Abstract
Alexander disease is a neurodegenerative disorder of the central white matter caused by dominant mutations in GFAP, the gene encoding glial fibrillary acidic protein. Magnetic resonance imaging pattern recognition studies have established characteristic radiologic phenotypes for this disorder. In some cases, however, genetically confirmed cases do not express these features, and several reports have identified “atypical” radiologic findings in Alexander disease patients. Here, the authors report 3 genetically confirmed Alexander disease cases with focal central white matter lesions that, upon longitudinal clinical and radiologic evaluation, appear to reflect an atypical Alexander disease magnetic resonance imaging phenotype and not another pathophysiologic process such as encephalitis, infarction, or neoplasm.
Keywords: Alexander disease, white matter, leukodystrophy, glial fibrillary acidic protein (GFAP)
Alexander disease is a neurodegenerative disorder caused by dominant missense mutations in GFAP, the gene encoding glial fibrillary acidic protein, an intermediate filament protein expressed in astrcoytes.1 Typical magnetic resonance imaging (MRI) features include (1) predominance of frontal white matter signal abnormalities, signal changes in (2) periventricular rim, (3) brainstem, and (4) basal ganglia/thalamus, and (5) contrast enhancement in particular structures.2 Identification of 4 of these 4 criteria is diagnostic of Alexander disease.2 However, patients whose diagnosis is confirmed by GFAP gene sequencing do not always fulfill these criteria and may have atypical MRI findings,3 including predominant or isolated posterior fossa involvement without supratentorial white matter findings, multifocal tumor-like brainstem lesions or brainstem atrophy, diffuse signal changes involving basal ganglia/thalamus, and periventricular garland-like structures. Here, we report the new imaging finding of focal cerebral white matter lesions in Alexander disease.
Methods
Clinical histories and radiologic examinations for 3 patients with genetically confirmed Alexander disease diagnoses were reviewed by a group of researchers that includes a child neurologist (AV) and a neuroradiologist (NK). This study was approved by the Children’s National Medical Center Institutional Review Board, and each subject’s family provided written informed consent for the use of these patients’ case histories.
Case Summaries
Case 1
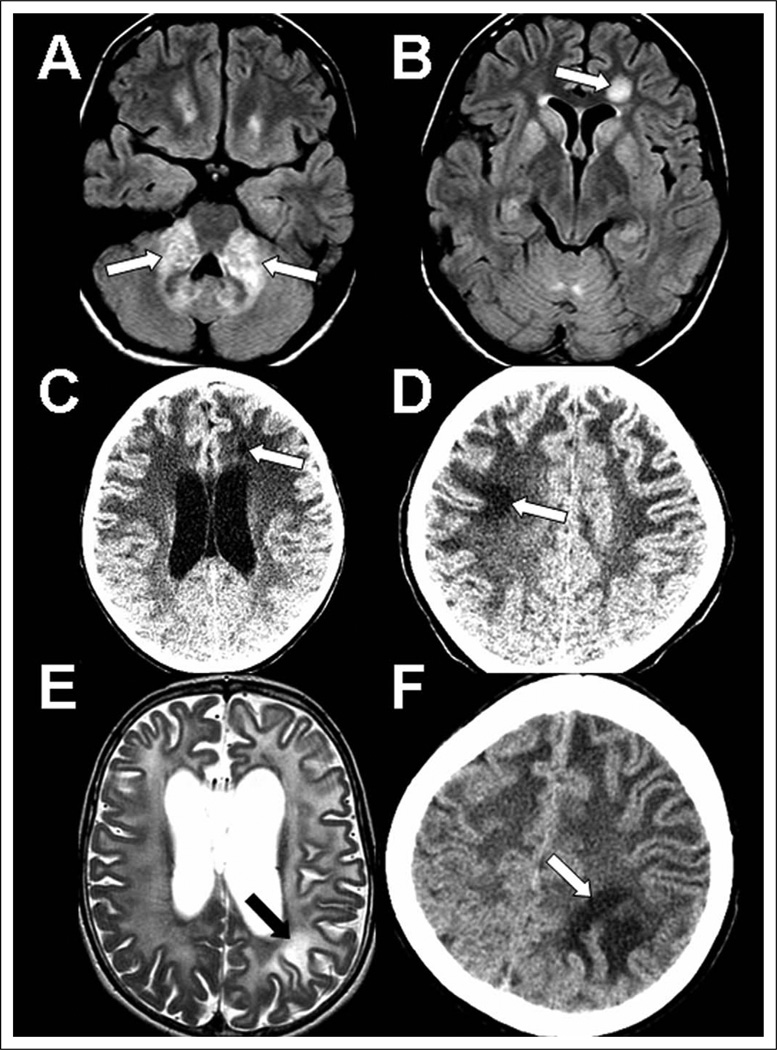
Patient 1 is a 14-year-old girl with episodic emesis and idiopathic short stature since infancy. Examination revealed mild dextroconvex scoliosis, dysarthria, and dysphonia. Brain MRI at age 17 months was reportedly normal but was repeated at age 13 years due to migraines, revealing atypical brain stem findings initially raising concern for malignancy (Figures 1A and B). Fluid-attenuated inversion recovery (FLAIR) images showed abnormal bright signal in the medulla and bilaterally in the cerebellar peduncles, pons, cerebellar white matter, and dentate hila. Contrast-enhanced T1-weighted images confirmed abnormal signal in the middle cerebellar peduncles, with associated expansion in this region. Supratentorially, nonenhancing FLAIR signal increase surrounding the frontal horns and a small enhancing focus in the left frontal periventricular white matter were seen. In addition, a focal 14-mm nonenhancing FLAIR bright lesion was seen in the left frontal deep white matter. Mild diffuse FLAIR bright signal was detected in caudate, putamen, and medial thalami. GFAP sequencing revealed a novel de novo pathogenic heterozygous mutation (c. 868 C>G, p.Q290E).
Figure 1.
Focal white matter lesions in 3 Alexander disease cases. Multiple imaging studies showing focal lesions in case 1 (A, B), case 2 (C, D), and case 3 (E, F). A–B, noncontrast axial FLAIR images at age 13 showing bright lesions with mass effect in the middle cerebellar peduncles (A) and a left frontal focal well-defined lesion (B). C–D, noncontrast axial CT images: first, at age 6 years, there is a small left frontal hypodense lesion (C), and then by age 9 years there is good visualization of an additional right superior frontal hypodense lesion (D). E–F, axial T2 MRI (E) and noncontrast axial CT image (F) showing some left parietal white matter that is more bright in signal than is the surrounding abnormal white matter at age 6 years (E) and has distinctively abnormal appearance in comparison to surrounding white matter on CT at age 7 years (F). CT, computed tomography; FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging.
Case 2
Patient 2 is a 10-year-old girl who presented at age 4 years with failure to thrive, swallowing difficulties, and focal hemiparesis after traumatic injury. Brain MRI revealed Alexander disease—typical radiologic features.2 She developed progressive encephalopathy, seizures, hydrocephalus, bulbar dysfunction, severe scoliosis, and obstructive sleep apnea. Physical examination revealed severe encephalopathy, with cranial nerves remarkable for roving eye movements, nystagmus, and palatal myoclonus. Motor examination was notable for truncal hypotonia and appendicular hypertonia, contractures, and limited lower extremity motion. She showed brisk deep tendon reflexes and bilateral Babinski reflexes. In addition to early diagnostic MRIs, she underwent several noncontrast computed tomography studies for ventriculomegaly (Figures 1 C and D). At age 6 years, she developed a left frontal hypodense white matter lesion, which became smaller by age 9 years. At age 7 years, she developed a right frontal hypodense subcortical lesion, which became more hypodense and better visualized by age 9 years. GFAP gene sequencing revealed a known de novo pathogenic mutation (c.729 C>T, p.R239C),1 associated with early onset and aggressive course.1
Case 3
Patient 3 is a 10-year-old girl who presented at age 7 months with focal motor dysfunction and seizures after varicella infection. Magnetic resonance imaging was typical for Alexander disease. Over subsequent years, she developed gastroesophageal reflux disease, intractable vomiting, bulbar dysfunction, central apnea, severe scoliosis, spastic quadriparesis, and epilepsy. She was severely encephalopathic and did not use words or respond to simple commands. Cranial nerve examination revealed nystagmus and lack of eye tracking. Motor examination revealed truncal hypotonia and appendicular hypertonicity. Upper and lower extremity deep tendon reflexes were brisk, with clonus and bilateral Babinski reflexes. Initial brain MRI (Figures 1 E and F) was performed at age 22 months, revealing a left parietal subcortical signal on diffusion-weighted imaging, with no corresponding apparent diffusion coefficient dark signal. The lesion was not seen on T2-weighted imaging due to generally abnormal bright white matter signal and did not enhance with gadolinium on T1 images. On follow-up MRI at age 2 years, the lesion was not seen on T2 but, at age 6 years, showed brighter T2 signal than did the surrounding white matter. On computed tomography at age 7 years, the lesion was obvious and remained stable over 6months. GFAP gene sequencing revealed a novel de novo pathogenic mutation (c. 270 A>G, p. K86E).
Discussion
We describe atypical, focal MRI findings in 3 genetically confirmed Alexander disease patients. In all 3, focal abnormalities raised concern for focal processes unrelated to Alexander disease, such as encephalitis, infarction, or neoplasm, but these concerns were not supported clinically or on subsequent imaging studies. We suggest that these lesions can manifest in some cases as radiologic features of Alexander disease, and although their presence does not constitute a diagnosis of Alexander disease per se, they do not necessarily mean a second diagnosis of neoplasm, infection, or vascular insult exists in addition to genetically confirmed Alexander disease. Further studies are needed to investigate the pathophysiologic basis and clinical significance of these focal lesions.
Acknowledgment
The cases presented were followed at Children’s National Medical Center (CNMC). We would like to thank CNMC’s Department of Neurology, Neuroradiology Program, and Center for Genetic Medicine for their support of this project.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Adeline Vanderver is supported by a research grant from the Dana Foundation, the FP7 Seventh Framework, and the CETT program and by a K08-funded grant from the National Institute of Neurological Disorders and Stroke. She has been supported in the past by a fellowship from the American Academy of Neurology Foundation and by a grant from the Pelizaeus-Merzbacher Disease Foundation.
Footnotes
Author Contributions
PB contributed to study design, reviewed case histories, and prepared and edited the manuscript.MJP reviewed case histories and prepared and edited the manuscript. JC reviewed case histories and prepared and edited the manuscript. JL reviewed case histories and prepared and edited the manuscript. NK read and interpreted MRI scans and provided editorial feedback during manuscript preparation. AV contributed to study design, reviewed case histories, read and interpreted MRI scans, and provided editorial feedback on manuscript preparation.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by the Children’s National Medical Center Institutional Review Board. The family of each patient provided written informed consent for participation in this study.
References
- 1.Brenner M, Johnson AB, Boespflug-Tanguy O, et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- 2.van der Knaap MS, Naidu S, Breiter SN, et al. Alexander disease: diagnosis with MR imaging. AJNR Am J Neuroradiol. 2001;22:541–552. [PMC free article] [PubMed] [Google Scholar]
- 3.van der Knaap MS, Salomons GS, Li R, et al. Unusual variants of Alexander’s disease. Ann Neurol. 2005;57:327–338. doi: 10.1002/ana.20381. [DOI] [PubMed] [Google Scholar]