Abstract
NSCLC arises in the complex environment of chronic inflammation. Depending on lung immune polarization, infiltrating immune cells may either promote or suppress tumor growth. Despite the importance of the immune microenvironment, current staging techniques for NSCLC do not take into consideration the immune milieu in which the neoplasms arise. T-cell subset content was compared between paired tumor-bearing and contralateral lungs, patient and control peripheral blood. The relationship between T-cell subset distribution and survival were evaluated. CD4 and CD8+ T cells were subsetted by CD45RA/CD27 and analyzed for expression of activation, adhesion, and homing markers. Strikingly, T-cell content was indistinguishable between lungs. Compared with peripheral blood, naïve CD4 and CD8 T cells were rare in BAL. CD4+ BAL T cells showed increased CD95 (higher apoptotic potential) and CD103 expression (epithelial adhesion), but decreased CD38 (activation) and CCR7 expression (lymph node homing). CD8+ BAL T cells showed increased CD103 expression and decreased CD28 expression (co-stimulation). Differences in CD28, CD95, and CCR7 expression were more pronounced within memory cells, while differences in CD4+ CD103 expression were more prominent in effector/memory cells. Of these populations, the absence of lung CD4 T cells with an effector-like phenotype (CD45RA+/CD27−) emerged as a predictor of favorable outcome. Patients with a low proportion (≤0.44%) had 90% 5-year survival (n = 10, median survival 2,343 days), compared with 0% (n = 9, median survival 516 days) of patients with a higher proportion. Further study is required to confirm this association prospectively and define the function of this subpopulation.
Keywords: Non-small cell lung cancer, Bronchoalveolar lavage, T-cell subsets, Biomarkers, Prognosis
Introduction
Lung cancer is the second most common cancer and the leading cause of cancer deaths, accounting for 30% of male and 26% of female cancer deaths [1]. With treatment, clinical stage 1A non-small cell lung cancer (NSCLC) has a 5-year survival of 61%, while stage IV disease has a 5-year survival of only 1% [2]. Current lung cancer diagnosis and staging focus only on the detection and anatomic distribution of the tumor. Even the most advanced efforts in molecular staging focus on the characteristics of the tumor, to the exclusion of host factors of potential biological significance, such as the immunological milieu in which the tumor developed. The ability of epithelial tumors to subvert the immune response by a number of mechanisms including induction of T-cell apoptosis [3], secretion of immunosuppressive cytokines (reviewed in [4]), and modulation of immune polarization [5–7] is well appreciated. Inflammatory cells in the tumor milieu may actually promote tumor growth through cytokine production [8, 9], matrix metalloproteinase secretion [10], and induction of angiogenesis [11]. Further, tumor may induce the immune system to self-suppress through recruitment of regulatory T cells [12–14].
The present study represents a 5-year survival analysis of patients undergoing lung resection for NSC lung cancer. At the time of resection or staging, BAL samples were subjected to detailed multiparameter immunophenotyping. Both the tumor-bearing and contralateral lungs were sampled in an effort to identify T-cell populations unique to the tumor-bearing lung. Data analysis revealed, counterintuitively, that the lungs behave immunologically as a single physiological unit, with no detectable differences in immunophenotypic profile between T cells infiltrating the tumor-bearing and contralateral lungs [15]. The central finding in NSCLC patients was a preponderance of activated, apoptosis prone, memory T cells in both lungs.
In the present study, with more than 5 years of patient follow-up, we determine whether differences in BAL T-cell composition observed among patients at the time of tumor resection are of prognostic significance.
Methods
Human subjects
Peripheral venous blood (5 ml) was collected into tubes containing heparin from beef lung (10 units/ml, Upjohn, Kalamazoo MI) and was obtained from 15 normal volunteers and 19 NSCLC patients. Bronchoalveolar lavage (BAL) samples from lungs ipsilateral (BALi) and contralateral (BALc) to the tumor were obtained from the same NSCLC patients prior to tumor resection (2 patients underwent biopsy only). Patients were stage 1A through 3B, with 15 having adenocarcinoma and 4 squamous cell carcinoma. Of the 19 patients, 5 were current smokers, 10 were former smokers (defined as a quit time of 1 year or greater), and 4 were never smokers. Patients had a mean age of 66.5 ± 8.8 (SD) and were followed for at least 5.5 years or until the time of death. Informed consent was obtained from all subjects according to protocols approved by the University of Pittsburgh Institutional Review Board (IRB#9502100 and IRB#970156).
Peripheral blood mononuclear cell (PBMC) preparation
Ten milliliters of heparinized blood from subjects was diluted 1:2 with Dulbecco’s Ca2 ++- and Mg2 ++-free phosphate-buffered saline (PBS-A). PBMCs were obtained by centrifugation over Ficoll-Hypaque gradients (Sigma Diagnostics, Inc., Saint. Louis MO), according to the manufacturer’s instructions. Harvested cells were washed 3 times with 40 ml of PBS-A prior to surface staining. The cell pellet was resuspended in a 15-ml polypropylene conical tube (Falcon, Becton–Dickinson, Franklin Lakes, NJ) in 3 ml of staining buffer (PBS-A containing 4% v/v newborn calf serum and 0.1% w/v NaN3).
Bronchoalveolar lavage cell preparation
After the administration of conscious sedation and topical anesthesia, an Olympus fiber optic bronchoscope was passed transnasally, advanced to a subsegment of the middle lung, and wedged. Bronchoalveolar lavage was conducted by instilling 2 aliquots of sterile, 37°C 0.9% NaCl solution with recovery by hand suction. Returned fluid was kept on ice until processing. Fluid was pooled, filtered through cotton gauze under vacuum, centrifuged at 400×g for 10 min, and resuspended in 3 ml of staining buffer.
Photomicrographs
Experiments were documented with photomicrographs of PBMC and BAL samples. Slides were prepared using a Cytospin-2 cytocentrifuge (Thermo Shandon, Pittsburgh, PA) and Wright–Giemsa stain with an Ames Hema-tek stainer. Photomicrographs were taken with a Nikon Labophot-2 microscope fitted with a Nikon Coolpix 950 digital camera.
Surface staining of PBMC and BAL cells
Resuspended cells (2−15 × 106) were centrifuged in a 15-ml polypropylene conical tube at 400 × g for 5 min at 4°C and decanted to a “dry” pellet. At this point, 2 μl of each fluorescent-labeled antibody was added, and the cell pellet was incubated for 20 min on ice. All samples were co-incubated with CD27 (PE-Cy5 conjugated), CD45 RA (ECD conjugated), and either CD4 (PE-Cy7 conjugated) or CD8 (PE-Cy7 conjugated). Stained cells were then split into 2–9 replicate tubes (depending on the number of cells recovered), providing multiple replicates for CD4 and CD8 CD45RA/CD27 determinations. Each replicate tube was further co-incubated with additional mAb combinations. In this report, we examined the expression of the following markers in CD4 and CD8 differentiation compartments: CD95 (PE conjugated) plus CD28 (FITC conjugated), CD54 (PE conjugated) plus CCR7 (FITC conjugated), CD29 (PE conjugated) plus CD38 (FITC conjugated), or CD31 (PE conjugated) plus CD103 (FITC conjugated). Stained cells were resuspended to a concentration of 5 × 106 cells/ml and fixed in 0.25% paraformaldehyde in staining buffer.
Flow cytometer setup and acquisition
Samples were acquired using a 5-color Beckman-Coulter FC500 flow cytometer (Beckman-Coulter Corp., Fullerton, CA). PMTs were balanced to predetermined target channels using FlowSet beads (Beckman-Coulter Corp., Fullerton, CA). The cytometer was quality controlled on a daily basis using FlowCheck beads to confirm laser alignment and fluidics and FlowSet beads to calibrate fluorescence measurement. The latter was critical to these studies and assures that cells of a given brightness appear in the same fluorescence channel when assayed on different days. Compensation was made with balanced PMTs using single-color stained PBMC and verified using a 5-color stained sample. Daily QC validated our use of the same target channels throughout the study. Acquisition of at least 50,000 events for both CD4+ and CD8+ T-cell subsets was completed at a maximum of 1,000 events/s. The fact that BAL samples are >90% myeloid cells necessitated acquisition of at least 1 × 106 events/sample. Data were analyzed using CXP (Beckman Coulter, Fullerton, CA) and VenturiOne (Applied Cytometry Systems, Dinnington, UK) software.
Statistical analysis
All samples were analyzed as percentages of total live cells within a subset. In cases where a region defining a particular analytic population contained less than 30 events, further subset analysis was not performed on that region. For rare event analysis in the CD4+ effector differentiation subset, statistical testing was conducted on log-transformed values. Normal and patient PBMCs were compared using the 2-sample student’s t test. A three-way comparison between patient PBMC, BALi, and BALc was performed by ANOVA for repeated measures, with Bonferroni correction of post hoc comparisons. Kaplan–Meier survival analysis (log-rank test) was used to compare prognosis within patient BAL and PBMCs after differentiation subsets, and CD4/CD8 ratios were stratified into high and low groups. Covariate comparisons between high and low groups were completed using chi-squared analysis (Table 1). All statistical testing was 2-sided. Statistical analysis and graphics generation were performed using SYSTAT version 12 (Systat Software, San Jose, CA).
Table 1.
CD4+ Effector (CD45RA+/CD27−) percent is an independent prognostic indicator in NSCLC
| Parameter | Low-risk group (low CD45RA+/CD27−%) | High-risk group (high CD45RA+/CD27−%) | P value |
|---|---|---|---|
| Survival (%) | 90.0 | 0.0 | 0.0000 |
| Median/maximum survival (days) | 2,343/2,447 | 516/1,751 | |
| Age (mean ± SD) | 63.8 ± 8.9 | 69.5 ± 8.0 | 0.16 |
| Gender (male/female) | 7/3 | 3/6 | 0.11 |
| Adenocarcinoma/squamous cell carcinoma | 10/0 | 5/4 | 0.02 |
| Stage (1/2/3/unknown) | 5/3/2/0 | 4/3/1/1 | 0.89 |
| Smoking Hx (current/former/never) | 2/6/2 | 3/4/2 | 0.76 |
| Surgery (segmentectomy/lobectomy/pneumonectomy/biopsy only) | 3/5/1/1 | 4/3/1/1 | 0.90 |
| Adjuvant chemo/rad (no/yes) | 5/5 | 6/3 | 0.46 |
| CD4/CD8 ratio (>1.27/≤1.27) | 3/7 | 6/3 | 0.11 |
| CD4+%naive (>0.65/≤0.65) | 7/3 | 3/6 | 0.11 |
Patients were stratified by BAL CD4+/CD45RA+/CD27− status (high or low) and demographic or clinical parameters were compared using the chi-square test in all cases, except for survival (log-rank test) and age (Student’s 2-tailed t test) comparisons. Samples with unknown stage were eliminated from the chi-square analysis of stage
Results
Analytical strategy
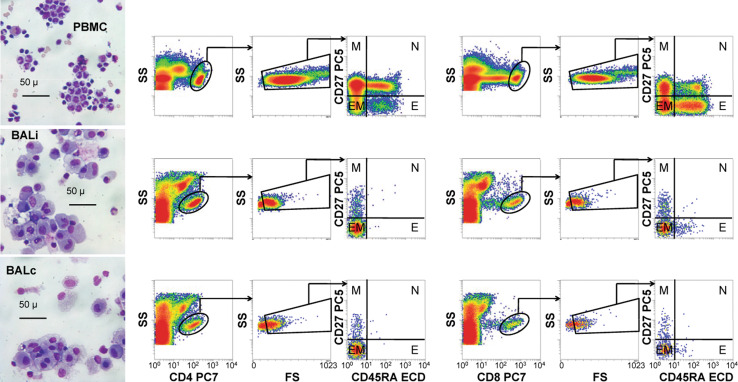
T cells were first classified as either CD4+ or CD8+, on the basis of marker expression plus side scatter (Fig. 1). The CD8dim population, which is contaminated with NK cells [16], was excluded. Next, low light scatter (apoptotic) events were eliminated based on FS (forward scatter) versus SS (side scatter). CD4+ and CD8+ lymphocytes with characteristic light scatter properties were identified among non-apoptotic cells and divided into four discrete differentiation compartments, which have been designated: memory (M, CD27+/CD45RA−), naïve (N, CD27+/CD45RA+), effector/memory (EM, CD27−/CD45RA−), and effector/terminally differentiated (E, CD27−/CD45RA+) [17–22].
Fig. 1.
Microscopy and flow cytometry gating strategy. Wright–Giemsa-stained cytocentrifuge preparations were photographed using a ×40 objective (left column). The images were viewed to assess the general quality and contents of each sample before flow cytometric analysis. The flow gating strategy for these samples involved the selection of either CD4+ or CD8+ events, elimination of low light scatter (apoptotic) events, and classification into memory (M), naïve (N), effector/memory (EM), or effector/terminally differentiated (E) subsets (middle and right panels). Patient ipsilateral bronchoalveolar lavages (BALi), patient contralateral bronchoalveolar lavages (BALc), patient peripheral blood mononuclear cells (PBMC), and normal PBMCs (not shown) were analyzed in this way
T-cell differentiation compartments in peripheral blood and BAL fluid from patients with NSCLC
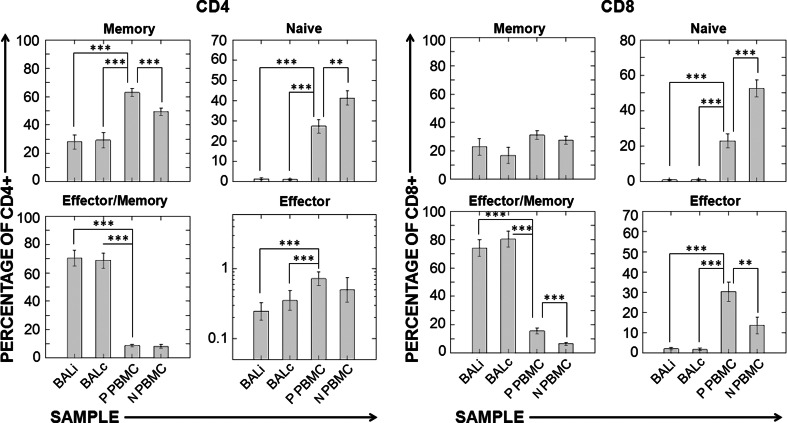
In both CD4+ and CD8+ lymphocytes, the distributions of differentiation compartments were indistinguishable between BALi and BALc. Both of these samples differed considerably from both patient and control PBMCs, with BAL showing a moderate decrease in CD4/CD8 ratio and a marked increase in the proportion of the effector/memory subset in both CD4+ and CD8+ T cells (Fig. 2). Additionally, patients had a lower proportion of naïve CD4+ and CD8+ T cells than control subjects.
Fig. 2.
T-cell differentiation compartments in BAL samples and peripheral blood. CD4+ and CD8+ cells were classified as memory, naïve, effector/memory, or effector as shown in Fig. 1. Ipsilateral and contralateral BALs were compared with each other and with patient PBMC (ANOVA for repeated measures, Bonferroni-corrected post hoc test). Patient PBMC and control PBMC were compared using the Student’s two-tailed t test. Sample size was n = 19 for patients and n = 15 for normal healthy volunteers. Abbreviations BALi patient ipsilateral bronchoalveolar lavage, BALc patient contralateral bronchoalveolar lavage, P PBMC patient peripheral blood mononuclear cells, N PBMC normal healthy volunteer PBMC. ***P < 0.001, **P < 0.01
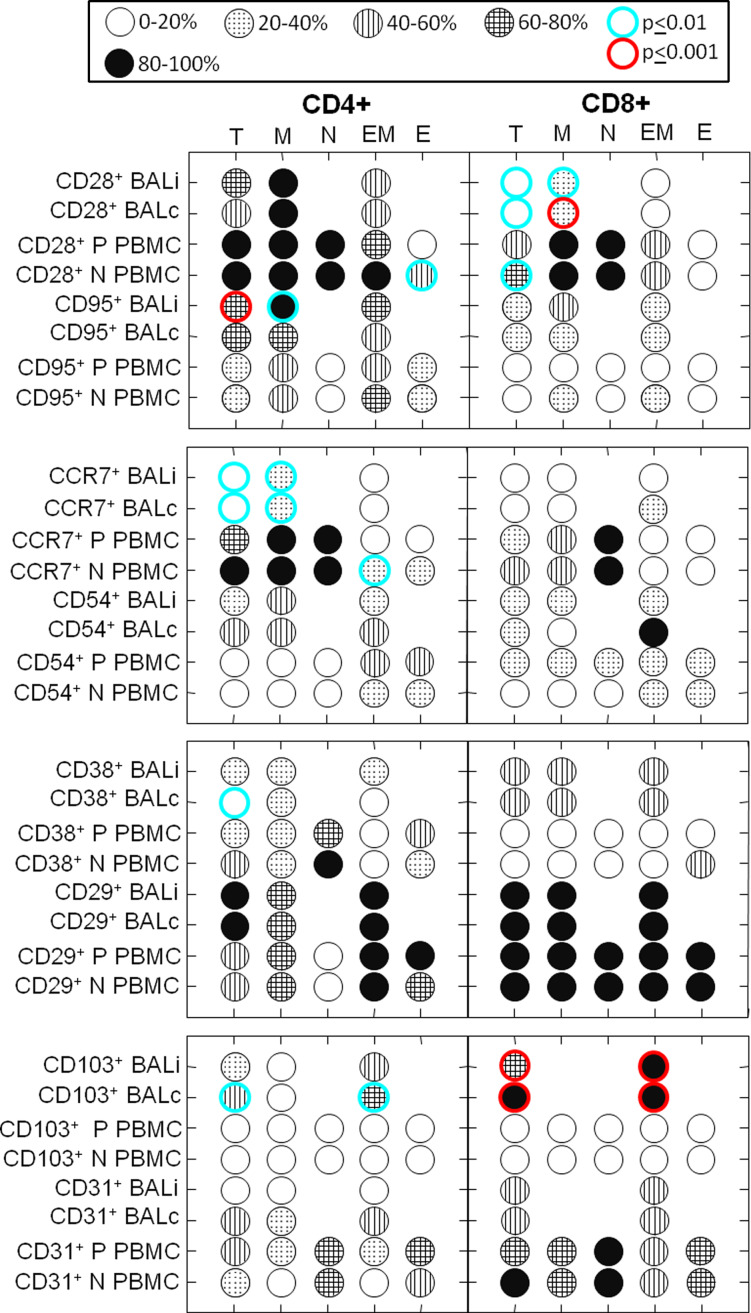
A panel of T-cell subset markers were assessed within the four differentiation compartments and displayed as an icon chart in Fig. 3. We sought to determine the profile of T cells infiltrating the tumor-bearing and the contralateral lung and the peripheral circulation of patients with NSCLC. To this end, we used a panel of T-cell markers in naïve, memory, effector/memory, and effector differentiation compartments of CD4+ and CD8+ T cells: (CD28 (co-stimulatory) [23, 24], CD95 (apoptosis prone) [23, 25], CCR7 [26], CD38 [27](activation, immunoregulation), CD29 [28], CD31 [29], CD103 [30], and CD54 [31] (adhesion molecules). As was determined at the level of the differentiation compartments (Fig. 2), there was no detectable difference between BALi and BALc (P > 0.05, ANOVA for repeated measures, Bonferroni corrected post hoc test) among the battery of markers shown in Fig. 3.
Fig. 3.
BALi and BALc T cells are phenotypically indistinguishable. The proportion of cells positive for a given marker (rows) within a given differentiation compartment (columns) is shown in a “Consumer Reports” icon graphic, where darker shading indicates greater prevalence. In cases where data were sparse (<30 cells in the column category), no further subsetting was performed and no circle is shown. Columns represent CD4 and CD8 differentiation compartments based on the expression of CD45RA and CD27. Rows indicate marker expression and the source of cells. BAL performed on lungs ipsilateral and contralateral to the tumor was compared with each other and with patient PBMC (ANOVA for repeated measures, Bonferroni corrected post hoc test). Patient PBMC and control PBMC were compared using Students two-tailed t test. In no cases did ipsilateral and contralateral BALs differ significantly from each other (P > 0.05 for all). All comparisons shown here with lighter (P < 0.01) or darker circle outlines (P < 0.001) are significantly different compared with patient PBMC. In the on-line color version of this figure, these are shown as aqua and red, respectively. Due to the large number of comparisons made, a result was not considered significant unless P < 0.01. Sample size for CD28 and CD95 patients samples was n = 11, all other patient samples were n = 4. Normal healthy volunteer sample size was n = 15. Abbreviations BALi patient ipsilateral bronchoalveolar lavage, BALc patient contralateral bronchoalveolar lavage, P PBMC patient peripheral blood mononuclear cells, N PBMC normal healthy volunteer PBMC, T total T cells, M memory, N naïve, EM effector/memory, E effector/terminally differentiated
Patient BAL T cells differed from their peripheral counterparts in multiple ways. CD4+ BAL T cells showed increased CD95 (higher apoptotic potential) and CD103 expression (epithelial adhesion), but decreased CD38 (activation) and CCR7 expression (lymph node homing). CD8+ BAL T cells showed increased CD103 expression and decreased CD28 expression (co-stimulatory molecule). Differences in CD28, CD95, and CCR7 expression were more pronounced within the memory compartment, while the differences in CD103 expression were more prominent in CD4+ effector/memory cells. When compared with normal volunteers, patient peripheral blood T cells had decreased CD28 expression among CD4+ effectors, less CCR7 expression among CD4+ effector/memory cells, and less CD28 expression among total CD8+ T cells. There were no statistical differences noted in CD29, CD31, or CD54 expression between all comparisons.
Prognostic markers
CD4+ and CD8+ content (expressed as a ratio) and the proportion of CD4+ and CD8+ cells distributed among the four differentiation compartments were tested for prognostic significance. The median value was determined for each parameter measured in each sample (BALi, BALc, and PBMC). For each parameter, patients were stratified into those above versus below or equal to the median. These strata were used to perform Kaplan–Meier survival analysis, where the median and maximum follow-up times for patients surviving at the time of analysis (n = 9 of 19) were 1,751 and 2,447 days, respectively. Differentiation compartment percentages and CD4/CD8 ratios were measured multiple times for the same patient (i.e., in multiple tubes measuring CD4 and CD8 in combination with other markers). These data were pooled across tubes, increasing the number of events for subset analysis. Data were analyzed separately for each lung. Additionally, since T-cell subset data ipsilateral and contralateral to the tumor were consistently indistinguishable (Figs. 2, 3, 4), ipsilateral, and contralateral subset data were further pooled using a flow cytometry event weighted average for analysis of prognostic significance.
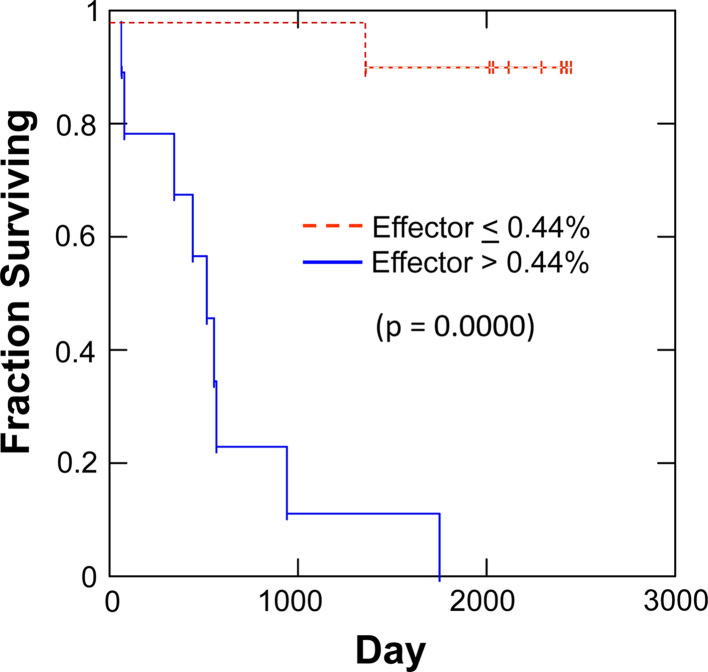
Fig. 4.
A low CD4+ effector (CD45RA+/CD27−) percentage in BAL fluid at the time of surgical resection is a favorable prognostic indicator. Nineteen patients were studied. Results from BALi and BALc were pooled due to the lack of statistical differences between the 2 groups in any of the comparisons made in Figs. 2 or 3. All data points were stratified into high and low groups with values greater than the median considered high and values less than or equal to the median considered low. Kaplan–Meier survival analysis with the log-rank hypothesis test was used to compare survival between high and low groups
When stratified on the pooled CD4/CD8 ratio, the CD4-low group had greater raw survival (70.0% vs. 22.2%). Kaplan–Meier analysis of pooled data revealed no significant difference in mean survival time (1,811 vs. 1,166 days, P = 0.09, log-rank test). The effector (CD45RA+/CD27−) compartment was rare between both BALi and BALc CD4+ T cells (BALi/BALc pooled geometric mean = 0.34%, lower 95% CI = 0.21%, upper 95% CI = 0.57%). Despite the scarcity of the CD45RA+/CD27− CD4 effectors, their presence provided a dramatic prognostic indicator. When BALi and BALc CD4+CD45RA+/CD27− effector T-cell percentages were analyzed separately as prognostic indicators, both were correlated with survival (P = 0.05 and 0.003, log-rank test, respectively). When data from ipsilateral and contralateral lungs were pooled by taking a weighted average, results were even more striking (Fig. 4). Patients with a proportion of CD4 effector cells, above the median (0.44% of BAL CD4+ T cells), had 0.0% 5-year survival (516 day median survival), compared with those below or equal to the median (90.0% 5-year survival, 2,343 day median survival, P = 0.0000, log-rank test). All demographic parameters shown in Table 1 were also evaluated as covariates of CD4 effector cell content by Cox proportional hazards regression. None were statistically significant. Of multiple demographic and clinical parameters, only histology (adenocarcinoma vs. squamous cell carcinoma) was weakly correlated with the CD4+/CD45RA+/CD27− stratum (P = 0.02, chi-square, Table 1).
The BAL CD4 naive (CD45RA+/CD27+) compartment was also weakly correlated with survival. Patients with a proportion of these cells above the median (0.65% of BAL CD4+ T cells) had poorer survival (22% 5-year median survival, 555-day median survival) than those below or equal to the median (70.0% 5-year survival, 2,256 day median survival, P = 0.05, log-rank test). All other survival analyses within patient PBMCs and BALs were not statistically significant.
Discussion
There is no standard approach to the management of resectable NSCLC. Prognostic indicators which have been proposed to guide therapy include pathological staging, mediastinoscopy, PET imaging, and endoscopic ultrasonic techniques. Although some of these methodologies are of proven value, none take into consideration the immune milieu in which the neoplasms arise. The relationship between the tumor and local immunity is complex. The composition of immune cells localized to the lung may predict response as an indicator of the ability of the immune system to eliminate residual disease after therapy. Alternatively, the extent to which local T-cell subsets are skewed may reflect the degree to which the immune system is effectively subverted, directly by the tumor or indirectly by environmental effects such as smoke exposure. In either case, there is potential to gain early prognostic information. Bronchoalveolar lavage provides a non-invasive means of sampling the immune environment of the lung. In the current study, BAL was used preoperatively to assess the distribution of T cells in the tumor-bearing and contralateral lungs of patients with resectable NSCLC.
Because BAL T cells are selected for their tissue tropism, their subset distribution differed considerably from that observed in patient or healthy control peripheral blood. Notably, BAL T-cell subsets were characterized by a paucity of naïve cells, greater susceptibility to apoptosis, resistance to co-stimulation (CD28−), reduced activation (CD38−), increased epithelial adhesion (CD103), and decreased lymph node homing (CCR7−).
Despite the differences between BAL and peripheral blood, the most striking finding was that it was not possible to distinguish between the tumor-bearing and contralateral lungs on the basis of T-cell subset distribution. Why this should be the case is not obvious. Local immune perturbations of one lung cannot directly propagate to the contralateral lung because the lungs are anatomically separate with respect to vascular and lymphatic supply. The independent nature of the lungs is particularly evident in the pattern of tumor metastasis. Spread to the contralateral lung is relatively rare in comparison with liver and brain [32]. One explanation is that the immune environment in both lungs is affected by a common factor, either external (such as tobacco smoke) or systemic (such as cytokines secreted by the tumor into the peripheral circulation). Alternatively, in analogy to sympathetic ophthalmia [33], immune cells may be responding to autoantigens present in both lungs that were revealed by tumor-induced tissue damage to one lung.
Despite the striking similarities between ipsilateral and contralateral lungs, there was sufficient heterogeneity among patients to stratify them on the basis of CD4/CD8 distribution and within these subsets, on differentiation compartments. Two potential prognostic predictors emerged from this analysis: BAL CD4 effector content (a strong predictor) and CD4 naïve content (a weak predictor). Having low proportions of the rare CD4+/CD45RA+/CD27− effector/terminally differentiated compartment was a very strong predictor of a favorable outcome (P = 0.000009, Mantel log-rank test). The median value and cutpoint for stratification of this subset was 0.44% of CD4+ cells. Five-year actuarial survival of patients with values below or equal to this cutpoint was 90%, compared with 0% of patients above the cutpoint. This association is supported by two lines of evidence that converged during data analysis: (1) CD4 effector determinations performed in independent samples from tumor-bearing and contralateral lungs were both predictive of survival (pooled data were strongest); (2) the original observation was made using CD4 effector determinations from a single tube; the observation held up (and was strengthened) when subset data from 2 to 9 tubes (replicate in CD4, CD45RA and CD27 but disparate in 2 other markers) were pooled as event-weighted averages. That the presence of such a minor subset at the time of tumor resection is highly correlated with poor survival months and later is suggestive of a direct biological role. Although the function of CD4+/CD45RA+/CD27− cells has not been established, they have been termed CD4 effector/terminally differentiated here because of their phenotypic correspondence to the well-characterized CD8+ compartment [17], which we have shown to be apoptosis prone and T-cell receptor zeta deficient in head and neck cancer patients [34]. Consensus thinking is that among CD4+ T cells, the CD45RA+/CD27− compartment corresponds to the most antigen experienced CD28−/CCR7− subset. These cells are characterized by short telomeres, high turnover, a skewing toward IL-2 negative/IFN-γ+ cytokine response, and expression of cytotoxic effector proteins [22]. In the context of local response to NSCLC, this CD4 differentiation compartment may act as an immune modulator similar to regulatory T cells (T-regs) [35]. Cells of this phenotype comprise a small minority of classical regulatory T cells [36], which are conventionally defined as CD25+, FoxP3+, CD39+ [37]. Although the function of the CD4 effector/terminally differentiated subset is undefined in NSCLC, the possibilities exist that: (1) its presence inhibits local antitumor effector responses; (2) it creates a local environment favorable for tumor growth (e.g., angiogenesis signals or tumor growth factors); and/or [3] (3) Its presence is indicative of an exhausted and inefficacious local immune response. Whatever the ultimate function of these cells, it is conceivable that they play a role in polarizing the lung milieu in favor of the tumor.
We also observed that a relative increase in the proportion CD4+ T cells (CD4/CD8 ratio ≤1.27) was weakly associated with an unfavorable outcome (22% vs. 70% 5-year survival, P = 0.09). This is consistent with the observation that a high CD4% in NSCLC pleural effusions is associated with a poorer prognosis [38]. Other research indicates that a high degree of CD8+, but not CD4+ T-cell tumor infiltration, is associated with a favorable prognosis in NSCLC [39]. On the other hand, some groups have found that high absolute number of tumor-infiltrating CD4+ and CD8+ T cells is a favorable prognostic indicator in NSCLC [40, 41]. However, the mechanistic significance of a high pulmonary CD4/CD8 ratio in the progression of NSCLC is unknown.
Clearly if the CD4 effector/terminally differentiated content of BAL fluid is to be recommended as a prognostic marker, these studies must be repeated prospectively. The present study represents a retrospective reanalysis of data collected more than 5 years ago in an unsuccessful attempt to define a local immune response unique to the tumor-bearing lung. If the present observation concerning the significance of BAL CD4 effector/terminal differentiated T cells holds, it could provide an independent prognostic indicator at the time of clinical presentation. Although in the present study most BALs were collected immediately prior to tumor resection, they could be performed routinely during staging, before a therapeutic modality is chosen.
Acknowledgments
The authors would like to thank Drs. Theresa Whiteside and Jill Siegfried for their careful reading and critique of this manuscript. This work was supported by the Heart Lung and Esophageal Institute of the University of Pittsburgh Medical Center, and the University of Pittsburgh Lung Cancer SPORE: NCI P50-CA90440.
Contributor Information
A. D. Donnenberg, Phone: +412-623-7780, FAX: +412-623-7778, Email: donnenbergad@upmc.edu
V. S. Donnenberg, Email: donnenbergvs@upmc.edu
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1718. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 3.Gastman BR, Atarashi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–5364. [PubMed] [Google Scholar]
- 4.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–S283. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F. Quantitative analysis of Th1, Th2 and TGF-&bgr;1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer. 1998;77:7–12. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–3853. [PubMed] [Google Scholar]
- 8.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Batra RK, Lin Y, Sharma S, Dohadwala M, Luo J, Pold M, Dubinett SM. Non-small cell lung cancer-derived soluble mediators enhance apoptosis in activated T lymphocytes through an IκB kinase-dependent mechanism. Cancer Res. 2003;63:642–646. [PubMed] [Google Scholar]
- 10.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 11.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et Biophysica Acta (BBA) Rev Cancer. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 13.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 14.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S, Whiteside TL. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg VS, Luketich JD, Landreneau RJ, Popovich AM, Donnenberg AD (2004) Influx and apoptosis of activated effector memory T cells in both lungs of patients with unilateral non-small cell lung cancer (NSCLC). In: Journal of clinical oncology, 2004 ASCO annual meeting proceedings, vol 22, no. 14S (July 15 Supplement), New Orleans, LA, p 7373
- 16.Perussia B, Fanning V, Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983;131:223–231. [PubMed] [Google Scholar]
- 17.Baars PA, Ribeiro Do Couto LM, Leusen JH, Hooibrink B, Kuijpers TW, Lens SM, van Lier RA. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27− human T cells. J Immunol. 2000;165:1910–1917. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- 18.Baars PA, Maurice MM, Rep M, Hooibrink B, van Lier RA. Heterogeneity of the circulating human CD4+ T cell population. Further evidence that the CD4+CD45RA-CD27− T cell subset contains specialized primed T cells. J Immunol. 1995;154:17–25. [PubMed] [Google Scholar]
- 19.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8+ T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–5550. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 20.De Jong R, Brouwer M, Hooibrink B, van der Pouw-Kraan T, Miedema F, van Lier RAW. The CD27− subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- 21.Hamann DR, Baars PA, Rep MHG, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RAW. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytom A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 23.Heriberto P-G, Dolores A-C, Hector F-V, Juan Jose M, Jose Sullivan L-G. Effector, memory and naive CD8+ T cells in peripheral blood and pleural effusion from lung adenocarcinoma patients. Lung Cancer (Amsterdam, Netherlands) 2005;47:361–371. doi: 10.1016/j.lungcan.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins M, Taylor P, Norton S, Urdahl K. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10:1408–1417. doi: 10.1096/fasebj.10.12.8903511. [DOI] [PubMed] [Google Scholar]
- 28.Woods ML, Shimizu Y. Signaling networks regulating {beta}1 integrin-mediated adhesion of T lymphocytes to extracellular matrix. J Leukoc Biol. 2001;69:874–880. [PubMed] [Google Scholar]
- 29.Watt SM, Gschmeissner SE, Bates PA. PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymphoma. 1995;17:229–244. doi: 10.3109/10428199509056827. [DOI] [PubMed] [Google Scholar]
- 30.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/S0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 31.Rothlein R, Dustin M, Marlin S, Springer T. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 32.Mountain CF, Carr DT, Anderson WA. A system for the clinical staging of lung cancer. Am J Roentgenol Radium Ther Nucl Med. 1974;120:130–138. doi: 10.2214/ajr.120.1.130. [DOI] [PubMed] [Google Scholar]
- 33.Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye (Lond) 1997;11(Pt 2):213–216. doi: 10.1038/eye.1997.54. [DOI] [PubMed] [Google Scholar]
- 34.Kuss I, Donnenberg AD, Gooding W, Whiteside TL. Effector CD8+CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rallón NI, López M, Soriano V, García-Samaniego J, Romero M, Labarga P, García-Gasco P, González-Lahoz J, Benito JM. Level, phenotype and activation status of CD4+FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin Exp Immunol. 2009;155:35–43. doi: 10.1111/j.1365-2249.2008.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi K, Saito S, Kamamura Y, Katakawa M, Monden Y. Prognostic value of CD4+ lymphocytes in pleural cavity of patients with non-small cell lung cancer. Thorax. 2001;56:639–642. doi: 10.1136/thorax.56.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang X, Xia X, Wang C, Gao F, Shan N, Zhang L, Zhang LA. High number of CD8+ T cells infiltrated in NSCLC Tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol 18:24–28 [DOI] [PubMed]
- 40.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]