17-hydroxyprogesterone caproate, which is a synthetic progestin with a potential safety signal.
S. Ananth Karumanchi (Beth Israel Deaconess Medical Center and Harvard Medical School) talked about the role of several angiogenic growth factors and their inhibitors that contribute to preeclampsia in women who present prematurely with severe disease.37 In particular, he focused his attention on vascular endothelial growth factor (VEGF) signaling and discussed how impairment of VEGF signaling may lead to preeclamptic signs and symptoms. He also discussed strategies to treat preeclampsia by depleting proteins that interfere with critical angiogenic factors, such as VEGF, to shift the balance of factors toward proangiogenesis.
Session VII: Environment, hormone action, and pharmacological/endocrine disruptors
Chair: L. David Wise (Merck)
There are numerous consumer products on the market for which safety data are lacking. The FDA, academic institutions, and nongovernmental organizations are conducting studies to address the safety of these compounds for fetal development and postnatal health. Liang Ma (Washington University School of Medicine) described his research into the mechanism of action of diethylstilbestrol (DES), a synthetic estrogen that was widely used as an antimiscarriage drug between 1940 and 1970 but was later shown to induce reproductive tract malformations and other defects.38 Ma's data show that DES represses key developmental genes such as Hoxa10, inhibits cell death, reduces cell proliferation in the uterine epithelium, induces accumulation of lipid droplets, and promotes upregulation of the glucose pathway. Ma predicts that the metabolic defects caused by DES exposure could also be present in other estrogen-responsive tissues, such as fatty tissue, and thus may contribute to obesity later in life.39
Patricia Hunt (Washington State University) discussed reproductive tract abnormalities induced by BPA. Hunt's investigation of BPA was prompted by an observation that chromosomal abnormalities occur in mice that consume water from BPA bottles.40 Treating mice with BPA at fetal and perinatal stages increased chromosomal recombination, increased multioocyte follicle formation in females, and affected spermatogenesis in males. Similar effects were detected in experiments in rhesus monkeys.41 Future studies will analyze the effect of BPA on other organs, including the brain.
L. David Wise described the guidelines issued by the International Conference on Harmonization (http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html) that outline a series of studies set by the United States, the European Union, and Japan for examining the effects of drugs on several stages of prenatal and postnatal development. These guidelines cover studies on fertility and early development (from fertilization until implantation), embryo-fetal development (from implantation until just before birth), and prenatal and postnatal development (from implantation until weaning). Additional guidelines address the need and design of juvenile toxicity (from birth until after sexual maturity) studies. Wise's talk detailed the design and types of data collected in the embryo-fetal studies in rodents and rabbits.42 Although labor intensive, these studies provide the most suitable data for determining risk information for women of childbearing age.
Sessions VIII and IX: Panel and general audience discussion
Moderator: Patricia Hunt (Washington State University)
Energy remained high during the meeting's final session, with panel members and the audience deliberating on the question, How can we better predict, assess, and lower the risk of, and/or prevent environmental and genetic factors that predispose to, adverse fetal outcomes and preterm birth? John Rogers (U.S. EPA) pointed out the challenges of developing defined in vitro and in vivo tests to better assess developmental exposures over critical windows of development. Rogers noted that it is also important to agree upon what defines adverse endpoints, such as gene expression, phenotypic changes, and functional developmental changes. He also noted that it is important to incorporate functional challenges to reveal effects, for example, providing a high-fat diet for studies of putative environmental obesogens. Fred vom Saal went on to add that we need to incorporate appropriate developmental assays that include long-latency outcomes.
Thad Schug (National Institute of Environmental Health Sciences (NIEHS)) explained how a collaborative group of environmental health scientists and green chemists have developed a tiered endocrine disruption screening protocol (TiPED) (Fig. 3) that is very different from current regulatory endocrine disruption screening programs.43 The protocol is designed for chemists to use in early stages of chemical development and is completely voluntary. It involves a time- and cost-sensitive approach to toxicology testing, with assay complexity and cost increasing as one proceeds through testing tiers. The protocol starts with computer modeling and ends with mammalian testing. A positive test anywhere in the protocol means the chemical is problematic and needs further evaluation or redesign. The group has developed guiding principles for assays and guidelines for proper laboratory testing. The protocol will be online on December 6, 2012 and in print in January 2013 in the U.K. Royal Society of Chemistry's Green Chemistry journal; a real-time online version will soon be available.
Figure 3.

Tiered protocol for endocrine disruption (TiPED). The approach to using this tiered system runs from the simplest, fastest, and cheapest on the top (Tier 1) to the most expensive on the bottom (Tier 5). Failure to find EDC activity in one tier then leads to testing at the next level tier.
Schug noted that the NIEHS, the National Toxicology Program (NTP), and the FDA have formed the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) program to support a perinatal two-year good laboratory practices (GLP) chronic toxicity study on BPA.44 In addition to the core elements of a GLP-compliant study, the study involves academic research partners and will incorporate a wide range of doses and disease-relevant endpoints that have not been used in any previous GLP-compliant BPA toxicity study. The consortium is making all experimental data available via the Chemical Effects in Biological Systems (CEBS) database system developed by the National Toxicology Program at NIEHS. CEBS is a public resource, comprising integrated depositions of data from academic, industry, and governmental laboratories,45 and a tool designed to better coordinate multilevel studies and support meta-analysis.
Toward the end of the session members of the panel and the audience engaged in an active discussion on the experimental challenges for determining the health implications of low-dose effects and nonmonotonic dose responses in relation to endocrine-active chemicals.2 Debate centered on whether current observations in the literature are sufficient to reexamine the ways in which chemicals are tested for endocrine-disrupting properties and how risk to human health may be managed. To this end, the NEIHS cosponsored a workshopa that aimed to define research needs required to move closer to scientific agreement on low-dose effects and nonmonotonic dose responses for endocrine-active substances. Rogers noted that the EPA has created a workgroup to develop a response to a recent article on low-dose nonmonotonic dose responses, and an agency position on how such dose responses should be considered for risk-assessment purposes.
In her closing remarks, Fisher said the research presented at the meeting will ultimately lead to developments that "will improve pregnancy outcomes and our ability to treat and prevent disorders that emerge later in life." "It's exciting to see how far we have come in our understanding of the complex interactions between genes and our environment occurring in utero," added Fisher.
Acknowledgments
This meeting report presents research featured at the June 11-12, 2012 Fetal Programming and Environmental Exposures: Implications for Prenatal Care and Preterm Birth conference, presented by the New York Academy of Sciences and Cincinnati Children's Hospital Medical Center. The conference was supported by Cincinnati Children's Hospital Medical Center and Academy Friend Sponsors, Abcam Inc., Quartzy, Watson Pharmaceuticals, Inc., and the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine at Weill Cornell Medical College. The conference was also supported by educational grants from the Burroughs Wellcome Fund and the March of Dimes Foundation (Grant No. 4-FY12-545). Funding for this conference was also made possible, in part, by Grant number 1R13ES021699-01 from the National Institute of Environmental Health Sciences (NIEHS) and the National Institute on Drug Abuse (NIDA). The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. government.
Sponsored by the New York Academy of Sciences and Cincinnati Children's Hospital Medical Center, with support from the National Institute of Environmental Health Sciences (NIEHS), the National Institute on Drug Abuse (NIDA), and Life Technologies, "Fetal Programming and Environmental Exposures: Implications for Prenatal Care and Preterm Birth" was held on June 11-12, 2012 at the New York Academy of Sciences in New York City. The meeting, comprising individual talks and panel discussions, highlighted basic, clinical, and translational research approaches, and highlighted the need for specialized testing of drugs, consumer products, and industrial chemicals, with a view to the unique impacts these can have during gestation. Speakers went on to discuss many other factors that affect prenatal development, from genetics to parental diet, revealing the extraordinary sensitivity of the developing fetus.
Footnotes
Conflicts of interests
The authors declare no conflicts of interest.
References
- 1.vom Saal FS, et al. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandenberg LN, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes. Dev. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyman A, et al. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungheim ES, et al. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungheim ES, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011;95:1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungheim ES, et al. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil. Steril. 2011;96:880–883. doi: 10.1016/j.fertnstert.2011.07.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int. J. Dev. Biol. 2010;54:341–354. doi: 10.1387/ijdb.082768dh. [DOI] [PubMed] [Google Scholar]
- 10.Franco HL, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin N, et al. Mig-6 is required for appropriate lung development and to ensure normal adult lung homeostasis. Development. 2009;136:3347–3356. doi: 10.1242/dev.032979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubel CA, et al. GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene. Expr. Patterns. 2012;12:196–203. doi: 10.1016/j.gep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Duggavathi R, et al. Liver receptor homolog 1 is essential for ovulation. Genes. Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nancy P, et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann DS, et al. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008;51:1058–1065. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- 16.Barcena A, et al. Human placenta and chorion: potential additional sources of hematopoietic stem cells for transplantation. Transfusion. 2011;51(Suppl 4):94S–105S. doi: 10.1111/j.1537-2995.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 18.Arnaud P, Feil R. Epigenetic deregulation of genomic imprinting in human disorders and following assisted reproduction. Birth Defects Res. C. Embryo. Today. 2005;75:81–97. doi: 10.1002/bdrc.20039. [DOI] [PubMed] [Google Scholar]
- 19.Aghajanova L, et al. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol. Reprod. 2012;86:1–21. doi: 10.1095/biolreprod.111.092775. [DOI] [PubMed] [Google Scholar]
- 20.Soares MJ, et al. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborty D, et al. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc. Natl. Acad. Sci. USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent LN, et al. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardo AS, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Challis J. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 2001;357:1798. doi: 10.1016/S0140-6736(00)04910-2. [DOI] [PubMed] [Google Scholar]
- 25.Challis JR. Endocrine disorders in pregnancy: Stress responses in children after maternal glucocorticoids. Nat. Rev. Endocrinol. 2012;8:629–630. doi: 10.1038/nrendo.2012.183. [DOI] [PubMed] [Google Scholar]
- 26.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnin A, Levitt P. Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 2012;37:299–300. doi: 10.1038/npp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolcun-Filas E, Schimenti JC. Genetics of meiosis and recombination in mice. Int. Rev. Cell. Mol. Biol. 2012;298:179–227. doi: 10.1016/B978-0-12-394309-5.00005-5. [DOI] [PubMed] [Google Scholar]
- 29.Hartford SA, et al. Minichromosome maintenance helicase paralog MCM9 is dispensible for DNA replication but functions in germ-line stem cells and tumor suppression. Proc. Natl. Acad. Sci. USA. 2011;108:17702–17707. doi: 10.1073/pnas.1113524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang GQ, et al. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 2008;28(46):12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross GA, et al. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc. Natl. Acad. Sci. USA. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plunkett J, et al. Primate-specific evolution of noncoding element insertion into PLA2G4C and human preterm birth. BMC Med. Genomics. 2010;3:62. doi: 10.1186/1755-8794-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plunkett J, et al. An evolutionary genomic approach to identify genes involved in human birth timing. PLoS Genet. 2011;7:e1001365. doi: 10.1371/journal.pgen.1001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan SS, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet. Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am. J. Obstet. Gynecol. 2012;206:124. doi: 10.1016/j.ajog.2011.12.003. e1-e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet. Gynecol. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- 37.Lely AT, et al. Circulating Lymphangiogenic Factors in Preeclampsia. Hypertens Pregnancy. 2012 Sep 7; doi: 10.3109/10641955.2012.697953. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin Y, et al. Neonatal diethylstilbestrol exposure alters the metabolic profile of uterine epithelial cells. Dis. Model Mech. 2012;19:870–880. doi: 10.1242/dmm.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L. Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. 2009;20:357–363. doi: 10.1016/j.tem.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muhlhauser A, et al. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol. Reprod. 2009;80:1066–1071. doi: 10.1095/biolreprod.108.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt PA, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wise LD, et al. Embryo-fetal developmental toxicity study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86:418–428. doi: 10.1002/bdrb.20214. [DOI] [PubMed] [Google Scholar]
- 43.Willett CE, Bishop PL, Sullivan KM. Application of an integrated testing strategy to the U.S. EPA endocrine disruptor screening program. Toxicol. Sci. 2011;123:15–25. doi: 10.1093/toxsci/kfr145. [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum LS, et al. Consortium-Based Science: The NIEHS's Multipronged, Collaborative Approach to Assessing the Health Effects of Bisphenol A. Environ Health Perspect. 2012 doi: 10.1289/ehp.1205330. doi:10.1289/ehp.1205330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fostel JM. Towards standards for data exchange and integration and their impact on a public database such as CEBS (Chemical Effects in Biological Systems) Toxicol. Appl. Pharmacol. 2008;233:54–62. doi: 10.1016/j.taap.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Lim HJ, Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J. Clin. Invest. 2010;120:1004–1015. doi: 10.1172/JCI41210. [DOI] [PMC free article] [PubMed] [Google Scholar]


