Abstract
Body composition analysis has become a useful tool in both clinical and research settings. Its use in the pediatric population is complicated by the rapid periods of growth and physical development that are characteristic of infancy, childhood, and adolescence. A thorough understanding of the changing nature of body composition during this time is essential for choosing the most appropriate measurement technique for a given individual, population, or clinical question. Growing evidence suggests that tissues such as fat, muscle, and bone are intimately involved in the regulation of whole body energy metabolism. This knowledge, when coupled with advancements in imaging techniques such as MRI and PET-CT, offers the possibility of developing new models of “functional” body composition. These models may prove to be especially important when assessing malnutrition and metabolic risk in patients with chronic disease.
Keywords: Body Composition, Obesity, Fat Mass Index, Lean Body Mass Index, Body Mass Index, Metabolic Syndrome
Body composition analysis is an important tool for the pediatric endocrinologist with applications in both clinical and research settings. The goal of this review is to outline basic concepts underlying the assessment of body composition in the pediatric population. Particular attention will be paid to the challenges of using these techniques during periods of rapid growth and development. The use of body composition to accurately assess obesity and metabolic risk will be highlighted, as will its use in specific chronic disease groups. Finally, new techniques for the qualitative assessment of adipose tissue will be discussed with a focus on future directions.
Basic Concepts
Body composition assessment aims to quantify the amount and relative proportions of body tissue compartments, and in some cases, their cellular, molecular, and atomic components. The five-level model of human body composition developed by Wang et al. (1) defines a series of interrelated, increasingly complex levels (Table 1) that provide an organizational framework for approaching questions related to body composition and identifying appropriate methods of analysis. For example, an investigator interested in changes in bone mass during growth and development may start with an anthropometric measure such as height as a general measure of bone at the whole body level. Alternatively, total body bone mineral content obtained using dual energy x-ray absorptiometry (DXA) provides an accurate measurement at the tissue level. Total body calcium can be estimated from total body bone mineral content (calcium(gm)=0.34 x bone mineral content(gm)) based on the known composition of hydroxyapatite, yielding a measure (total body calcium) at the atomic level (2).
Table 1.
The five-level model of body composition1
| Level | Information Obtained | Methods of Determination |
|---|---|---|
| I. Atomic | Elemental: O,C,H, N, Ca, P, S, K, Na, Cl | Neutron Activation, total body potassium counting with 40K |
| II. Molecular | Water, protein, lipid, osseous and extraosseous mineral, glycogen | Total body water, neutron activation, magnetic resonance spectroscopy |
| III. Cellular | Fat, body cell mass, extracellular fluid, extracellular solids | Total body water, isotope dilution for sodium bromide, total body potassium counting with 40K |
| IV. Tissue | Adipose, skeletal, muscle, organs | Hydrodensitometry, dual energy X-ray absorptiometry, bioelectric methods, computed tomography, magnetic resonance imaging |
| V. Whole Body | Height, weight, circumferences, segment lengths, skinfold thickness | Anthropometry |
Adapted from Zemel and Barden 2004 (3)
Beyond the five-level model, approaches to body composition analysis can be organized according to the number of compartments described. Two-compartment models divide the body into fat mass (FM) and fat free mass (FFM) such that total body mass = FM + FFM. Two compartment methods include anthropometry, densitometry, bioelectric impedance, or isotope dilution for total body water. The density of FM and FFM usually are assumed to be constant. This may be a reasonable assumption for FM, which is defined by the ether-extractable lipid fraction of the body(4). FFM, however, is a complex tissue compartment composed of skeletal muscle, organs, bone, and supporting tissue. FFM hydration and the contribution of osseous mineral to FFM in particular are known to introduce uncertainty in the estimation of FFM. This is an especially important consideration in children, as these components of FFM change with growth and development (5–7), and in disease states.
Three-compartment models divide body mass further into FM, non-osseous lean body mass (LBM) and bone mass such that total body mass = FM + LBM + bone mass. DXA offers a quick, convenient means of three compartment analysis. FM, LBM, and bone mass have unique tissue densities and therefore attenuate energy beams differently, allowing for accurate quantification of each tissue. Because DXA measures bone mineral content directly, this method eliminates one of the major sources of variability inherent in the estimation of the FFM in the two-compartment model. Though often used interchangeably in the literature, it is important to note that FFM and LBM differ in that LBM contains a small amount (2–3% of body mass) of essential lipid (8).
Multi-component models using methods or combinations of methods to measure FM + three or more components of FFM have also been developed (Table 2). The accuracy of body composition assessment improves with the number of components measured as there is less dependency on the assumption that FFM density is constant (9). For example, the formula for a four-component model might include density values for fat, water, mineral and protein (0.9007 g/mL, 0.9937g/ml, 3.038 g/mL, 1.34 g/mL, respectively) compared to a two-compartment model which would include density values for only FM and FFM (0.9007 g/mL, 1.100 g/mL, respectively) (10).
Table 2.
Examples of multi-compartment models of body composition1
| Model | Components Measured | Techniques | Assumptions |
|---|---|---|---|
| Two-Compartment | Fat mass + fat free mass | Anthropometry, underwater weighing, isotope dilution for total body water, bioelectric methods | Constant densities of fat mass and fat free mass |
| Three-Compartment | Fat mass + lean body mass + bone | DXA | Constant densities of fat mass and lean body mass |
| Fat mass + total body water + non-aqueous solids | Underwater weighing, Bod Pod, isotope dilution for total body water | Fixed ratio of protein to minerals | |
| Four-Compartment | Fat mass + lean body mass + intracellular water + extracellular water | DXA, isotope dilution for total body water and sodium bromide (or bioelectric methods for intra- and extracellular water | |
| Fat mass + lean body mass + total body water + protein | DXA, underwater weighing, isotope dilution for total body water | Fixed ratio of K, 2H20 or 2H180 and NaBr in cellular components | |
| Fat mass + body cell mass + extracellular water + extracellular solids | DXA, isotope dilution for total body water, total body potassium counting with 40K | ||
| Five Compartment | Fat mass + lean body mass + total body protein + total body nitrogen + glycogen | Neutron Activation | Glycogen estimated from total body protein or by magnetic resonance spectroscopy |
| Fat mass + total body protein + total bone mineral + extracellular water + intracellular water | Neutron Activation |
Adapted from Zemel and Barden 2004 (3)
A further consideration beyond the compartment models discussed above is the distribution of adipose tissue on the body (fat patterning). Traditionally fat patterns have been described as “android” with greater trunk fat and less extremity fat and “gynoid” with greater hip and extremity fat and less trunk fat (11). Observations that the risk of cardiometabolic disease may vary based upon these patterns of fat distribution have raised important questions regarding qualitative differences among fat deposits.
Visceral fat, located in the trunk, is thought to be more metabolically active than subcutaneous fat and is a strong risk factor for insulin resistance, type 2 diabetes and cardiovascular disease (12–14). Conversely, lower extremity subcutaneous fat has been found to be associated with increased insulin sensitivity and may be protective against the development of cardiometabolic disease by suppressing the release of free-fatty acids (15, 16). Anthropometric measures such as waist circumference provide simple means of estimating fat distribution that supplement measures of excess adiposity such as BMI, but ultimately cannot definitively differentiate between visceral and subcutaneous adipose tissue (17). Single slice CT and MRI are currently the most frequently used methods for quantifying visceral fat. Recent advances in software technology permit estimation of visceral fat mass, area and volume using DXA(18).
Ectopic fat deposition within and around lean tissue, organs and bone may impair local tissue function and whole body glucose and lipid metabolism via lipotoxicity, impaired blood flow, and cytokine release (19). Studies in adults and children using CT and MRI to assess fat deposition in the thigh have found that adipose tissue below the fascia lata, infiltrating muscle groups (intramuscular adipose tissue, IMAT), and within myocytes (intramyocellular lipid) are all associated with insulin resistance and type 2 diabetes, while subcutaneous adipose tissue is not (20–25). Intrahepatic lipid deposition may hinder glucose metabolism and has been shown to be inversely associated with insulin sensitivity in obese children (26). Accumulation of adipose tissue within the bone marrow may also be detrimental, and has been found to be positively correlated with visceral adipose tissue and inversely associated with bone mineral density in adults (27, 28).
Finally, distinctions between brown and white adipose tissue may be important when considering fat mass. Brown adipose tissue (BAT) is highly vascularized, rich in mitochondria and highly metabolically active. Its primary function is to maintain body temperature upon cold exposure through non-shivering thermogenesis, a mechanism whereby uncoupling of the mitochondrial respiratory chain leads to the generation of heat instead of ATP (29). BAT is most abundant during infancy, a period of increased susceptibility to hypothermia owing to low skeletal muscle content and high body surface area relative to volume (30). It is now known that functional BAT persists beyond infancy, distributed primarily in the supraclavicular, neck, paravertebral, and suprarenal areas of the body (30, 31). The presence and volume of functional BAT increases with puberty and has been found to be positively correlated with muscle volume, amount of cortical bone, and bone size (32–34). BAT activity has also been found to be negatively correlated with BMI and percentage body fat in children and adults, suggesting a possible link between BAT activity and disordered weight gain (35, 36). The quantification and activity of BAT is typically determined using 18F-fluorodeoxyglucose positron-emission tomography (PET) integrated with CT.
Measurement and Interpretation of Body Composition
There is no in vivo gold-standard for the measurement of body composition in children. One or more methods may be appropriate for use based upon the individual (or population) of interest and the type of information that is desired. A description of the measurement techniques appropriate for a given level or compartment of body composition is available in Tables 1 and 2. Measurement techniques typically increase in difficulty, expense and potential risk to the individual as greater levels of detail are achieved. With the exception of cadaveric studies, most other methods of body composition analysis are indirect and rely on assumptions that have the potential to introduce bias into the results. A detailed technical discussion of the different methodologies is beyond the scope of this review but is available elsewhere (2, 3, 37, 38).
In situations where the goal is simply to quantify a given tissue or body compartment, the measurements attained using the methods outlined above may suffice. Often, however, the goal is to use body composition analysis as a means to describe populations or assess risk of disease in an individual patient. There are a number of indices currently in use that allow for the use of body composition analysis in this manner. Each comes with unique benefits and drawbacks that must be carefully considered in relation to the population and question of interest. Table 3 provides a list of indices for which there are reference data in the pediatric population – an important consideration for interpretation of body composition results.
Table 3.
Sources of Body Composition Reference Data in Children and Adolescents
| Measure | Source | Comment |
|---|---|---|
| Percentage Body Fat | Ogden 2011 (62) | Percentage body fat estimated by DXA, available for ages 8–19 (NHANES) |
| FFMI | Kelly et al. 2009 (63) | Described as LBMI, actually FFMI estimated by DXA, available for ages 8-adult (NHANES) |
| Waist Circumference | Fernandez (64) | Available for ages 2–19, includes group-specific percentiles for Whites, Blacks, and Mexican Americans |
| Bone Mineral Content, Bone Mineral Density | Zemel et al. 2011 (65) | Total body, lumbar spine, total hip, femoral neck, forearm for African Americans and non-African Americans ages 5–20 (Bone Mineral Density in Childhood Study) includes equation for adjusting for height |
| Baxter-Jones et al | Longitudinal reference ranges for bone mineral content, for ages 8 to 25 years for African Americans, Whites, Asians and Hispanics from Canada and the U.S. {Baxter-Jones, 2010 #4032} | |
| Total Body Water and other components | Ellis et al | Reference ranges for total body water, total body potassium and total bone mineral content for European-Americans, African Americans and Mexican Americans based on 856 healthy children, ages 5 to 18y {Ellis, 2000 #210} |
| Fomon et al. 1982 | Reference ranges for composition and density of fat free body mass (protein, total body water, intra- and extracellular water, osseous and non-osseous mineral, carbohydrate, total body potassium and density of fat-free mass for birth to 10 years of age {Fomon, 1982 #55} | |
| Butte et al. | Reference ranges for fat mass, fat-free mass, percent body fat, and composition and density of fat free mass for infants, birth to 24 months of age based on a longitudinal sample of 76 infants.{Butte, 2000 #211} | |
| Density and hydration of lean tissue | Wells et al. | Based on 533 individuals (91% white), ages 4–23 y in the U.K. {Wells, 2010 #499} |
Weight for length is a simple index commonly used for infants and reference data is applicable for use in diverse populations across the world (39). Waist circumference is another simple anthropometric measure that may be useful as estimates of overall and visceral adiposity and has been associated with cardiometabolic risk factors in childhood (40, 41). Lack of a standard measurement technique and a difficulty in measurement among obese individuals is a limitation of this technique (42–44). Upper arm fat and muscle areas, calculated from measurements of upper arm circumference and triceps skin fold thickness, have been used to predict body composition and nutritional status but these vary as a function of age and body size, and are based on several approximations that may limit accuracy (45).
The body mass index (BMI,) calculated as weight (kg)/stature (m)2 is the most widely used index in children and adults. BMI is easily obtained from simple anthropometric measures and has established reference standards (46, 47) making it an attractive screening tool for the assessment of both malnutrition and excess adiposity (48). Of note, the CDC BMI reference standards excluded children from contemporary NHANES surveys because of the obesity epidemic. Therefore, the charts do not represent the present distributions of BMI among current U.S. children. An important underlying assumption of BMI is that weight scales to height2, and therefore BMI is independent of height. This assumption has generally been found to be true in adult populations (48, 49) but not in children, where greater height is associated with greater BMI (50–54). Another assumption of BMI is that individuals of different stature but the same BMI (or BMI percentile) have identical fractional body composition (55). This assumption also has been challenged in the pediatric population, where the proportions of body mass attributable to FM and FFM are highly variable and dependent upon age and pubertal maturation (56).
Compartment specific indices such as the fat mass index (FMI, fat mass(kg)/stature(m)2), fat-free mass index (FFMI, fat-free mass(kg)/stature (m)2), and lean body mass index (LBMI, lean body mass(kg)/stature(m)2) have been proposed as more accurate indicators of adiposity and malnutrition. FM and FFM can be estimated using techniques for assessing two-compartment models, while (non-osseous) LBM requires a three-compartment approach (table 2). There is still an assumption that FM, FFM, and LBM scale to height2 in these indices, however the compartments of total body mass can be assessed independently. FMI and FFMI were found to be more sensitive indicators of nutrition status compared to BMI or percent body fat when applied to data from the Minnesota Semi-Starvation Study (57). Analyses of FMI and FFMI in children have revealed that increases in BMI during childhood are largely driven by increases in FFMI and not FMI, suggesting that BMI may not accurately represent adiposity in all situations (53, 58, 59). The use of FMI, FFMI, and LBMI in children is limited due to a lack of robust reference data.
Percent body fat (fat mass(kg)/body mass(kg)* 100) can be obtained from body composition methods that estimates fat mass and provides more valuable information than BMI by differentiating between fat and fat free mass. A study comparing BMI to percent body fat estimated by DXA found that less than half of children and adolescents defined as overweight by BMI (BMI ≥ 85th percentile) had high adiposity defined by percent body fat (60). The use of percent body fat estimated from skinfold thicknesses has also been shown to discriminate the presence of absence of metabolic syndrome in children and adolescents with moderate accuracy (61). The use of percent body fat is limited by the fact that it does not take into account the effects of height, body proportion, and the independent contributions of absolute amounts of fat and fat free mass to health and disease.
Growth and Development
Body composition changes dramatically over the lifespan in humans. Careful consideration of these underlying changes must be taken into account when applying and interpreting body composition analyses in the pediatric population.
Infancy is a time of rapid growth and is associated with marked changes in compartment, tissue, and chemical composition. In infants, extracellular water and organ mass comprise a larger proportion of body mass compared to children and adults (66). This results in results in a higher hydration of fat-free mass and can bias estimates of body composition (67). Fat mass as a proportion of body weight is also higher in infants. Percent body fat in humans peaks between 3–6 months of age, near 29% in males and 32% in females (68). Sex differences in infant body composition extend beyond percent body fat as males have been shown to have greater fat-free mass, total body water, total body potassium and bone mineral content (68).
Growth during childhood progresses at a slower pace with less pronounced changes in body composition. The sex differences in percent body fat observed during infancy continue through this period. A small increase in the rate of weight-, height-, and body breadth-gain is observed in the mid-childhood growth spurt occurring around ages 6–8. A rebound in body mass occurs at approximately the same time. BMI peaks near the end of infancy, declines in early childhood before reaching a nadir around age 5–6, then increases throughout the remainder of childhood, adolescence, and adulthood. The timing of this BMI rebound may be genetically regulated (69).
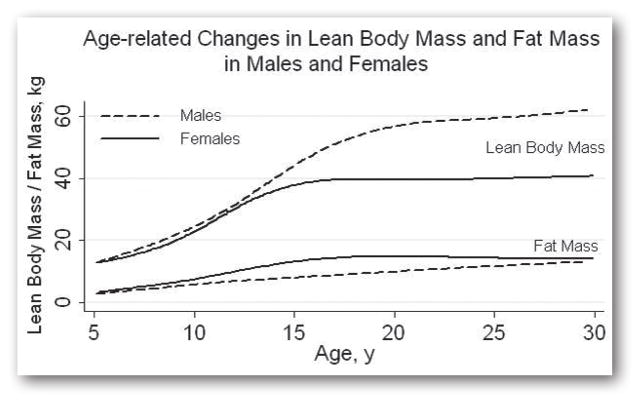
The profound changes in body compartments, chemical, and tissue composition occurring during adolescence are primarily due to the effects of gonadal sex steroids. The adolescent growth spurt results in rapid increases in body mass and height. Existing sex-differences in percent body fat become more pronounced. Females gain more fat mass relative to lean mass, in part due to the growth of breast tissue and the gradual development of the female body shape with fat deposition at the hips and thighs. Many males experience a pre-pubertal fat spurt followed by rapid gains in lean body mass and reductions in fat at the extremities (this includes the triceps, a site of skin fold thickness measurement). These sex-specific changes in body composition are illustrated in Figure 1. Bone mineralization, cortical density and trabecular density all increase during adolescence, with 40% of peak bone mass accruing during this time (70–72).
Figure 1.
Body composition differences between males and females are present at all ages, but pronounced differences emerge in adolescence with greater lean body mass in males and greater fat mass in females
Body composition continues to change through adulthood, although these changes are less pronounced than those seen during infancy, childhood and adolescence. Adults continue to gain weight throughout adulthood in most westernized societies, a phenomenon not always observed in traditional non-westernized societies. Increases in weight and BMI throughout adulthood are largely attributable to increases in fat mass as both FMI and percent body fat were found to increase with age in a cross sectional analysis of the US population (63).
Applications for Obesity and Metabolic Disease
The prevalence of obesity in the pediatric population has increased dramatically over the past few decades. Currently, 17% of American children and adolescents are identified as obese (73). The societal implications of this obesity epidemic have led to a renewed interest in the study and use of body composition to develop screening tools which can accurately identify patients at risk for the development of obesity-related disease. Excessive weight gain affects children of all ages and a careful consideration of the changes in body composition during growth and development is essential when considering which method of body composition analysis to use.
There is growing evidence that body composition during infancy and early childhood predicts obesity and risk of cardiometabolic disease in later life. Infancy is a period of transition, and both the pre- and post-natal environments contribute to body composition during this time. Birth weight is the most readily obtainable measure of fetal growth and has been studied extensively. A recent meta-analysis found that infants with high birth weight (> 4000 grams) had increased risk for the development of obesity later in life (74). Intrauterine growth restriction is also associated with subsequent obesity, and is a strong risk factor for the development of metabolic syndrome, insulin resistance, and cardiovascular disease (75, 76). Interpretation of these studies is complicated by the fact that birth weight has been consistently shown to be associated with subsequent lean mass; however, its association with fat mass is less clear (77). Studies offering more detailed assessments of body composition at birth are lacking; the recent development of air displacement plethysmography devices designed especially for infants may offer a safe and easy approach for this vulnerable population (78).
Consideration of the rate of weight gain during early infancy may be particularly important in predicting future BMI, fat mass and central fat distribution (79–82). Efforts to maximize catch-up growth in small for gestational age infants may lead to altered body composition and metabolic risk. Small for gestational age infants experiencing rapid catch up weight gain during the first two years of life showed increased whole body and central adiposity, decreased lean mass, and increased insulin resistance at 4 years of age compared to average for gestational age infants (83).
Early nutrition source, in particular, has been shown to impact measures of infant and childhood body composition. Compared to breast fed infants, formula fed infants have increased weight velocity and FFM during the first year of life, though these differences do not persist into the second year (84). Breast feeding has been shown to reduce the risk of future obesity in a dose-dependent manner, with infants who breast fed for at least 26 weeks having a 51% reduction obesity risk at age 9 compared to a 38% reduction for those breastfed 13–25 weeks (85). Feeding mode may also be important, as bottle feeding led to increased weight gain over the course of the first year of life, irrespective of whether it was with breast milk or formula (86).
During childhood, the timing of the body mass rebound may influence the risk of future obesity and cardiometabolic disease. Children who experience this rebound earlier have been shown to be at higher risk for obesity and the development of complications such as type 2 diabetes (87, 88). Rate of weight gain continues to be an important risk factor, and rapid gain in BMI during childhood has been found to be more strongly associated with coronary events in adulthood than a given level of BMI at any age (89).
Adolescence is heralded by the onset of puberty, a process that has a profound impact on glucose homeostasis, lipid metabolism and cardiovascular function. Insulin resistance, blood pressure, and cholesterol all increase during puberty, which may make this a period of increased risk for the development of metabolic syndrome (90–92). The prevalence of metabolic syndrome has been reported to vary from 10 to 12% in the adolescent population. Earlier pubertal development has been associated with increased risk for metabolic syndrome in young adulthood (93–95). The components of the metabolic syndrome (abdominal obesity, insulin resistance, dyslipidemia, hypertension) are all thought to result from the presence of excess adiposity, however clarifying the nature of this relationship has been difficult. This may be in part due to limitations of the commonly used measures of adiposity during this time of rapidly changing body composition.
BMI is currently the most widely used method of identifying children and adolescents with excess adiposity and related risk for the development of metabolic disease. Children and adolescents are classified as overweight if their BMI is between the 85th and 95th percentile for age and sex and obese if BMI is greater than the 95th percentile (96). BMI was developed as means of assessing obesity in populations and may not be an accurate screening tool for identifying individual patients at risk for the development of cardiometabolic disease. A cross-sectional analysis of children in the Bogalusa heart study revealed that the optimal cutoff for BMI to identify the presence of metabolic risk factors varied from the 50th to the 57th percentile across sex and racial groups (97). The fact that such a low percentile for BMI is required to maximize sensitivity and specificity suggests that BMI may fail as a screening tool for metabolic disease. The risk of metabolic syndrome for a given BMI was also found to differ significantly between white and black obese adolescents, which may have been attributable in part to lower levels of visceral adipose tissue in blacks (98). This study not only illustrated the potential for BMI to misclassify individuals in terms of metabolic risk, but also its lack of generalizability across racial groups in part due to the fact that it does not account for fat distribution.
These limitations of BMI have led investigators to evaluate other indices of body composition for use as screening tools for metabolic syndrome in youth. Cutoffs for waist circumference were found to be similar to those for BMI and have similar sensitivity and specificity (97). A cross-sectional study using contemporary data from NHANES (which includes more obese children than those represented in the BMI charts) determined that the optimal cutoffs for percent body fat to identify metabolic syndrome were the 85th percentile for males and the 68th percentile for girls (61). At this time, it remains to be seen which index will perform best as a screening tool for identifying cardiometabolic disease. Comparison studies using longitudinal data are needed to answer this question.
Applications for Specific Populations
With continued advances in medical care, the number of children surviving and suffering from chronic disease is increasing across all age groups. This represents another population that may benefit from a more comprehensive approach to body composition analysis. These are children who may be defined as normal by weight or BMI, but who in fact may have excess adiposity and are at increased risk for the development of cardiometabolic disease or have lean mass deficits leading to impaired body function.
Survivors of childhood cancer and stem cell transplantation are at risk for altered body composition due to corticosteroid exposure, radiation and chemotherapy leading to endocrine dysfunction, immobility, and nutritional deficiency. Studies have shown that while there is no difference in BMI Z scores of these patients compared to healthy controls, they have significant deficits of lean mass and excesses of fat mass (99, 100). Another study of childhood cancer survivors suggested that these differences in body composition may be associated with metabolic disease, finding a prevalence of metabolic syndrome of twice that of the general population, even though only 17% were identified as overweight or obese by BMI (101).
Chronic inflammation affects growth and may lead to deficits in lean body mass. Children and young adults with incident Crohn’s disease had deficits in lean mass (males and females) and fat mass (females only) at diagnosis (102). These deficits improved with treatment and lean mass was shown to be correlated with reduction of inflammatory markers (103). Chronic kidney disease is also associated with lean mass deficits, especially of the leg, which may be indicative of skeletal muscle wasting (104).
Nutritional status is an important predictor of morbidity and mortality in cystic fibrosis, and is associated with pulmonary function, exercise tolerance, and linear growth in longitudinal studies of children (105, 106). BMI is currently the most often used measure of nutritional status in this population, however it may not identify individuals with deficits in FFM which may be a better predictor of pulmonary function(107). One cross-sectional study of 50 children with mild lung disease found that BMI was more strongly correlated with pulmonary function than either FM or FFM (108). Further studies are needed to fully understand the relationships between body composition, nutritional status, and clinical outcomes in cystic fibrosis.
Future Directions
The term “functional body composition” has been coined to describe approaches to body composition analysis that go beyond the simple quantification of body tissue and aim to integrate body components within the broader regulatory systems of the human body(109). Fat, muscle, and bone are now understood to be important regulators of whole body energy metabolism. The refinement and application of cutting-edge techniques such as MRI and PET-CT will allow for deeper investigations into the nature of adipose tissue. Factors such as fat distribution and metabolic activity can then be incorporated into current models of body composition and may explain some of the individual and population specific variation in risk of disease. Ultimately the goal should be to develop new definitions of obesity and underweight that are based upon metabolic and physiologic function, rather than statistical prevalence.
Footnotes
Disclosure
The authors in this article declare no conflict of interest.
References
- 1.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: A new approach to organizing body-composition research. Am J Clin Nutr. 1992;56:19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Ellis KJ. Human body composition: In vivo methods. Physiol Rev. 2000;80:649–680. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 3.Zemel B, Barden E. Measuring body composition. In: Hauspie RC, CN, Molinari L, editors. Methods in human growth research. Cambridge: Cambridge Universiy Press; 2004. pp. 141–176. [Google Scholar]
- 4.Forbes G. Human body composition: Growth, aging, nutrition and activity. New York: Springer-Verlag; 1987. p. 350. [Google Scholar]
- 5.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 6.Lohman TG, Slaughter MH, Boileau RA, Bunt J, Lussier L. Bone mineral measurements and their relation to body density in children, youth and adults. Hum Biol. 1984;56:667–679. [PubMed] [Google Scholar]
- 7.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: Implications for body composition assessment. Am J Physiol. 1993;265:E88–95. doi: 10.1152/ajpendo.1993.265.1.E88. [DOI] [PubMed] [Google Scholar]
- 8.WDM, FLK . Essentials of exercise physiology. Baltimore: Lippincott, Williams, and Wilkins; 2006. p. 767. [Google Scholar]
- 9.Wells JC, Fuller NJ, Dewit O, Fewtrell MS, Elia M, Cole TJ. Four-component model of body composition in children: Density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- 10.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: Revision of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 11.Vague J. La differenciation sexuelle facteur determinent des formes de l’obesite. Presse Med. 1947;55:339. [PubMed] [Google Scholar]
- 12.Despres JP, Arsenault BJ, Cote M, Cartier A, Lemieux I. Abdominal obesity: The cholesterol of the 21st century? Can J Cardiol. 2008;24 (Suppl D):7D–12D. doi: 10.1016/s0828-282x(08)71043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan SK, Kriketos AD, Poynten AM, Furler SM, Thompson CH, Kraegen EW, Campbell LV, Chisholm DJ. Insulin action, regional fat, and myocyte lipid: Altered relationships with increased adiposity. Obes Res. 2003;11:1295–1305. doi: 10.1038/oby.2003.176. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: Link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007;30:2091–2097. doi: 10.2337/dc07-0203. [DOI] [PubMed] [Google Scholar]
- 15.Amati F, Pennant M, Azuma K, Dube JJ, Toledo FG, Rossi AP, Kelley DE, Goodpaster BH. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 2012;20:1115–1117. doi: 10.1038/oby.2011.401. [DOI] [PubMed] [Google Scholar]
- 16.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 17.Turcato E, Bosello O, Di Francesco V, Harris TB, Zoico E, Bissoli L, Fracassi E, Zamboni M. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: Their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord. 2000;24:1005–1010. doi: 10.1038/sj.ijo.0801352. [DOI] [PubMed] [Google Scholar]
- 18.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy x-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20:1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010;34 (Suppl 2):S4–17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 22.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: Effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 23.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: A 1h-13c nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S. The “obese insulin-sensitive” adolescent: Importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. 2005;90:3731–3737. doi: 10.1210/jc.2004-2305. [DOI] [PubMed] [Google Scholar]
- 25.Brumbaugh DE, Crume TL, Nadeau K, Scherzinger A, Dabelea D. Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: The epoch study. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, Chalew S, Gordon S, Smith SR, Newcomer BR, Goran M, Sothern M. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. Nonobese prepubertal children Obesity (Silver Spring) 2012;20:371–375. doi: 10.1038/oby.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, Albu J, Engelson E, Kotler D, Pi-Sunyer X, Gilsanz V. Mri-measured pelvic bone marrow adipose tissue is inversely related to dxa-measured bone mineral in younger and older adults. Eur J Clin Nutr. 2012 doi: 10.1038/ejcn.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with igf-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 30.Tews D, Wabitsch M. Renaissance of brown adipose tissue. Horm Res Paediatr. 2011;75:231–239. doi: 10.1159/000324806. [DOI] [PubMed] [Google Scholar]
- 31.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 32.Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 2011;158:722–726. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–609. e601. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 36.Drubach LA, Palmer EL, 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: Detection, epidemiology, and differences from adults. J Pediatr. 2011;159:939–944. doi: 10.1016/j.jpeds.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: Advances in models and methods. Annu Rev Nutr. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 38.Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11:566–572. doi: 10.1097/MCO.0b013e32830b5f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Who child growth standards: Growth velocity based on weight, length, and head circumference: Methods and development. 2009. Who multicentre growth refernce study group. [Google Scholar]
- 40.Wang J. Standardization of waist circumference reference data. Am J Clin Nutr. 2006;83:3–4. doi: 10.1093/ajcn/83.1.3. [DOI] [PubMed] [Google Scholar]
- 41.Esmaillzadeh A, Mirmiran P, Azizi F. Clustering of metabolic abnormalities in adolescents with the hypertriglyceridemic waist phenotype. Am J Clin Nutr. 2006;83:36–46. doi: 10.1093/ajcn/83.1.36. quiz 183–184. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, Horlick M, Kotler D, Laferrere B, Mayer L, Pi-Sunyer FX, Pierson RN., Jr Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77:379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 43.Bosy-Westphal A, Booke CA, Blocker T, Kossel E, Goele K, Later W, Hitze B, Heller M, Gluer CC, Muller MJ. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a caucasian population. J Nutr. 2010;140:954–961. doi: 10.3945/jn.109.118737. [DOI] [PubMed] [Google Scholar]
- 44.Johnson ST, Kuk JL, Mackenzie KA, Huang TT, Rosychuk RJ, Ball GD. Metabolic risk varies according to waist circumference measurement site in overweight boys and girls. J Pediatr. 2010;156:247–252. e241. doi: 10.1016/j.jpeds.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Rolland-Cachera MF, Brambilla P, Manzoni P, Akrout M, Sironi S, Del Maschio A, Chiumello G. Body composition assessed on the basis of arm circumference and triceps skinfold thickness: A new index validated in children by magnetic resonance imaging. Am J Clin Nutr. 1997;65:1709–1713. doi: 10.1093/ajcn/65.6.1709. [DOI] [PubMed] [Google Scholar]
- 46.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. Cdc growth charts: United states. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 47.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 49.Heymsfield SB, Heo M, Thomas D, Pietrobelli A. Scaling of body composition to height: Relevance to height-normalized indexes. Am J Clin Nutr. 2011;93:736–740. doi: 10.3945/ajcn.110.007161. [DOI] [PubMed] [Google Scholar]
- 50.Cole TJ. Weight/heightp compared to weight/height2 for assessing adiposity in childhood: Influence of age and bone age on p during puberty. Ann Hum Biol. 1986;13:433–451. doi: 10.1080/03014468600008621. [DOI] [PubMed] [Google Scholar]
- 51.Telford RD, Cunningham RB. Reformulation of bmi and percent body fat to remove the height bias in 8-year-olds. Obesity (Silver Spring) 2008;16:2175–2181. doi: 10.1038/oby.2008.332. [DOI] [PubMed] [Google Scholar]
- 52.Franklin M. Comparison of weight and height relations in boys from 4 countries. Am J Clin Nutr. 1999;70:157–162. doi: 10.1093/ajcn/70.1.157s. [DOI] [PubMed] [Google Scholar]
- 53.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107:344–350. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 54.Metcalf BS, Hosking J, Fremeaux AE, Jeffery AN, Voss LD, Wilkin TJ. Bmi was right all along: Taller children really are fatter (implications of making childhood bmi independent of height) earlybird 48. Int J Obes (Lond) 2011;35:541–547. doi: 10.1038/ijo.2010.258. [DOI] [PubMed] [Google Scholar]
- 55.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: New insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of bmi to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 57.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 58.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, Czerwinski SA, Towne B, Siervogel RM. Do changes in body mass index percentile reflect changes in body composition in children? Data from the fels longitudinal study. Pediatrics. 2006;117:487–495. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
- 59.Eissa MA, Dai S, Mihalopoulos NL, Day RS, Harrist RB, Labarthe DR. Trajectories of fat mass index, fat free-mass index, and waist circumference in children: Project heartbeat! Am J Prev Med. 2009;37:S34–39. doi: 10.1016/j.amepre.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, Borrud LG. High adiposity and high body mass index-for-age in us children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurson KR, Eisenmann JC, Welk GJ. Development of youth percent body fat standards using receiver operating characteristic curves. Am J Prev Med. 2011;41:S93–99. doi: 10.1016/j.amepre.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Ogden CL, Li Y, Freedman DS, Borrud LG, Flegal KM. Smoothed percentage body fat percentiles for u.S. Children and adolescents, 1999–2004. Natl Health Stat Report. 2011:1–7. [PubMed] [Google Scholar]
- 63.Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from nhanes. PLoS One. 2009;4:7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of african-american, european-american, and mexican-american children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 65.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bechard LWE, Ellis K. Body composition and growth. In: Duggan CWJ, Walker W, editors. Nutrition in pediatrics. BC Decker Inc; 2008. p. 932. [Google Scholar]
- 67.Olhager E, Flinke E, Hannerstad U, Forsum E. Studies on human body composition during the first 4 months of life using magnetic resonance imaging and isotope dilution. Pediatr Res. 2003;54:906–912. doi: 10.1203/01.PDR.0000088064.63106.5E. [DOI] [PubMed] [Google Scholar]
- 68.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: An updated reference. Pediatr Res. 2000;47:578–585. doi: 10.1203/00006450-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN, Cecil J, Sandling JK, Syvanen AC, Kaakinen M, Beilin LJ, Millwood IY, Bennett AJ, Laitinen J, Pouta A, Molitor J, Davey Smith G, Ben-Shlomo Y, Jaddoe VW, Palmer LJ, Pennell CE, Cole TJ, McCarthy MI, Jarvelin MR, Timpson NJ. Association between common variation at the fto locus and changes in body mass index from infancy to late childhood: The complex nature of genetic association through growth and development. PLoS Genet. 2011;7:e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: A longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 71.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, Guo XR. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes Rev. 2011;12:525–542. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 75.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–212. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome x): Relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 77.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 78.Wells JC. Body composition in infants: Evidence for developmental programming and techniques for measurement. Rev Endocr Metab Disord. 2012;13:93–101. doi: 10.1007/s11154-012-9213-9. [DOI] [PubMed] [Google Scholar]
- 79.Chandler-Laney PC, Gower BA, Fields DA. Gestational and early life influences on infant body composition at one year. Obesity (Silver Spring) 2012 doi: 10.1002/oby.20236. [DOI] [PubMed] [Google Scholar]
- 80.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 81.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of african americans. Am J Clin Nutr. 2003;77:1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 82.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: Evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 83.Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- 84.Butte NF, Wong WW, Hopkinson JM, Smith EO, Ellis KJ. Infant feeding mode affects early growth and body composition. Pediatrics. 2000;106:1355–1366. doi: 10.1542/peds.106.6.1355. [DOI] [PubMed] [Google Scholar]
- 85.McCrory C, Layte R. Breastfeeding and risk of overweight and obesity at nine-years of age. Soc Sci Med. 2012;75:323–330. doi: 10.1016/j.socscimed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 86.Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med. 2012;166:431–436. doi: 10.1001/archpediatrics.2011.1665. [DOI] [PubMed] [Google Scholar]
- 87.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: Causes and consequences for obesity in children and adults. Int J Obes (Lond) 2006;30 (Suppl 4):11–17. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- 88.Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: Findings from the helsinki birth cohort study (hbcs) Am J Clin Nutr. 2011;94:1799–1802. doi: 10.3945/ajcn.110.000638. [DOI] [PubMed] [Google Scholar]
- 89.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 90.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 91.Harrell JS, Jessup A, Greene N. Changing our future: Obesity and the metabolic syndrome in children and adolescents. J Cardiovasc Nurs. 2006;21:322–330. doi: 10.1097/00005082-200607000-00014. [DOI] [PubMed] [Google Scholar]
- 92.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 93.Jolliffe CJ, Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the adult treatment panel iii and international diabetes federation criteria. J Am Coll Cardiol. 2007;49:891–898. doi: 10.1016/j.jacc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 94.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in american adolescents: Findings from the third national health and nutrition examination survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 95.Widen E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen AL, Laitinen J, Pouta A, Kaprio J, Jarvelin MR, Peltonen L, Palotie A. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care. 2012;35:850–856. doi: 10.2337/dc11-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010:1–5. [PubMed] [Google Scholar]
- 97.Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:198–205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- 98.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome x in obese black versus white adolescents: Race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 99.Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr. 2010;92:55–60. doi: 10.3945/ajcn.2010.29201. [DOI] [PubMed] [Google Scholar]
- 100.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel BS, Shults J, Thayu M, Leonard MB. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160:122–128. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sohn YB, Kim SJ, Park SW, Kim SH, Cho SY, Lee SH, Yoo KH, Sung KW, Chung JH, Koo HH, Jin DK. The metabolic syndrome and body composition in childhood cancer survivors. Korean J Pediatr. 2011;54:253–259. doi: 10.3345/kjp.2011.54.6.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thayu M, Shults J, Burnham JM, Zemel BS, Baldassano RN, Leonard MB. Gender differences in body composition deficits at diagnosis in children and adolescents with crohn’s disease. Inflamm Bowel Dis. 2007;13:1121–1128. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, Howard KM, Ryan A, Leonard MB. Determinants of changes in linear growth and body composition in incident pediatric crohn’s disease. Gastroenterology. 2010;139:430–438. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: Analysis of the cystic fibrosis foundation national cf patient registry. J Pediatr. 2000;137:374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 106.Klijn PH, van der Net J, Kimpen JL, Helders PJ, van der Ent CK. Longitudinal determinants of peak aerobic performance in children with cystic fibrosis. Chest. 2003;124:2215–2219. doi: 10.1378/chest.124.6.2215. [DOI] [PubMed] [Google Scholar]
- 107.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr. 2012 doi: 10.1016/j.clnu.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Pedreira CC, Robert RG, Dalton V, Oliver MR, Carlin JB, Robinson P, Cameron FJ. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39:276–280. doi: 10.1002/ppul.20162. [DOI] [PubMed] [Google Scholar]
- 109.Muller MJ, Bosy-Westphal A, Later W, Haas V, Heller M. Functional body composition: Insights into the regulation of energy metabolism and some clinical applications. Eur J Clin Nutr. 2009;63:1045–1056. doi: 10.1038/ejcn.2009.55. [DOI] [PubMed] [Google Scholar]