Abstract
It has been shown previously that norbinaltorphimine (norBNI) and 5΄-guanidinonaltrindole (5΄-GNTI), long-acting kappa opioid receptor (KOPR) antagonists, cause frenzied scratching in mice [1;2]. In the current study, we examined if zyklophin, a short-acting cyclic peptide KOPR antagonist, also elicited scratching behavior. When injected s.c. in the nape of the neck of male Swiss-Webster mice, zyklophin at doses of 0.1, 0.3 and 1 mg/kg induced dose-related hindleg scratching of the neck between 3 and 15 min after injection. Pretreating mice with norBNI (20 mg/kg, i.p.) at 18–20 hr before challenge with zyklophin (0.3 mg/kg) did not markedly affect scratching. Additionally, KOPR −/− mice given 0.3 mg/kg of zyklophin displayed similar levels of scratching as wild-type animals. The absence of KOPR in KOPR −/− mice was confirmed with ex vivo radioligand binding using [3H]U69,593. Taken together, our data suggest that the presence of kappa receptors is not required for the excessive scratching caused by zyklophin. Thus, zyklophin, similar to the structurally different KOPR antagonist 5΄-GNTI, appears to act at other targets to elicit scratching and potentially the sensation of itch.
Keywords: itch, scratching, kappa opioid receptor, kappa opioid receptor antagonist, zyklophin
Introduction
The non-peptide KOPR antagonists 5΄-GNTI and norBNI (see Fig. 1 for structures) have long durations of action, with their effects lasting for at least 3 weeks (reviewed in [3]). Zyklophin is a novel short-acting dynorphin analog with 11 amino acids (see Fig. 1 for structure) that acts as a KOPR antagonist and is able to cross the blood-brain barrier following systemic administration [4]. This compound was first synthesized in 2005 [5] and its pharmacological properties characterized in 2009 [4]. Zyklophin has a duration of action of less than 12 hr and is selective for the KOPR (Ki ratio of 1/194/>330 for kappa/mu/delta opioid receptors). The unusual short duration of action and recent report of this KOPR antagonist warrant further investigation into its pharmacology.
Figure 1.
Chemical structures of 5΄-guanidinonaltrindole (GNTI), norbinaltorphimine (norBNI) and [N-benzylTyr1,cyclo(D-Asp5,Dap8)]Dyn A-(1–11)NH2 (zyklophin).
Cowan and colleagues have shown previously that 5΄-GNTI injected into the nape of the neck induces quick-onset dose-dependent, frenzied scratching in Swiss-Webster mice that is not blocked by pretreatment with norBNI, a selective KOPR antagonist or naloxone, a non-selective opioid antagonist [2]. norBNI, another other long-acting non-peptide KOPR antagonist, also induces repetitive scratching [1]. Kappa receptors are not the only opioid receptors involved in the sensation of itch. At the spinal level, mu opioid receptor agonists such as morphine and fentanyl induce scratching [6;7].
While KOPR antagonists appear to be inducers of scratch, it is interesting to note that KOPR agonists are able to suppress the incidence of scratching. Nalfurafine, a KOPR agonist, has been shown to act on the KOPR to block scratching in a dose-dependent manner. Previous studies have shown that nalfurafine attenuates scratching induced by 5΄-GNTI [2] as well as scratching induced by substance P and histamine [8], morphine [9], compound 48/80 [10] and chloroquine [11]. These data indicate that a decrease in scratching by KOPR agonism is not specific for 5΄-GNTI-induced scratching.
The pathophysiology of itch is complex and not well-understood (for reviews, see [12;13]). There are many substances that evoke scratching behavior and also many receptors and neural pathways that are implicated. Briefly, in a 2006 review of the neurobiology of itch, Ikoma and colleagues [12] identified at least 4 different types of itch: itch caused by skin disorders, itch caused by systemic disorders, psychogenic itch and neuropathic itch. The implicated systems in skin disorders are histamine, interleukins, prostaglandins and proteases. In systemic disorders, opiates and possibly interleukins are implicated. In psychogenic itch, serotonin and noradrenaline are involved, and finally, neuropathic itch is caused by damage to nerve fibers, proteases and neuropeptides including substance P. More recently, the gastrin-releasing peptide receptor (GRPR), also known as the BB2 (bombesin) receptor has been identified as a mediator of itch in the dorsal horn of the spinal cord [14]. The natriuretic polypeptide b (Nppb) has been found to be implicated in the GRP itch circuitry [15], though its exact role has not yet been fully elucidated [16]. In a recent publication, Kim and Yosipovitch [13] listed substances potentially involved in chronic stress-induced itch, which include, in addition to those mentioned above, nerve growth factor, acetylcholine, adrenocorticotropin hormone, corticotropin-releasing hormone, kinins, vasoactive intestinal peptide, and chemoattractants. Of this extensive (but not exhaustive) list, most have a role in pruritus signaling, whether it is directly on pruriceptors on nerve endings, or indirectly on nerve fiber sensitivity, activation of mast cells, or over-activation of the sympathetic nervous system.
norBNI and 5΄-GNTI are non-peptides, so a study on a peptide was of great interest. Additionally, no short-acting KOPR antagonists have been investigated as scratch-inducers to date. Thus, the two aims of the study were (1) to examine if zyklophin caused scratching following s.c. injection into the nape of the neck of Swiss-Webster mice and (2) to investigate the role of the KOPR in zyklophin-induced scratching behavior using norBNI pretreatment to block KOPR and also in mice lacking the KOPR, which was confirmed with [3H]U69,593 binding.
Materials and Methods
Animals
For all the scratching studies, unless otherwise noted, male Swiss-Webster mice weighing 25–30 g (Ace Laboratories, Boyertown, PA) were used. For the KOPR−/− study, male KOPR−/− mice (25–30 g) were used. These mice, of C57BL6/J background, were originally generated by Kieffer and her colleagues [17]. Breeding pairs were purchased from Jackson Laboratories (Bar Harbor, ME) and KOPR−/− × KOPR−/− mating was carried out in the Animal Facility at Temple University School of Medicine. Wild-type C57BL6/J mice of the same weight, obtained from Jackson Laboratories, were used as the controls. Mice were housed under a 12 hr light/dark cycle with food and water available ad libitum. Experiments were conducted between noon-6:00 PM. Experimental procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Mice were allowed to acclimate to the laboratory setting for 3–4 days before the experiments. The animals were habituated to individual shielded rectangular observation boxes (18 cm × 23 cm × 25 cm) for at least 1 hr before the experiment. All animals were drug-naïve and used only once.
Compounds
Zyklophin {[Nα-benzylTyr1,cyclo(d-Asp5,Dap8)]-dynorphin A-(1−11)NH2} was synthesized according to published procedures [5;18]. norBNI was provided by the NIDA Drug Supply Program. Naloxone HCl was purchased from Sigma-Aldrich (St. Louis, MO).
Effect of zyklophin on overt behavior of mice
We used submaximal s.c. doses of zyklophin (0.1, 0.3 and 1 mg/kg) which were based on previous kappa opioid antagonist studies [2] as well as zyklophin data of Aldrich et al. [4].
On the day of the experiment, mice were acclimated to the observation boxes and experiments were performed as previously described [11]. Injections with zyklophin (0.25 ml/25 g) were performed s.c. in the nape of the neck of the mice (n=6–12). Observations of bouts of scratching (defined as lifting the hind leg to scratch the neck) were recorded over a period of 30 min starting one min after injection. This procedure was followed for all zyklophin injections (in Swiss-Webster, wild-type C57BL6/J, and KOPR −/− mice).
Effect of norBNI pretreatment on zyklophin-induced scratching
Mice were injected with saline or norBNI (20 mg/kg, i.p.), used in this instance as a selective kappa opioid receptor antagonist, according to the 2011 study of Inan et al. [19]. Eighteen to 20 hr later, the animals were acclimated in individual boxes and injected with zyklophin (0.3 mg/kg, s.c., nape of neck). One minute after zyklophin injection, mice were observed for 30 min and the number of scratching bouts was recorded.
Ex vivo receptor binding study
For confirmation of the deletion of the kappa receptor in KOPR −/− mice, both knockout mice and the wild-type counterparts were kept for two weeks after the injection of zyklophin to give sufficient time for elimination of the peptide. Mice were euthanized with CO2 gas and the brains removed. The forebrain was collected and weighed. For homogenization, ice-cold 50 mM Tris-HCl and 1 mM EDTA buffer, pH 7.4 was used in a 1:6 w/v ratio with a Fisher F60 Sonic Dismembrator for 20 s. Knockout and wild-type samples were run side by side. Binding was performed in 50 mM Tris-HCl buffer containing 1 mM EGTA (pH 7.4). The selective KOPR agonist [3H]U69,593 (2 nM) was used with 200 µl homogenate for a final volume of 1 mL. Naloxone (10 µM) was used to define nonspecific binding. The reaction mixture was incubated for 1 hr at room temperature and terminated by filtration under reduced pressure with GF/B filters presoaked with 0.1 mg/ml BSA and 0.2% polyethyleneimine. Filters were washed three times with ice-cold 50 mM Tris-HCl buffer containing 0.15 M NaCl (pH 7.4). Radioactivity on filters was determined by liquid scintillation counting.
Data analysis
All data were analyzed for significance with the Student’s t-test in GraphPad Prism 6.0 (La Jolla, CA). Statistical significance was defined as P ≤ 0.05. All data are expressed as values ± S.E.M.
Results
Zyklophin causes scratching in a dose-dependent manner
Zyklophin induced scratching by 1 min after s.c. injection into the nape of the neck of male Swiss-Webster mice. The incidence of scratching was dose-related (0.1, 0.3 and 1 mg/kg) over the 30 min observation period (Fig. 2). Most of the scratching occurred within 15 min of injection and was essentially over after 30 min.
Figure 2.
Zyklophin induced scratching in a dose-dependent manner when injected s.c. into the nape of neck in male Swiss-Webster mice. Each value represents mean ± S.E.M. (n=6–12). Mice injected with saline had < 5 bouts of scratching/30 min.
Pretreatment with norBNI does not attenuate zyklophin-induced scratching
Mice pretreated with norBNI (20 mg/kg, i.p.) 18–20 hr before s.c. injection of 0.3 mg/kg zyklophin did not show a statistically significant (P=0.3887) decrease in scratching behavior, compared with saline pretreatment (Fig. 3). This dose of norBNI, given i.p. 18–20 hr before saline, did not cause scratching (data not shown).
Figure 3.
Pretreatment of mice with norBNI (20 mg/kg, i.p.), 18–20 hr before zyklophin (0.3 mg/kg, s.c.), did not attenuate zyklophin-induced scratching. Each value represents mean ± S.E.M. (n=6).
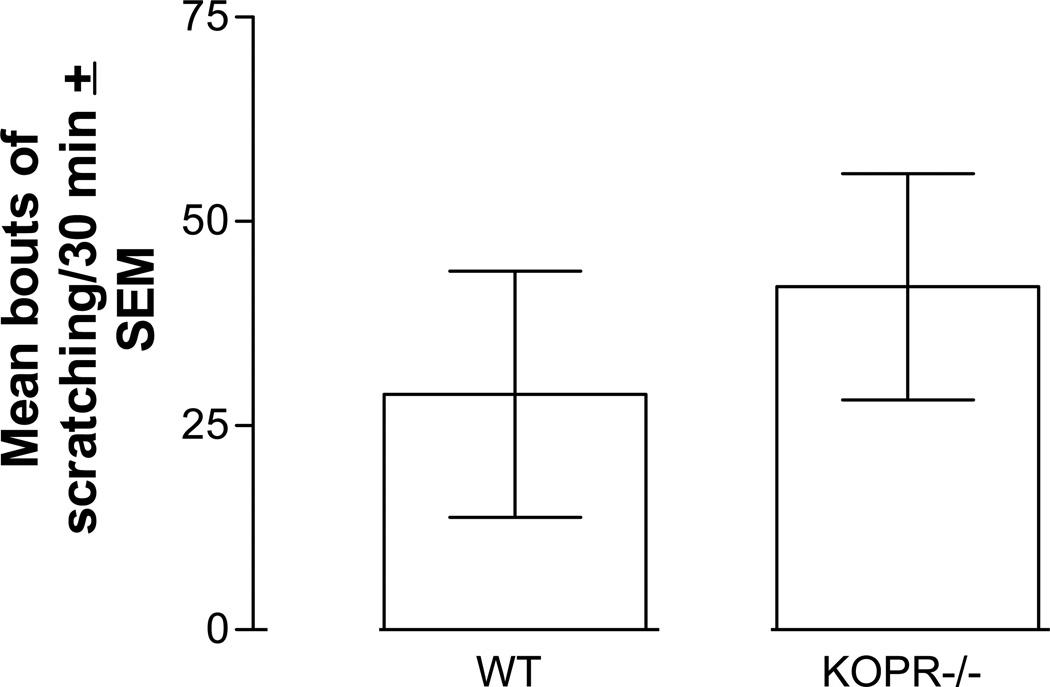
Zyklophin-induced scratching persists in KOPR −/− mice
KOPR −/− mice injected with zyklophin (0.3 mg/kg) did not show a statistically significant (P=0.5998) lower level of scratching behavior in comparison to wild-type C57BL6/J mice (Fig. 4). The number of scratches in C57BL6/J mice was much fewer than that observed in Swiss-Webster mice given the same dose of zyklophin. To confirm deletion of the KOPR, [3H]U69,593 ex vivo radioligand binding was performed on brain homogenates. There was no specific binding of [3H]U69,593 in brains of KOPR −/− mice, while there were appreciable levels of specific binding in the wild-type animals (684 ± 178 dpm/1.3 mg protein).
Figure 4.
Deletion of the KOPR did not attenuate zyklophin (0.3 mg/kg s.c.)-induced scratching in C57BL6/J male mice. Each value represents mean ± S.E.M. (n=6).
Discussion
We found that zyklophin (0.1–1 mg/kg), a short-acting KOPR antagonist, elicited dose-dependent scratching when injected s.c. in the nape of the neck of mice. Most of the scratching was observed between +3 and +15 min. Pretreating mice with norBNI mice at 18–20 hr to block the KOPR before the standard dose of zyklophin (0.3 mg/kg) did not markedly affect the incidence of scratching. Additionally, KOPR −/− mice given 0.3 mg/kg of zyklophin did not display decreased scratching when compared to wild-type animals. The absence of kappa receptors in KOPR−/− mice was confirmed with ex vivo radioligand binding using [3H]U69,593.
The observation that zyklophin-promoted scratching is not mediated by the KOPR coincide with that made by Inan [20], who found that scratching induced by 0.3 mg/kg 5΄-GNTI was not blocked by norBNI pretreatment and was not reduced in KOPR −/− mice, compared with wild-type mice.
There appears to be a strain difference, as C57BL6/J wild-type mice displayed less scratching behavior compared to Swiss-Webster mice at the same dose of zyklophin. Strain differences between C57BL6/J and Swiss-Webster mice have been documented in several areas, including anxiety [21], autonomic response to stress [22], and antinociceptive response to morphine [23]. This may be another area in which strain differences are present, but more data needs to be collected to solidify this observation.
Taken together, our data suggest that the presence of KOPR is not required for the excessive scratching caused by zyklophin. Thus, zyklophin, norBNI and 5΄-GNTI most likely induce scratching through an off target effect. It is interesting to note that even though zyklophin induces scratching qualitatively similar to 5΄-GNTI, the latter compound induces more vigorous scratching of longer duration [2].
Parenthetically, to examine the role of substance P and TRPA1 in preventing zyklophin-induced scratching, we injected mice with the selective neurokinin 1 receptor antagonist, RP 67580 (5 and 10 mg/kg, i.p.), and the selective TRPA1 antagonist HC-030031 (75 mg/kg, i.p.), respectively 1 hr before zyklophin. Neither compound markedly influenced the incidence of peptide-induced scratching (DiMattio, Cowan and Liu-Chen, unpublished observations). Thus, at present, the molecular targets at which zyklophin acts to elicit scratching remain to be determined. Although both zyklophin and 5΄-GNTI are selective kappa opioid antagonists, there is no evidence that both compounds act on the same targets to induce scratching.
Acknowledgments
This work was supported by NIH grants DA017302, T32DA007237 and P30 DA013429 and a PA Department of Health grant.
Abbreviations
- 5΄-GNTI
5΄-guanidinonaltrindole
- norBNI
norbinaltorphimine
- KOPR
kappa opioid receptor
Contributor Information
K.M. DiMattio, Email: kelly.dimattio@temple.edu.
T.V. Yakovleva, Email: tyakovl@ku.edu.
J.V. Aldrich, Email: jaldrich@ku.edu.
A. Cowan, Email: acowan@temple.edu.
L.Y. Liu-Chen, Email: lliuche@temple.edu.
References
- 1.Kamei J, Nagase H. Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur. J. Pharmacol. 2001;418:141–145. doi: 10.1016/s0014-2999(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 2.Inan S, Dun NJ, Cowan A. Nalfurafine prevents 5'-guanidinonaltrindole-and compound 48/80-induced spinal c-fos expression and attenuates 5'-guanidinonaltrindole-elicited scratching behavior in mice. Neuroscience. 2009;163:23–33. doi: 10.1016/j.neuroscience.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS. J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patkar KA, Yan X, Murray TF, Aldrich JV. [Nalpha-benzylTyr1,cyclo(D-Asp5,Dap8)]-dynorphin A-(1–11)NH2 cyclized in the "address" domain is a novel kappa-opioid receptor antagonist. J. Med. Chem. 2005;48:4500–4503. doi: 10.1021/jm050105i. [DOI] [PubMed] [Google Scholar]
- 6.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Ellis DJ, Millar WL, Reisner LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after cesarean section. Anesthesiology. 1990;72:981–986. doi: 10.1097/00000542-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur. J. Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- 9.Wakasa Y, Fujiwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, Nagase H. Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life Sci. 2004;75:2947–2957. doi: 10.1016/j.lfs.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen L-Y. Comparison of pharmacological activities of three distinct {kappa} ligands (Salvinorin A, TRK-820 and 3FLB) on {kappa} opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J. Pharmacol. Exp. Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 11.Inan S, Cowan A. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur. J. Pharmacol. 2004;502:233–237. doi: 10.1016/j.ejphar.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat. Rev. Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Yosipovitch G. An aberrant parasympathetic response: a new perspective linking chronic stress and itch. Exp. Dermatol. 2013;22:239–244. doi: 10.1111/exd.12070. [DOI] [PubMed] [Google Scholar]
- 14.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 15.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XY, Wan L, Huo FQ, Barry D, Li H, Zhao ZQ, Chen ZF. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol. Pain. 2014;10:4. doi: 10.1186/1744-8069-10-4. (Epub 01/18/2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patkar KA, Murray TF, Aldrich JV. The effects of C-terminal modifications on the opioid activity of[N-benzylTyR1)]dynorphin A-(1–11) analogues. J. Med. Chem. 2009;52:6814–6821. doi: 10.1021/jm900715m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inan S, Dun NJ, Cowan A. Investigation of gastrin-releasing peptide as a mediator for 5 '-guanidinonaltrindole-induced compulsive scratching in mice. Peptides. 2011;32:286–292. doi: 10.1016/j.peptides.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inan S. Pharmacological and neuroanatomical analysis of GNTI-induced repetitive behavior in mice[Ph.D. Thesis] Philadelphia, PA: Temple University; 2010. http://digital.library.temple.edu/cdm/compoundobject/collection/p245801coll10/id/75332/rec/2, archived at: http://www.webcitation.org/66yCyVaZH, [Google Scholar]
- 21.Morgan MA, Pfaff DW. Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav. Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 22.van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der GJ, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 23.Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine decreases early peritoneal innate immunity responses in Swiss-Webster and C57BL6/J mice through the inhibition of mast cell TNF-alpha release. J Neuroimmunol. 2011;232:101–107. doi: 10.1016/j.jneuroim.2010.10.017. [DOI] [PubMed] [Google Scholar]