Abstract
Problem
Maternal anti-fetal rejection is a mechanism of disease in spontaneous preterm labor. The objective of this study was to determine whether the presence of human leukocyte antigen (HLA) panel-reactive antibodies (PRA) during the second trimester increases the risk for spontaneous preterm delivery.
Methods of Study
This longitudinal case-control study included pregnant women with spontaneous preterm deliveries (n=310) and control patients with normal term pregnancies (n=620), matched for maternal age and gravidity. Maternal plasma samples obtained at 14-16, 16-20, 20-24, and 24-28 weeks of gestation were analyzed for HLA Class I and Class II PRA positivity using flow cytometry. The fetal HLA genotype and maternal HLA alloantibody epitope were determined for a subset of patients with positive HLA PRA.
Results
1) Patients with spontaneous preterm delivery were more likely to exhibit HLA Class I (adjusted OR=2.54, p<0.0001) and Class II (adjusted OR=1.98, p=0.002) PRA positivity than those delivering at term; 2) HLA Class I PRA positivity for patients with spontaneous preterm delivery between 28-34 weeks (adjusted OR=2.88; p=0.001) and after 34 weeks of gestation (adjusted OR=2.53; p<0.0001) was higher than for those delivering at term; 3) HLA Class II PRA positivity for patients with spontaneous preterm delivery after 34 weeks of gestation was higher than for those delivering at term (adjusted OR=2.04; p=0.002); 4) multiparous women were at higher risk for HLA Class I PRA positivity than nulliparous women (adjusted OR=0.097, p<0.0001 for nulliparity); 5) nulliparous women had a higher rate of HLA Class I PRA positivity with advancing gestational age (p=0.001); and 6) 78% of women whose fetuses were genotyped had allo-antibodies specific against fetal HLA class I antigens.
Conclusions
Pregnant women with positive HLA class I or class II PRA during the second trimester are at an increased risk for spontaneous preterm delivery due to antibody-mediated maternal anti-fetal rejection.
Keywords: Flow cytometry, preterm birth, rejection, transplantation
Introduction
Preterm birth occurs in 5 to 13% of deliveries, and is the leading cause of perinatal mortality and morbidity worldwide.1-5 The economic impact cannot be over-emphasized, costing $26 billion annually in the United States alone.6 Preterm birth can be the result of the spontaneous onset of labor in intact/pre-labor rupture of membranes or indicated for maternal or fetal complications such as preeclampsia and intrauterine growth restriction.2,7
Spontaneous preterm parturition is syndromic in nature,7-12 which means that activation of the common pathway of parturition can be caused by multiple pathologic processes such as intrauterine infection/acute inflammation,13-38 vascular lesions leading to relative utero-placental ischemia,39-46 uterine over-distension in multiple gestations,47 stress,48, 49 cervical disease,37,38,50-56 a decline in progesterone action,57-64 and immune-related disorders.65-69
Among immune-related disorders, two distinct categories have been identified: 1) an allergic-like phenomenon69 and 2) maternal anti-fetal rejection.70-72 Recently, our group reported that chronic chorioamnionitis, a placental lesion reflecting maternal anti-fetal cellular rejection, is common in spontaneous preterm delivery.70,73 This evidence suggests that rejection is associated with preterm parturition in a fraction of cases. A major challenge is to identify patients with ongoing maternal anti-fetal rejection during pregnancy. The diagnosis of chronic chorioamnionitis can only be made after the delivery of the placenta, and therefore, biomarkers in maternal blood are highly desirable to identify patients at risk for this pathologic process.
Allograft rejection results from cellular and humoral (antibody-mediated) immune response by the recipient of a graft.74-79 The major histocompatibility complex (MHC) Class I and Class II molecules include the human leukocyte antigen (HLA) and have been implicated in the rejection of solid organs,77,79-83 as well as bone marrow.84 The presence of pre-formed donor-specific HLA antibodies in the circulation of the recipient is a risk factor for graft rejection.79,85-88 Hence, the detection of such circulating antibodies is undertaken before transplantation for donor selection to assess the likelihood of a successful graft.88-90 In addition, the detection of these antibodies after transplantation has been used to implement therapies to reduce the degree of sensitization such as immunoglobulin administration and plasmapheresis.88, 89
The standard method which consisted of a serologically based complement cytotoxicity assay has been replaced with solid phased assays, such as the HLA panel-reactive antibodies (PRA) using flow cytometry analysis.91, 92 The HLA PRA assay detects anti-HLA Class I and Class II antibodies. We have reported a strong association between chronic chorioamnionitis and maternal IgG HLA Class I PRA positivity.93 Moreover, we have shown a strong correlation between maternal and umbilical cord plasma HLA PRA positivity, indicating that circulating antibodies in the mother can cross the placenta.93
Recently, we found that HLA PRA in maternal and fetal sera can be associated with C4d deposition on the umbilical vein endothelium and that this occurs more frequently after spontaneous preterm labor/delivery than in normal delivery at term.70 Activation of complement by the detection of C4d (a degradation product of the C4b) is indicative of acute humoral graft rejection.94-98 We also reported an association between positive maternal HLA PRA and the diagnosis of chronic chorioamnionitis by histopathological examination of the placenta.70, 93
The objective of this study was to determine whether the presence of anti-HLA antibodies detected by HLA PRA in maternal blood in the second trimester of pregnancy is a risk factor for spontaneous preterm delivery in asymptomatic pregnant women.
Methods
Study population
A case-control study was designed to include pregnant women whose plasma samples were obtained one or more times during the second trimester (between 14 and 28 weeks of gestation). Cases consisted of pregnant women who subsequently developed spontaneous preterm labor with intact or ruptured membranes and had a preterm birth. All women in the control group delivered at term.
Three hundred and ten patients with spontaneous preterm delivery were matched for maternal age (within 3 years) and gravidity (primigravidae vs. multigravidae) with 620 pregnant women with normal term deliveries (ratio=1:2). Two or more serial plasma samples were available in 255 patients who had a spontaneous preterm delivery and in 578 who delivered at term (controls). Spontaneous preterm labor was diagnosed as the presence of regular uterine contractions (at least 3 in 30 minutes) associated with cervical dilatation followed by a preterm delivery. Preterm prelabor rupture of membranes (PPROM) was diagnosed by sterile speculum examination when pooling of amniotic fluid in the vagina was observed or when positive nitrazine and ferning tests, performed if necessary, were confirmed before 37 completed weeks of gestation in the absence of labor. Women with hypertensive disorders during pregnancy, small-for-gestational-age neonates, multiple gestations, and fetal congenital anomalies were excluded from this study. All plasma samples were stored at -80°C until use.
All patients were Hispanic women who delivered at the Sótero del Río Hospital, Santiago, Chile. Maternal plasma samples were retrieved from the Bank of Biological Materials of Wayne State University/Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services. All patients provided written informed consent, and the Institutional Review Boards of the Sótero del Río Hospital (an affiliate of the Pontificia Universidad Católica de Chile), Wayne State University, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services approved the use of clinical data and the collection and utilization of biological samples for research purposes.
Flow cytometry of panel-reactive anti-HLA antibodies
The FlowPRA®-I screening test (One Lambda Inc., Canoga Park, CA, USA) and FlowPRA®-II screening test (One Lambda) were used for flow cytometric analyses of HLA Class I and Class II PRA in maternal plasma, according to the manufacturer's instructions. Serum and plasma samples gave concordant results for these tests.99 Briefly, HLA class I or class II microbeads were mixed with 20 μL of plasma, and incubated for 30 min at room temperature with gentle rotation. The beads were washed, centrifuged, and subsequently incubated with 100 μl of FITC-conjugated F (ab) 2 fragment of Fcγ fragment-specific goat anti-human IgG for 30 min. After 2 washes, 0.5 mL of fixing solution was added. The FL1 fluorescence of 5,000 events was analyzed using BD LSRII flow cytometry (BD Biosciences, San Jose, CA, USA). A sample with reactivity of 10% or more was considered to be positive for HLA PRA.88, 100
Luminex assay for fetal HLA specificity of maternal HLA antibodies
As maternal HLA antibodies specific to fetal HLA antigens can be considered analogous to specific antibodies in the setting of organ transplantation, fetal HLA specificity of maternal HLA antibodies was analyzed in 14 HLA Class I PRA-positive cases during the second trimester. This group was selected based on the availability of genomic DNA from umbilical cord blood. LABType® SSO typing kits (One Lambda) were used for the fetal HLA genotype and LABScreen® Single Antigen (One Lambda) for the epitope assessment of maternal HLA antibodis with Luminex assay (Luminex Corporation, Austin, TX, USA), according to the manufacturers' instructions.
Statistical analysis
For continuous variables, the Kolmogorov-Smirnov tests were used to examine distributions for normality. When data were not normally distributed, a Kruskal-Wallis analysis of variance test was used, and the Mann-Whitney U test was performed for multiple comparisons among groups. When the distribution was normal, one-way analysis of variance tests were used to compare differences, and post hoc tests were performed for the multiple comparisons. For categorical variables, proportions were compared with the χ2 test or Fisher's exact test. Medians and ranges were reported for continuous variables, whereas frequencies and percentages were calculated for categorical variables. Logistic regression analysis was performed to determine the magnitude of association between positive HLA PRA and the occurrence of spontaneous preterm delivery, adjusting for gestational age at sampling, fetal gender, parity, and history of previous preterm delivery which could influence spontaneous preterm delivery development. Maternal age and gravidity were adjusted in the matching process between case and control groups. Effect modification terms were used to assess subgroup effects.
The HLA PRA Class I reactivity level was also evaluated as a dichotomous variable (above or below 10%) using a generalized linear model assuming a binomial distribution with a logit link function. The effect and significance of the fixed effects were determined by generalized estimating equations,101 therefore allowing for longitudinal (and eventually correlated) HLA PRA Class I reactivity observations from the same individuals. Explanatory variables included as covariates in this model were the group factor (disease or normal), parity, and gestational age (GA); effect modification between parity, gestational age, baby gender and history of preterm delivery was also investigated. Model selection was performed using the ANOVA function in the Geepack package which allowed comparison of the quality of the data fit among different models. Basic statistical analyses were conducted using the SPSS Version 15.0 (SPSS, Inc., Chicago, IL, USA). Longitudinal analysis was conducted using the Geepack of R statistical software.102 All P values are two-sided, and a value of P<0.05 is considered to be statistically significant.
Results
Patient characteristics
Table I shows the demographics and clinical characteristics of the study population. The frequency of previous preterm delivery was significantly higher in women who had a spontaneous preterm delivery than in those who delivered at term (P<0.001).
Table I. Demographics of the study population.
| Term delivery (N=620) | Spontaneous preterm delivery (N=310) | P value | |
|---|---|---|---|
| Maternal age (year)* | 25 (15-44) | 26 (15-46) | 0.939 |
| Gravida (Primigravida) (%) | 36.8 | 36.8 | 1.000 |
| Para (Nullipara) (%) | 39.4 | 40.3 | 0.776 |
| Gestational age at 1st sample (weeks)* | 16.7 (14.1-25.3) | 17.1 (14.1-27.6) | 0.044 |
| Number of serial sampling* | 3 (1-4) | 2 (1-4) | <0.001 |
| Number of cases at 14-16 weeks | 219 | 98 | |
| Number of cases at 16-20 weeks | 446 | 215 | |
| Number of cases at 20-24 weeks | 476 | 199 | |
| Number of cases at 24-28 weeks | 509 | 213 | |
| History of previous preterm delivery (%) | 3.5 | 17.7 | <0.001 |
| Cigarette smoking (%) | 12.3 | 12.9 | 0.779 |
| Alcohol use (%) | 4.0 | 3.9 | 0.906 |
| Drug abuse (%) | 0.5 | 1.3 | 0.230 |
| Cesarean delivery (%) | 16.1 | 21.6 | 0.040 |
| Baby gender (Male) (%) | 54.4 | 58.1 | 0.283 |
| Gestational age at delivery (weeks)* | 39.7 (37.0-42.0) | 35.6 (22.0-36.9) | <0.001 |
| Birth weight (gm)* | 3400 (2530-4150) | 2590 (480-3930) | <0.001 |
Median (range)
HLA Class I PRA positivity in maternal blood as a risk factor for spontaneous preterm delivery
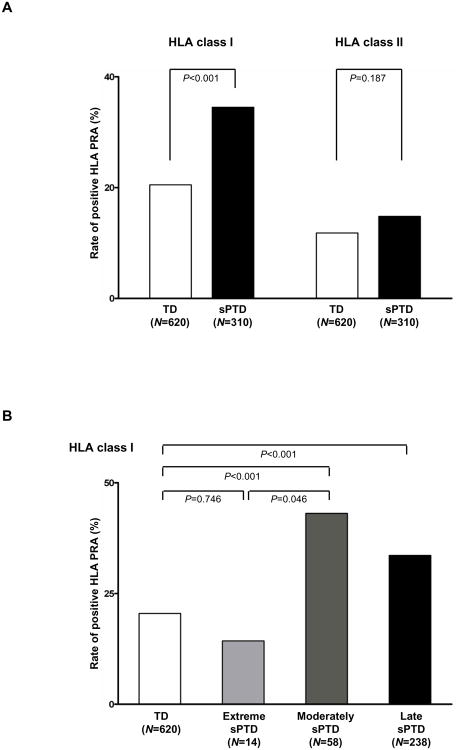
Figure 1A displays the differences in the rates of positive HLA Class I and Class II PRA between the two groups. The first maternal blood sample was used for a cross-sectional analysis. Patients who had a spontaneous preterm delivery had a higher rate of sero-positivity for HLA Class I PRA than those who delivered at term [34.5% (107/310) vs. 20.5% (127/620), p<0.001, see Figure 1A). However, the proportion of positive HLA Class II PRA was not significantly different between the two groups [14.8% (46/310) vs. 11.8% (73/620) p=0.18].
Figure 1. HLA PRA in maternal plasma.
(A) HLA class I and class II PRA positive rates in normal term delivery and spontaneous preterm delivery cases, (B) HLA class I PRA positive rate according to gestational age at delivery. HLA, human leukocyte antigen; PRA, panel-reactive antibody; TD, normal term delivery; sPTD, spontaneous preterm delivery; extreme sPTD, delivered before 28 + 0 weeks of gestation; moderate sPTD, delivered between 28 + 0-33 + 6 weeks of gestation; late sPTD, delivered between 34 + 0-36 + 6 weeks of gestation.
A positive maternal HLA Class I PRA during the second trimester was an independent risk factor for spontaneous preterm delivery after adjusting for confounding factors including gestational age at sampling, fetal gender, parity, and history of previous preterm delivery (odds ratio [OR] 2.40, 95% confidence interval [CI] 1.69-3.39). The sensitivity and specificity of positive HLA class I PRA in the identification of patients who subsequently had a spontaneous preterm delivery were 34.5% (107/310) and 79.5% (493/620), respectively. The rate of positive HLA Class I PRA did not differ between women who eventually had a spontaneous preterm delivery with intact membranes and those with preterm PPROM (35.1% vs. 33.3%).
Relationship between positive HLA PRA Class I and gestational age at delivery
Patients with a spontaneous preterm delivery were subdivided into three groups according to the gestational age at birth: 1) extremely preterm (<28 weeks), 2) moderately preterm (28-33 weeks), and 3) late preterm (34-37 weeks of gestation).2, 103 The rate of maternal HLA PRA Class I positivity did not differ between patients who had a spontaneous preterm delivery before 28 weeks of gestation [14.3% (2/14)] and those who delivered at term [20.5% (127/620) (p=0.7)]. However, patients who delivered between 28 and 34 weeks of gestation [43.1% (25/58)] and after 34 weeks of gestation [33.6% (80/238)] had a higher rate of positive maternal HLA PRA Class I than patients who delivered at term [20.5% (127/620)] (p<0.001, for each) (Figure 1B).
Temporal changes in maternal HLA PRA positivity: longitudinal analysis
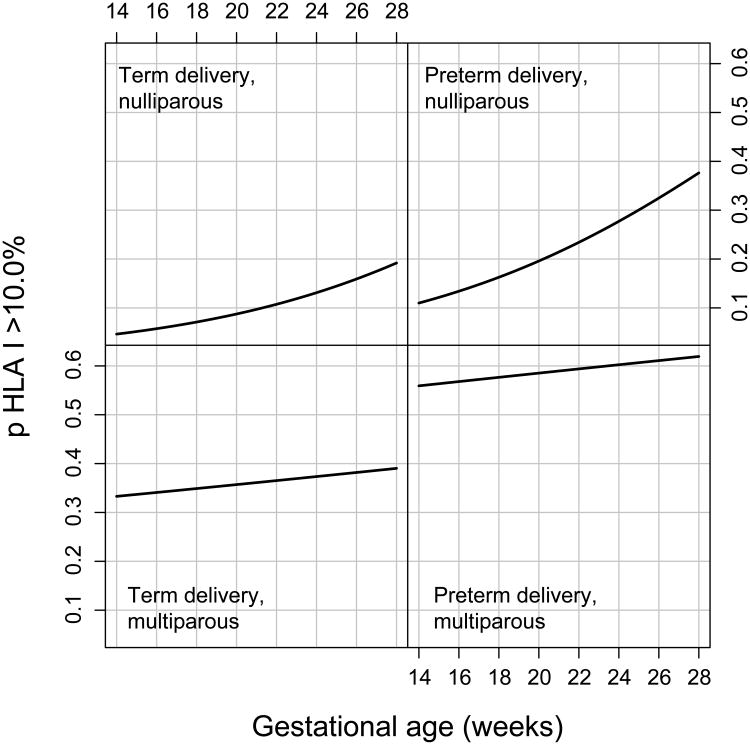
To determine whether there is a difference in the rate of positive maternal HLA PRA Class I and Class II between patients who subsequently had a spontaneous preterm delivery and those who delivered at term according to gestational age at maternal blood sampling, a longitudinal analysis was conducted. Spontaneous preterm delivery and parity status were associated with maternal sero-positivity for HLA PRA Class I after adjusting for fetal gender and a history of preterm delivery [(adjusted OR=2.54, p<0.0001) for spontaneous preterm delivery and an adjusted OR = 0.097, p<0.0001 for nulliparity)]. The association between spontaneous preterm delivery and sero-positivity status for HLA PRA Class I did not change with gestational age (no significant interaction). In contrast, nulliparous women were at a higher risk to have sero-positivity for HLA PRA Class I as gestational age increased (p<0.001) (Figure 2). Fetal gender and a history of preterm delivery did not significantly change sero-positivity status for HLA PRA Class I.
Figure 2. Illustration of effect modification between parity and case status over time.
The likelihood of a HLA PRA class I > 10% elevating among women with spontaneous preterm delivery compared to women with normal term delivery over time differed significantly by parity in multiparous women with spontaneous preterm delivery, exhibiting the greatest risk relative to nulliparous women over time. TD, normal term delivery; sPTD, spontaneous preterm delivery; OR, odds ratio.
There were no effect modification terms identified for patients with HLA PRA Class II positivity (cut-off level above 10%) using mixed-effects models, including gestational age, parity, and pregnancy outcome variables (preterm or term birth). Therefore, only the main effects were tested. Spontaneous preterm delivery and parity status were associated with HLA PRA class II sero-positivity after adjusting for fetal gender and a history of preterm delivery (adjusted OR=1.98, p=0.002 for spontaneous preterm delivery and an adjusted OR = 0.11, p<0.0001 for nulliparity). There was no significant change in the sero-positivity status as a function of gestational age.
HLA PRA Class I positivity was significantly associated with late preterm deliveries (34-36 6/7 weeks) (adjusted OR = 2.53, p<0.0001) and moderate preterm deliveries (28-33 6/7 weeks) (adjusted OR = 2.88, p=0.001) relative to those who delivered at term. HLA PRA Class I and II positivity did not significantly differ between extreme preterm deliveries relative to term deliveries. Women with late preterm deliveries were more likely to exhibit HLA PRA Class II positivity compared to those who delivered at term (adjusted OR = 2.04, P=0.002). Moderate preterm delivery was not statistically significant when associated with HLA class II PRA positivity (adjusted OR = 1.7, p=0.23).
Fetal HLA specificity of maternal PRA
Table II summarizes the information for fetal HLA genotyping and the assessment of fetal HLA specificity of maternal HLA antibodies. For HLA class I antigens, 78.6% (11/14) positive maternal HLA PRA Class I cases had HLA antibodies specific against fetal HLA class I antigens. For HLA PRA Class II, 66.7% (4/6) of positive maternal HLA PRA Class II cases and none of the 8 negative maternal HLA PRA Class II cases (0%) had HLA antibodies specific against fetal HLA class II antigens (p=0.015).
Table II. Fetal HLA specificity of maternal HLA antibodies.
| Case | HLA PRA* | Fetal Genotyping (HLA-) | HLA antibodies identified | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class I | Class II | A | B | C | DQ | DR | DR 51/ 52/ 53 | Class I | Class II | ||
| 1 | sPTD | 73.9 | 5.1 | 33, 68 | 14, 51 | 3, 8 | 2, 7 | 14, 17 | 52 | A23, A24, A25, A32, B13, B18, B27, B35, B37, B38, B44, B45, B46, B47, B49, B50, B51†, B52, B53, B57, B58, B59, B62, B63, B71, B72, B75, B76, B77, B78, B82, Cw9 | |
| 2 | sPTD | 98.5 | 0.5 | 2, 68 | 39, 48 | 1, 7 | 4, 9 | 8, 9 | 53 | A1, A3, A11, A23, A24, A25, A26, A29, A30, A31, A32, A33, A34, A36, A43, A66, A68†, A69, A74, A80, B7, B13, B27, B35, B37, B41, B42, B47, B48†, B51, B52, B55, B60, B61, B62, B67, B72, B73, B75, B78, B81, Cw2, Cw17 | |
| 3 | TD | 40.4 | 1.1 | 3, 24 | 38, 39 | 7, 12 | 5, 7 | 1, 16 | 51 | B7, B13, B27, B42, B48, B54, B55, B56, B60, B61, B67, B72, B73, B81, B82 | DP14, DR52 |
| 4 | TD | 85.1 | 60.9 | 1, 31 | 40 | 1, 2 | 6, 8 | 4, 13 | 52, 53 | A1, A3, A11, A23, A24, A25, A26, A29, A34, A36, A43, A66, A80, B44, B45, B73, B76, B82, Cw2†, Cw4, Cw5, Cw6, Cw7, Cw15, Cw17, Cw18 | DR8, DR11, DR12, DR13†, DR14, DR17, DR18, DR52† |
| 5 | sPTD | 94.2 | 1.5 | 29, 31 | 39, 44 | 7, 16 | 2, 8 | 4, 7 | 53 | A1, A23, A24, A25, A32, A80, B27, B35, B37, B38, B41, B44†, B45, B46, B47, B49, B50, B51, B52, B53, B57, B58, B59, B60, B61, B62, B63, B71, B72, B75, B76, B77, B82, Cw5 | DP11, DQ7, DR13 |
| 6 | TD | 99.9 | 1.5 | 3, 11 | 7, 49 | 7 | 2, 6 | 7, 13 | 52, 53 | A66, B7, B8, B13, B18, B27, B35, B38, B39, B41, B42, B45, B46, B47, B48, B50, B51, B52, B53, B54, B55, B56, B57, B59, B60, B61, B62, B63, B64, B65, B67, B71, B72, B73, B75, B76, B78, B81, B82, Cw2, Cw15, Cw16, Cw17 | |
| 7 | TD | 35.1 | 16.0 | 2 | 15, 35 | 1, 4 | 8, 9 | 4, 9 | 53 | B7, B13, B41, B42, B44, B45, B47, B49, B50, B55, B56, B60, B61, B62, B67, B81, B82 | DR7, DR18 |
| 8 | sPTD | 90.0 | 95.0 | 1, 24 | 18, 52 | 5, 12 | 2, 6 | 3, 15 | 51, 52 | A1†, A3, A11, A25, A26, A29, A30, A31, A32, A33, A34, A36, A43, A66, A74, A80, B44, B45, B46, B73, B76, Cw1, Cw6, Cw7, Cw8, Cw9, Cw10, Cw12†, Cw14, Cw16, Cw18 | DQ4, DQ5, DQ6†, DQ8, DQ9, DR1, DR4, DR7, DR9, DR10, DR15†, DR16 DR51†, DR53, DR103 |
| 9 | sPTD | 94.8 | 57.7 | 1, 23 | 8, 14 | 6, 8 | 2, 5 | 1, 3 | 52 | A2, A23, A24, A25, A32, A68, A69, B13, B27, B35, B37, B38, B41, B44, B45, B46, B47, B49, B50, B51, B52, B53, B55, B56, B57, B58, B59, B60, B61, B62, B63, B71, B72, B75, B77, B76, B77, B78, Cw10 | DQ7, DR1†, DR4, DR9, DR10, DR14, DR15, DR51, DR53, DR103 |
| 10 | sPTD | 66.0 | 17.2 | 29, 68 | 39, 44 | 7, 16 | 7, 8 | 4, 14 | 52, 53 | A1, A23, A24, A25, A32, B13, B18, B27, B35, B37, B38, B44†, B45, B47, B49, B50, B51, B52, B53, B57, B58, B59, B62, B63, B71, B72, B75, B76, B77, B78, B82 | DR4†, DR13 |
| 11 | sPTD | 84.3 | 1.0 | 23, 68 | 7, 50 | 5, 7 | 5, 7 | 13, 14 | 52 | A32, A66, A74, B7†, B8, B13, B18, B27, B37, B38, B39, B41, B42, B44, B46, B47, B48, B51, B52, B54, B55, B56, B57, B59, B60, B61, B62, B64, B65, B67, B71, B72, B73, B75, B76, B77, B78, B81, B82, Cw1, Cw2, Cw9, Cw10, Cw14, Cw15, Cw17 | DP11 |
| 12 | sPTD | 99.6 | 99.6 | 2, 31 | 39, 49 | 7 | 5 | 1 | – | A2†, A11, A23, A24, A25, A26, A32, A33, A34, A43, A66, A68, A69, B13, B18, B27, B35, B37, B38, B41, B42, B44, B45, B46, B47, B49†, B50, B51, B52, B53, B54, B55, B56, B57, B58, B59, B60, B61, B62, B63, B64, B65, B71, B67, B71, B72, B73, B75, B76, B77, B78, B82, Cw2, Cw5, Cw6, Cw9, Cw10, Cw15, Cw17, Cw18 | DP1, DP2, DP3, DP4, DP5, DP6, DP9, DP10, DP11, DP13, DP14, DP17, DP18, DP19, DP20, DP28, DQ2, DQ4, DQ7, DQ8, DQ9, DR4, DR7, DR8, DR9, DR11, DR12, DR13, DR15, DR16, DR51, DR52, DR103 |
| 13 | TD | 16.5 | 1.0 | 2, 24 | 39, 40 | 7, 15 | 6, 8 | 4, 15 | 51, 53 | B44, B45, B49, B50, B52, B62, B71, B72, B75, B76, B82 | DP2, DR8, DR9, DR13, DR18 |
| 14 | sPTD | 66.8 | 0.5 | 1, 29 | 8, 51 | 7, 16 | 7, 8 | 4, 13 | 52, 53 | A23, A24, A25, A32, B13, B18, B27, B35, B37, B38, B44, B45, B47, B49, B51†, B52, B53, B57, B58, B59, B63, B71, B72, B75, B77, B78, Cw1, Cw12, Cw15 | DQ2 |
, panel-reactivity (%)
, specific against fetal HLA antigens
HLA, human leukocyte antigen; PRA, panel-reactive antibodies; TD, normal term delivery; sPTD, spontaneous preterm delivery
Discussion
Principal findings of the study
(1) women with HLA PRA Class I or Class II positivity in the second trimester were significantly more likely to have a spontaneous preterm delivery than those who were HLA PRA negative;64 (2) the rate of HLA PRA Class I was higher in women who had a spontaneous preterm delivery after 28 weeks of gestation than in patients who delivered at term; (3) the rate of HLA PRA Class II positivity was higher in women who had a spontaneous preterm delivery after 34 weeks (late preterm birth) than in patients who delivered at term; and (4) multiparous women had a higher risk of sero-positivity for HLA PRA Class I and II than nulliparous women. However, nulliparous women were at higher risk for sero-positivity for HLA class I as gestational age increased.
Maternal anti-fetal rejection as a mechanism of disease in human pregnancy
The central paradigm of reproductive immunology is that the fetus is a semi-allograft, which is tolerated during pregnancy.104-113 The mechanisms for tolerance have been the subject of intensive investigation for decades and remain to be elucidated, although a considerable body of evidence supports a role for T-regulatory cells,110, 114-116 expression of non-classical MHC molecules of trophoblast cells,117, 118 tryptophan catabolism,119, 120 T-cell apoptosis,121 complement,122 and co-stimulatory molecules such as the programmed death ligand, among others.123-125
Our group has been concerned with the role of maternal anti-fetal rejection as a mechanism of disease in human pregnancy.70-72,93,126 Transplant rejection occurs when the recipient's immune system responds to antigenic differences between self and the graft.79 Antigenic differences encoded by MHC genes are the major cause of transplant rejection.79 Allo-recognition refers to immune responses to MHC antigens. Such antigens can elicit a B-cell response with the production of antibodies which mediates humoral rejection, as well as T-cell-mediated rejection (cellular rejection).79 Both mechanisms – humoral and cellular – have been described by our group in the context of maternal anti-fetal rejection.71 Chronic chorioamnionitis is an example of a cellular-mediated maternal anti-fetal rejection in which maternal T cells are in direct contact with the fetal trophoblast.73 Antibody-mediated rejection occurs when the mother develops antibodies against fetal antigens in the MHC system.127 The antibodies can cross the placenta and damage fetal cells by activating complement.128, 129
When fetal cells cross into the maternal circulation, they can elicit a maternal immune response to paternal antigens expressed in fetal cells. Although tolerance has been attributed to the expression of monomorphic MHCs by trophoblast, this cannot be invoked to explain tolerance to fetal white blood cells that cross the placenta into the maternal circulation. Humoral rejection requires the generation of antibodies that can cross the placenta and activate complement. We have previously demonstrated that these events occur in patients with histopathological evidence of maternal anti-fetal rejection in the placenta.
How can we identify the mother at risk for maternal anti-fetal rejection?
In transplant medicine, two major steps are taken to prepare recipients and potential donors.79,130 Genotyping for MHC is routinely performed to characterize the degree of incompatibility between potential donors and recipients.79,130 Also, the presence of anti-HLA antibodies is determined before transplantation because the presence of specific antibodies against antigens in the graft is associated with poor graft survival.79, 130 This is accomplished by testing with HLA PRA sero-positivity. This test is also used to monitor the development of antibodies by the recipient after transplantation and the results guide therapy.95, 130
HLA PRA sero-positivity can be employed during pregnancy to identify the mother sensitized against the MHC antigens. Indeed HLA PRA testing is equivalent to an indirect Coombs test to assess whether the mother has antibodies against red blood cell antigens. HLA PRA positivity needs to be followed by characterization of antibodies to determine their HLA antigen specificity. Moreover, for rejection to occur, these antigens must be present in the fetus and the antibodies able to cross the placenta. We have previously documented that HLA PRA positivity is associated with histological evidence of maternal anti-fetal rejection at the time of birth by examination of the placenta. However, it is unknown whether HLA PRA positivity in the mid-trimester is associated with adverse pregnancy outcome, and specifically, spontaneous preterm labor.
Maternal HLA PRA status and the risk of preterm delivery
Although the clinical significance of maternal HLA antibodies has been studied in the setting of recurrent spontaneous abortions,131,132 this is the first study to examine the relationship between second-trimester maternal HLA PRA status and subsequent spontaneous preterm delivery. Our findings reported herein indicate that mothers who are HLA PRA positive are more likely to have a spontaneous preterm delivery than those who are HLA PRA negative, and this is especially true for late preterm deliveries which account for the majority of all preterm births. The HLA PRA sero-positivity which conferred risk for spontaneous preterm delivery was both against Class I and Class II HLA. The magnitude of risk for preterm delivery conferred by HLA PRA positivity was greater for Class I than for class II.
In the present study, the HLA class I PRA positive rate in spontaneous preterm deliveries before 28 weeks of gestation was not different from that of pregnant women who delivered at term. However, patients with spontaneous preterm deliveries after 28 weeks of gestation had a higher rate of positive HLA class I PRA than women who delivered at term. This finding strongly suggests an association between maternal antibody-mediated anti-fetal rejection and late spontaneous preterm delivery. This is consistent with previous reports indicating that early preterm labor is associated with intra-amniotic infection and acute chorioamnionitis and funisitis.133-137 Our findings are also consistent with previous observations that mean gestational age at delivery for patients with chronic chorioamnionitis (a manifestation of maternal anti-fetal cellular rejection) is higher than in those with acute chorioamnionitis among spontaneous preterm deliveries.73
HLA PRA positivity, however, indicates only the presence of antibodies in the maternal circulation against a wide range of potential antigens belonging to the HLA family. In a subset of patients, we were able to demonstrate that the anti-HLA antibodies were against specific fetal antigens as demonstrated by characterization of the fetal genotype. These antigens are paternally inherited. The clinical utility and predictive value of maternal HLA alloantibodies for spontaneous preterm delivery need to be determined in future studies. The sensitivity of HLA PRA positivity to detect spontaneous preterm delivery was 34.5%. This is not unexpected as only a fraction of all preterm deliveries results from maternal anti-fetal rejection.
When do pregnant women become sensitized against fetal HLA antigens?
HLA sensitization can occur after a blood transfusion,138 organ transplantation,79,83 feto-maternal hemorrhage,139, 140 and fetal cell trafficking into the maternal circulation.140-142 It is possible, therefore, that sensitization occurred before or during the index pregnancy. Our finding that HLA PRA positivity was more common in parous than in non-parous women suggests that the likelihood of sensitization increases with a prior pregnancy. The observation that a positive sero-conversion of HLA PRA class I during the index pregnancy is more frequent with advancing gestational age indicates that the risk of sensitization increases with the duration of pregnancy. This is consistent with a previous report that the positive rate of anti-paternal/fetal cytotoxic antibody test increases as a function of gestational age in normal pregnant women.143 The finding that a large proportion of HLA PRA Class I positive pregnant women (77.7%) already had antibodies at the time of initial sampling indicates that sensitization begins before the second trimester.
Strengths and limitations of the study
There are two major strengths of this study: 1) It is the first longitudinal examination of HLA PRA positivity during pregnancy, and 2) there was a large number of patients studied. A limitation of this study is that the HLA PRA status prior to pregnancy or during the first trimester could not be evaluated. Because most HLA PRA Class I positive cases had HLA PRA Class I at their first blood sampling, HLA alloantibodies might not be related to the index pregnancy in a fraction of HLA PRA positive women in this study. HLA sensitization prior to pregnancy is likely to have immunological effects on the index pregnancy considering that the presence of either donor-specific or non-donor-specific HLA antibodies in the recipients is associated with the development of humoral and cellular rejection and poor graft outcome in organ transplantation.144, 145 In addition, the observation in this study that 78.6% of HLA PRA positive cases tested had fetal HLA-specific antibodies indicates that maternal HLA antibodies may have an effect on the fetuses regardless of the gestational age at which they are detected.
Conclusion
A positive HLA PRA Class I or Class II during the second trimester of pregnancy is a risk factor for spontaneous preterm delivery, especially for late preterm birth which represents 70% of all preterm births. These observations provide support for the concept that maternal anti-fetal rejection is a mechanism of disease in late spontaneous preterm births.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HSN275201300006C.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: final data for 2005. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2007;56:1–103. [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, McClure EM. The Epidemiology of Preterm Birth. In: Berghella V, editor. Preterm Birth Prevention & Management. Wiley-Blackwell; 2010. [Google Scholar]
- 4.Steer P. The epidemiology of preterm labour. BJOG : an international journal of obstetrics and gynaecology. 2005;112 Suppl 1:1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, Dias T, Potetz L, Davidoff MJ, Damus K, Petrini JR. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 7.Iams JD, Romero R, Creasy RK. Preterm Labor and Birth. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, editors. Maternal-Fetal Medicine: Principles and Practice. Philadelphia: Elsevier Saunders; 2009. pp. 544–582. [Google Scholar]
- 8.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG : an international journal of obstetrics and gynaecology. 2006;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moutquin JM. Classification and heterogeneity of preterm birth. BJOG : an international journal of obstetrics and gynaecology. 2003;110 Suppl 20:30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchely H, Bennett P, Thornton S, editors. Preterm Birth. London: RCOG Press; 2004. [Google Scholar]
- 11.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder M, Romero R, Lamont R, editors. Preterm Labor. New York, NY: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 12.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, Mazor M, Brekus CA, Hobbins JC. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. American journal of obstetrics and gynecology. 1990;163:757–761. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 14.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clinics in perinatology. 1995;22:281–342. [PubMed] [Google Scholar]
- 15.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomez R, Romero R, Mazor M, Ghezzi F, David C, Yoon BH, Elder MG, Lamont RF, Romero R. Preterm labour. Churchill Livingstone; 1997. The role of infection in preterm labour and delivery; pp. 85–125. [Google Scholar]
- 17.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biology of reproduction. 2002;67:1337–1341. doi: 10.1095/biolreprod67.4.1337. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375–379. [PubMed] [Google Scholar]
- 19.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. Journal of perinatal medicine. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh KJ, Yoon BH, Romero R, Park CW, Lee SM, Kim SM. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2010;201 [Google Scholar]
- 21.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Avila C, Sepulveda W. The role of systemic and intrauterine infection in preterm labor. In: Fuchs A, Fuchs F, Stubblefield P, editors. Preterm Birth: Causes, Prevention, and Management. New York: McGraw-Hill; 1993. [Google Scholar]
- 23.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. American journal of obstetrics and gynecology. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15 Suppl 2:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. American journal of obstetrics and gynecology. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell GH. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. American journal of obstetrics and gynecology. 1992;166:129–133. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. American journal of obstetrics and gynecology. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Seminars in perinatology. 1988;12:262–279. [PubMed] [Google Scholar]
- 30.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. American journal of obstetrics and gynecology. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Foundation symposium. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. discussion 220-203. [DOI] [PubMed] [Google Scholar]
- 32.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. American journal of obstetrics and gynecology. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 33.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, Shim SS. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. American journal of obstetrics and gynecology. 2001;185:1137–1142. doi: 10.1067/mob.2001.118162. [DOI] [PubMed] [Google Scholar]
- 34.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, Nhan-Chang CL, Erez O, Kim CJ, Espinoza J, Goncalves LF, Vaisbuch E, Mazaki-Tovi S, Hassan SS, Costerton JW. Detection of a microbial biofilm in intraamniotic infection. American journal of obstetrics and gynecology. 2008;198:135 e131–135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633 e631–638. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 38.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433 e431–438. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Preterm premature rupture of the membranes: a risk factor for the development of abruptio placentae. American journal of obstetrics and gynecology. 1987;156:1235–1238. doi: 10.1016/0002-9378(87)90153-0. [DOI] [PubMed] [Google Scholar]
- 40.Brar HS, Medearis AL, De Vore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: relationship to outcome. American journal of obstetrics and gynecology. 1989;161:1519–1522. doi: 10.1016/0002-9378(89)90916-2. [DOI] [PubMed] [Google Scholar]
- 41.Strigini FA, Lencioni G, De Luca G, Lombardo M, Bianchi F, Genazzani AR. Uterine artery velocimetry and spontaneous preterm delivery. Obstetrics and gynecology. 1995;85:374–377. doi: 10.1016/0029-7844(94)00420-I. [DOI] [PubMed] [Google Scholar]
- 42.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstetrics and gynecology. 2003;102:94–100. doi: 10.1016/s0029-7844(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 43.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 44.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Chaiworapongsa T, Espinoza J, Gonzalez R. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 45.Maehara K, Kanayama N, Maradny EE, Uezato T, Fujita M, Terao T. Mechanical stretching induces interleukin-8 gene expression in fetal membranes: a possible role for the initiation of human parturition. European journal of obstetrics, gynecology, and reproductive biology. 1996;70:191–196. doi: 10.1016/s0301-2115(95)02602-9. [DOI] [PubMed] [Google Scholar]
- 46.Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, Pacora P, Ogge G, Dong Z, Kim CJ, Yeo L, Hassan SS. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–1139. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph KS, Allen AC, Dodds L, Vincer MJ, Armson BA. Causes and consequences of recent increases in preterm birth among twins. Obstetrics and gynecology. 2001;98:57–64. doi: 10.1016/s0029-7844(01)01394-1. [DOI] [PubMed] [Google Scholar]
- 48.Hobel CJ. Stress and preterm birth. Clinical obstetrics and gynecology. 2004;47:856–880. doi: 10.1097/01.grf.0000142512.38733.8c. discussion 881-852. [DOI] [PubMed] [Google Scholar]
- 49.Lockwood CJ. Stress-associated preterm delivery: the role of corticotropin-releasing hormone. American journal of obstetrics and gynecology. 1999;180:S264–266. doi: 10.1016/s0002-9378(99)70713-1. [DOI] [PubMed] [Google Scholar]
- 50.Romero R, Mazor M, Gomez R. Cervix, incompetence and premature labor. Fetus. 1993;3 [Google Scholar]
- 51.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? American journal of obstetrics and gynecology. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 54.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–367. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 55.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 56.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 57.Henderson D, Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. American journal of obstetrics and gynecology. 2001;185:579–585. doi: 10.1067/mob.2001.116753. [DOI] [PubMed] [Google Scholar]
- 58.Iams JD. Supplemental progesterone to prevent preterm birth. American journal of obstetrics and gynecology. 2003;188:303. doi: 10.1067/mob.2003.194. [DOI] [PubMed] [Google Scholar]
- 59.Lei K, Chen L, Cryar BJ, Hua R, Sooranna SR, Brosens JJ, Bennett PR, Johnson MR. Uterine stretch and progesterone action. The Journal of clinical endocrinology and metabolism. 2011;96:E1013–1024. doi: 10.1210/jc.2010-2310. [DOI] [PubMed] [Google Scholar]
- 60.Marx SG, Wentz MJ, Mackay LB, Schlembach D, Maul H, Fittkow C, Given R, Vedernikov Y, Saade GR, Garfield RE. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–639. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 61.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. American journal of obstetrics and gynecology. 1994;171:231–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 62.Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. The Journal of clinical endocrinology and metabolism. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 63.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19:763–772. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 64.How H, Huang ZH, Zuo J, Lei ZM, Spinnato JA, 2nd, Rao CV. Myometrial estradiol and progesterone receptor changes in preterm and term pregnancies. Obstetrics and gynecology. 1995;86:936–940. doi: 10.1016/0029-7844(95)00306-C. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Kusanovic JP, Gomez R, Lamont R, Bytautiene E, Garfield RE, Mittal P, Hassan SS, Yeo L. The clinical significance of eosinophils in the amniotic fluid in preterm labor. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23:320–329. doi: 10.3109/14767050903168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R, Kusanovic JP, Munoz H, Gomez R, Lamont RF, Yeo L. Allergy-induced preterm labor after the ingestion of shellfish. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2010;23:351–359. doi: 10.3109/14767050903177193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacques SM, Qureshi F, Kim CJ, Lee JH, Giorgadze T, Mittal P, Hassan SS, Romero R. Eosinophilic/T-cell chorionic vasculitis: a clinicopathologic and immunohistochemical study of 51 cases. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2011;14:198–205. doi: 10.2350/10-07-0867-OA.1. [DOI] [PubMed] [Google Scholar]
- 68.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. IgE-independent mast cell activation augments contractility of nonpregnant and pregnant guinea pig myometrium. International archives of allergy and immunology. 2008;147:140–146. doi: 10.1159/000135701. [DOI] [PubMed] [Google Scholar]
- 69.Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. American journal of obstetrics and gynecology. 2004;191:1356–1361. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 70.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PloS one. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, Chaiworapongsa T, Dong Z, Mittal P, Hassan SS, Kim CJ. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mueller DL. Mechanisms maintaining peripheral tolerance. Nature immunology. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 75.Zachary AA, Leffell MS. Barriers to successful transplantation of the sensitized patient. Expert review of clinical immunology. 2010;6:449–460. doi: 10.1586/eci.10.14. [DOI] [PubMed] [Google Scholar]
- 76.Snanoudj R, Beaudreuil S, Arzouk N, de Preneuf H, Durrbach A, Charpentier B. Immunological strategies targeting B cells in organ grafting. Transplantation. 2005;79:S33–36. doi: 10.1097/01.tp.0000153298.48353.a4. [DOI] [PubMed] [Google Scholar]
- 77.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73:1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 78.Kim IK, Bedi DS, Denecke C, Ge X, Tullius SG. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation. 2008;86:889–894. doi: 10.1097/TP.0b013e318186ac4a. [DOI] [PubMed] [Google Scholar]
- 79.Ayala Garcia MA, Gonzalez Yebra B, Lopez Flores AL, Guani Guerra E. The major histocompatibility complex in transplantation. Journal of transplantation. 2012;2012:842141. doi: 10.1155/2012/842141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howell WM, Carter V, Clark B. The HLA system: immunobiology, HLA typing, antibody screening and crossmatching techniques. Journal of clinical pathology. 2010;63:387–390. doi: 10.1136/jcp.2009.072371. [DOI] [PubMed] [Google Scholar]
- 81.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69:319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 82.Ho EK, Vlad G, Colovai AI, Vasilescu ER, Schwartz J, Sondermeijer H, Burke E, Marboe CC, Itescu S, Suciu-Foca N, Mancini D. Alloantibodies in heart transplantation. Human immunology. 2009;70:825–829. doi: 10.1016/j.humimm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 83.Mahdi BM. A glow of HLA typing in organ transplantation. Clinical and translational medicine. 2013;2:6. doi: 10.1186/2001-1326-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buckley RH. 27. Transplantation immunology: organ and bone marrow. The Journal of allergy and clinical immunology. 2003;111:S733–744. doi: 10.1067/mai.2003.142. [DOI] [PubMed] [Google Scholar]
- 85.Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, Terasaki PI, Rose ML. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 86.Zeevi A, Girnita A, Duquesnoy R. HLA antibody analysis: sensitivity, specificity, and clinical significance in solid organ transplantation. Immunologic research. 2006;36:255–264. doi: 10.1385/IR:36:1:255. [DOI] [PubMed] [Google Scholar]
- 87.Rowshani AT, Bemelman FJ, Lardy NM, Ten Berge IJ. Humoral immunity in renal transplantation: clinical significance and therapeutic approach. Clinical transplantation. 2008;22:689–699. doi: 10.1111/j.1399-0012.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 88.Betkowski AS, Graff R, Chen JJ, Hauptman PJ. Panel-reactive antibody screening practices prior to heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002;21:644–650. doi: 10.1016/s1053-2498(01)00422-3. [DOI] [PubMed] [Google Scholar]
- 89.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000;69:1370–1374. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 90.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 91.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 92.Mihaylova A, Baltadjieva D, Boneva P, Ivanova M, Penkova K, Marinova D, Mihailova S, Paskalev E, Simeonov P, Naumova E. Clinical relevance of anti-HLA antibodies detected by flow-cytometry bead-based assays--single-center experience. Human immunology. 2006;67:787–794. doi: 10.1016/j.humimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duong Van Huyen JP, Fornes P, Guillemain R, Amrein C, Chevalier P, Latremouille C, Creput C, Glotz D, Nochy D, Bruneval P. Acute vascular humoral rejection in a sensitized cardiac graft recipient: diagnostic value of C4d immunofluorescence. Human pathology. 2004;35:385–388. doi: 10.1016/j.humpath.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 96.Frank R, Molina MR, Wald JW, Goldberg LR, Kamoun M, Lal P. Correlation of circulating donor-specific anti-HLA antibodies and presence of C4d in endomyocardial biopsy with heart allograft outcomes: A single-center, retrospective study. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:410–417. doi: 10.1016/j.healun.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Rudzinski E, Gilroy M, Newbill C, Morgan T. Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2013;16:7–13. doi: 10.2350/12-05-1195-OA.1. [DOI] [PubMed] [Google Scholar]
- 98.Troxell ML, Weintraub LA, Higgins JP, Kambham N. Comparison of C4d immunostaining methods in renal allograft biopsies. Clinical journal of the American Society of Nephrology : CJASN. 2006;1:583–591. doi: 10.2215/CJN.00900805. [DOI] [PubMed] [Google Scholar]
- 99.Norris PJ, Lee JH, Carrick DM, Gottschall JL, Lebedeva M, de Castro BR, Kleinman SH, Busch MP. Long-term in vitro reactivity for human leukocyte antigen antibodies and comparison of detection using serum versus plasma. Transfusion. 2009;49:243–251. doi: 10.1111/j.1537-2995.2008.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartel G, Wahrmann M, Exner M, Regele H, Schillinger M, Horl WH, Bohmig GA. Determinants of the complement-fixing ability of recipient presensitization against HLA antigens. Transplantation. 2007;83:727–733. doi: 10.1097/01.tp.0000256337.18347.aa. [DOI] [PubMed] [Google Scholar]
- 101.Liang KY, SL Z. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 102.Hojsgaard S, Halekoh U, Yan J. The R package Geepack for generalized estimating equations. Journal of Statistical Software. 2005;15:1–11. [Google Scholar]
- 103.Raju TN. The problem of late-preterm (near-term) births: a workshop summary. Pediatric Research. 2006;60:775–776. doi: 10.1203/01.pdr.0000246074.73342.1e. [DOI] [PubMed] [Google Scholar]
- 104.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 105.von Rango U. Fetal tolerance in human pregnancy--a crucial balance between acceptance and limitation of trophoblast invasion. Immunology letters. 2008;115:21–32. doi: 10.1016/j.imlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 106.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reproductive biology and endocrinology : RB&E. 2003;1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nature immunology. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 108.Aluvihare VR, Betz AG. The role of regulatory T cells in alloantigen tolerance. Immunological reviews. 2006;212:330–343. doi: 10.1111/j.0105-2896.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 109.Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PloS one. 2007;2:e382. doi: 10.1371/journal.pone.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terness P, Kallikourdis M, Betz AG, Rabinovich GA, Saito S, Clark DA. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 111.Makrigiannakis A, Karamouti M, Drakakis P, Loutradis D, Antsaklis A. Fetomaternal immunotolerance. Am J Reprod Immunol. 2008;60:482–496. doi: 10.1111/j.1600-0897.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 112.Betz AG. Immunology. Have you seen your mother, baby. Science. 2010;330:1635–1636. doi: 10.1126/science.1200406. [DOI] [PubMed] [Google Scholar]
- 113.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ernerudh J, Berg G, Mjosberg J. Regulatory T helper cells in pregnancy and their roles in systemic versus local immune tolerance. Am J Reprod Immunol. 2011;66 Suppl 1:31–43. doi: 10.1111/j.1600-0897.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- 115.Quinn KH, Parast MM. Decidual Regulatory T Cells in Placental Pathology and Pregnancy Complications. Am J Reprod Immunol. 2013 doi: 10.1111/aji.12077. [DOI] [PubMed] [Google Scholar]
- 116.Williams Z. Inducing tolerance to pregnancy. The New England journal of medicine. 2012;367:1159–1161. doi: 10.1056/NEJMcibr1207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 118.King A, Burrows TD, Hiby SE, Bowen JM, Joseph S, Verma S, Lim PB, Gardner L, Le Bouteiller P, Ziegler A, Uchanska-Ziegler B, Loke YW. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–387. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 119.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 120.Zhu BT. Development of selective immune tolerance towards the allogeneic fetus during pregnancy: Role of tryptophan catabolites (Review) International journal of molecular medicine. 2010;25:831–835. doi: 10.3892/ijmm_00000411. [DOI] [PubMed] [Google Scholar]
- 121.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–4128. [PubMed] [Google Scholar]
- 122.Bulla R, Bossi F, Fischetti F, De Seta F, Tedesco F. The complement system at the fetomaternal interface. Chemical immunology and allergy. 2005;89:149–157. doi: 10.1159/000087963. [DOI] [PubMed] [Google Scholar]
- 123.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nature medicine. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 124.Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernandez N, Arck PC. Dendritic cells: key to fetal tolerance? Biology of reproduction. 2007;77:590–598. doi: 10.1095/biolreprod.107.060632. [DOI] [PubMed] [Google Scholar]
- 125.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Critical reviews in immunology. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 126.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, Yoo W, Chaiworapongsa T, Mittal P, Hassan SS, Kim CJ. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tilburgs T, Scherjon SA, Claas FH. Major histocompatibility complex (MHC)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. Journal of reproductive immunology. 2010;85:58–62. doi: 10.1016/j.jri.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 129.Adeniyi-Jones SC, Ozato K. Transfer of antibodies directed to paternal major histocompatibility class I antigens from pregnant mice to the developing fetus. J Immunol. 1987;138:1408–1415. [PubMed] [Google Scholar]
- 130.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 131.Rogenhofer N, Toth B, Kiessig S, Hellstern P, Taborski U, Scholz S, Thaler CJ. Enzyme linked immunosorbent assay (ELISA) as screening method for anti-paternal allo-antibodies in patients with recurrent pregnancy loss (RPL) European journal of obstetrics, gynecology, and reproductive biology. 2008;136:155–159. doi: 10.1016/j.ejogrb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 132.Nielsen HS, Witvliet MD, Steffensen R, Haasnoot GW, Goulmy E, Christiansen OB, Claas F. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. Journal of reproductive immunology. 2010;87:67–73. doi: 10.1016/j.jri.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 133.Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30:56–61. doi: 10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 135.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Mental retardation and developmental disabilities research reviews. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 136.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 137.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 138.Rebibou JM, Chabod J, Alcalay D, Coussediere MC, Deteix P, Touchard G, Dupont I, Thevenin C, Chalopin JM, Tiberghien P. Flow cytometric evaluation of pregnancy-induced anti-HLA immunization and blood transfusion-induced reactivation. Transplantation. 2002;74:537–540. doi: 10.1097/00007890-200208270-00018. [DOI] [PubMed] [Google Scholar]
- 139.Cohen F, Zuelzer WW, Gustafson DC, Evans MM. Mechanisms of Isoimmunization. I. The Transplacental Passage of Fetal Erythrocytes in Homospecific Pregnancies. Blood. 1964;23:621–646. [PubMed] [Google Scholar]
- 140.Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion. 1990;30:344–357. doi: 10.1046/j.1537-2995.1990.30490273444.x. [DOI] [PubMed] [Google Scholar]
- 141.Sanfilippo F, Vaughn WK, Bollinger RR, Spees EK. Comparative effects of pregnancy, transfusion, and prior graft rejection on sensitization and renal transplant results. Transplantation. 1982;34:360–366. doi: 10.1097/00007890-198212000-00010. [DOI] [PubMed] [Google Scholar]
- 142.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA : the journal of the American Medical Association. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 143.Regan L, Braude PR, Hill DP. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod. 1991;6:294–298. doi: 10.1093/oxfordjournals.humrep.a137325. [DOI] [PubMed] [Google Scholar]
- 144.Rebellato LM, Ozawa M, Verbanac KM, Catrou P, Haisch CE, Terasaki PI. Clinical and anti-HLA antibody profile of nine renal transplant recipients with failed grafts: donor-specific and non-donor-specific antibody development. Clinical transplants. 2006:241–253. [PubMed] [Google Scholar]
- 145.Li X, Ishida H, Yamaguchi Y, Tanabe K. Poor graft outcome in recipients with de novo donor-specific anti-HLA antibodies after living related kidney transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2008;21:1145–1152. doi: 10.1111/j.1432-2277.2008.00755.x. [DOI] [PubMed] [Google Scholar]