Abstract
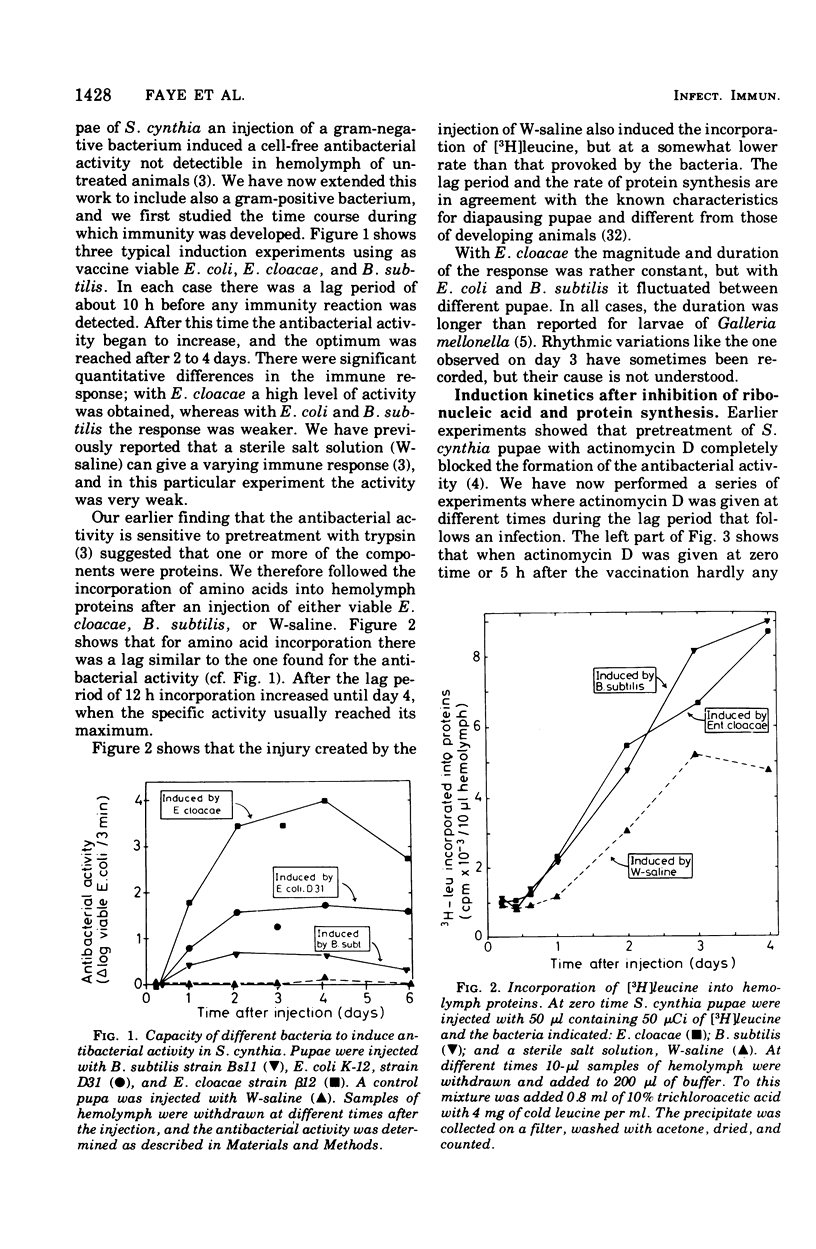
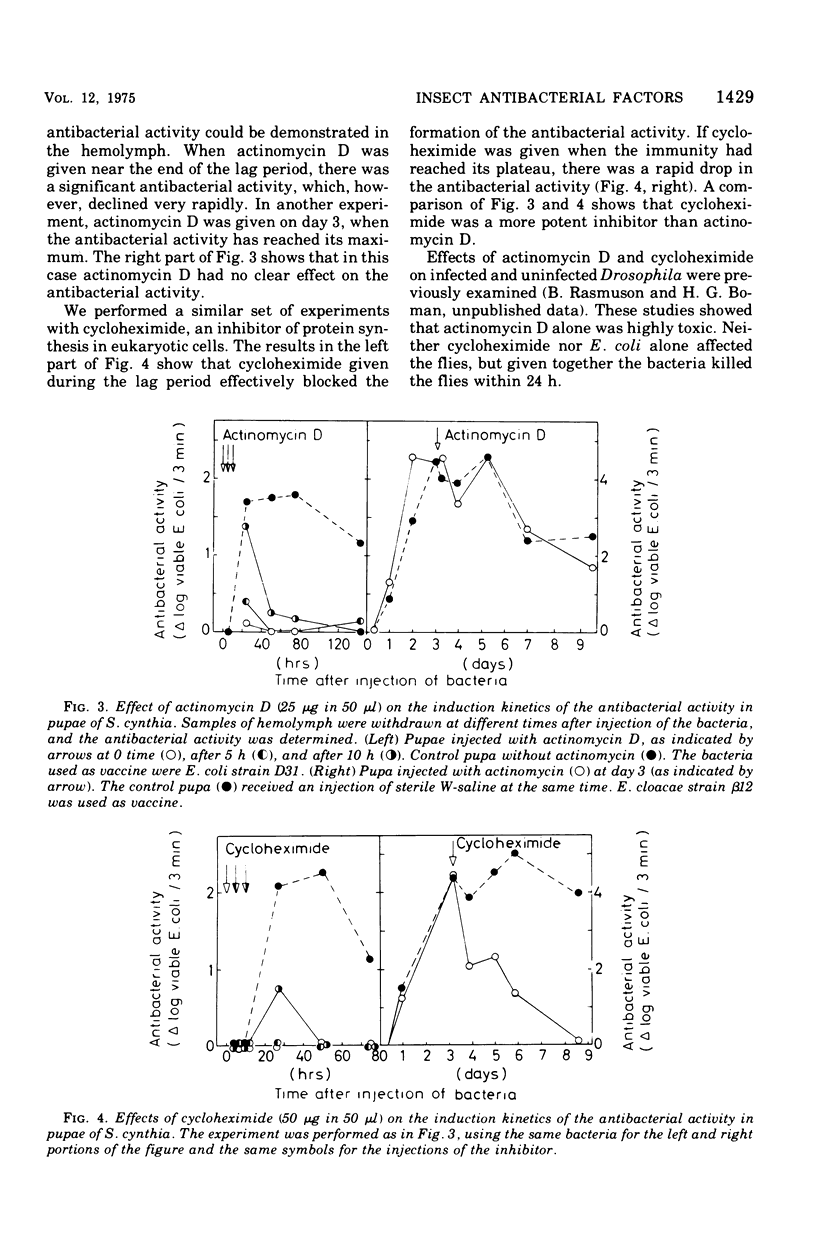
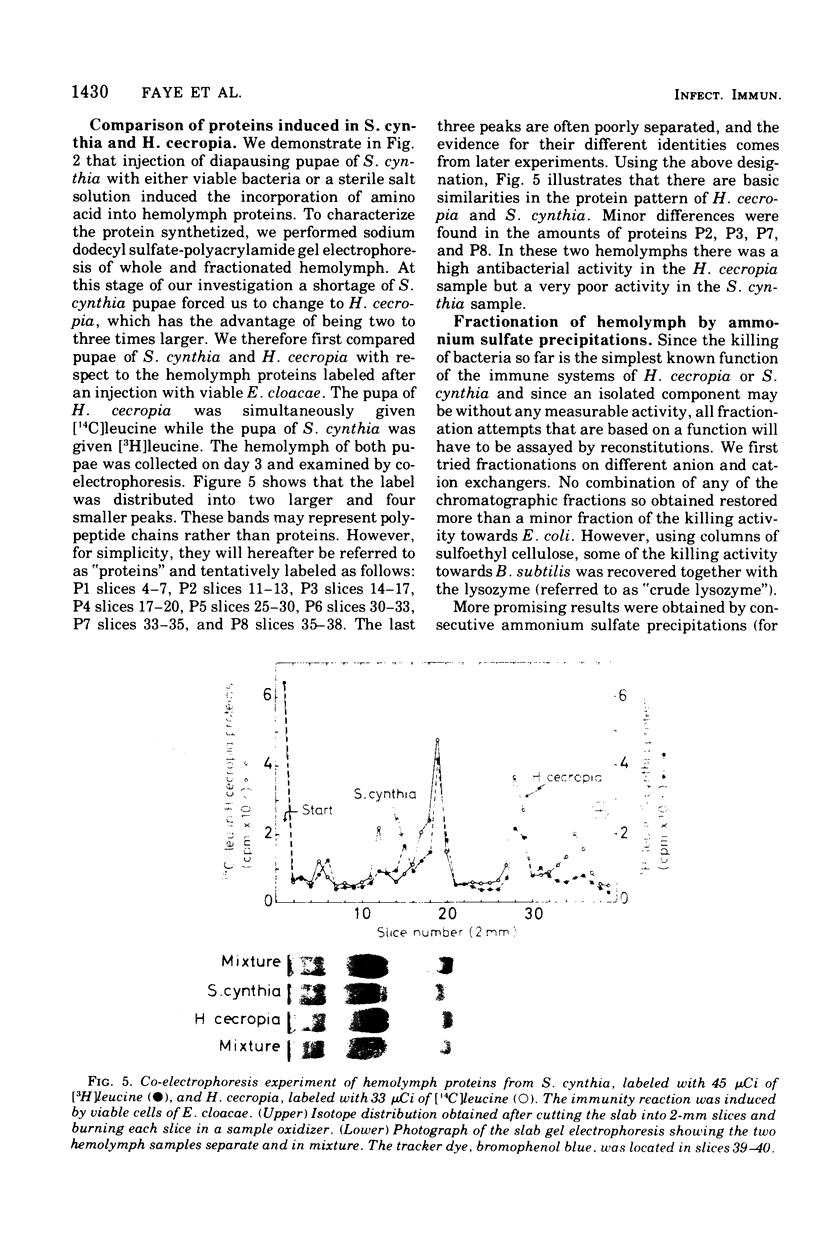
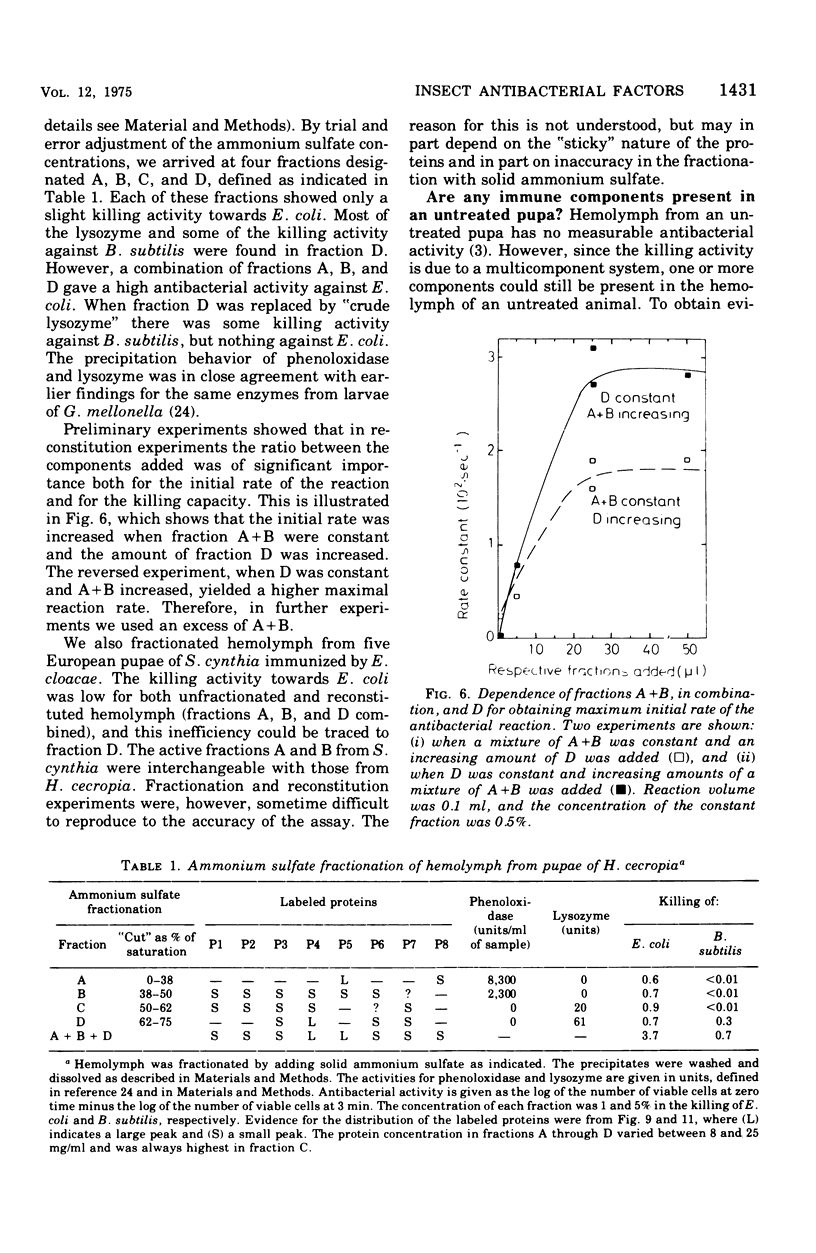
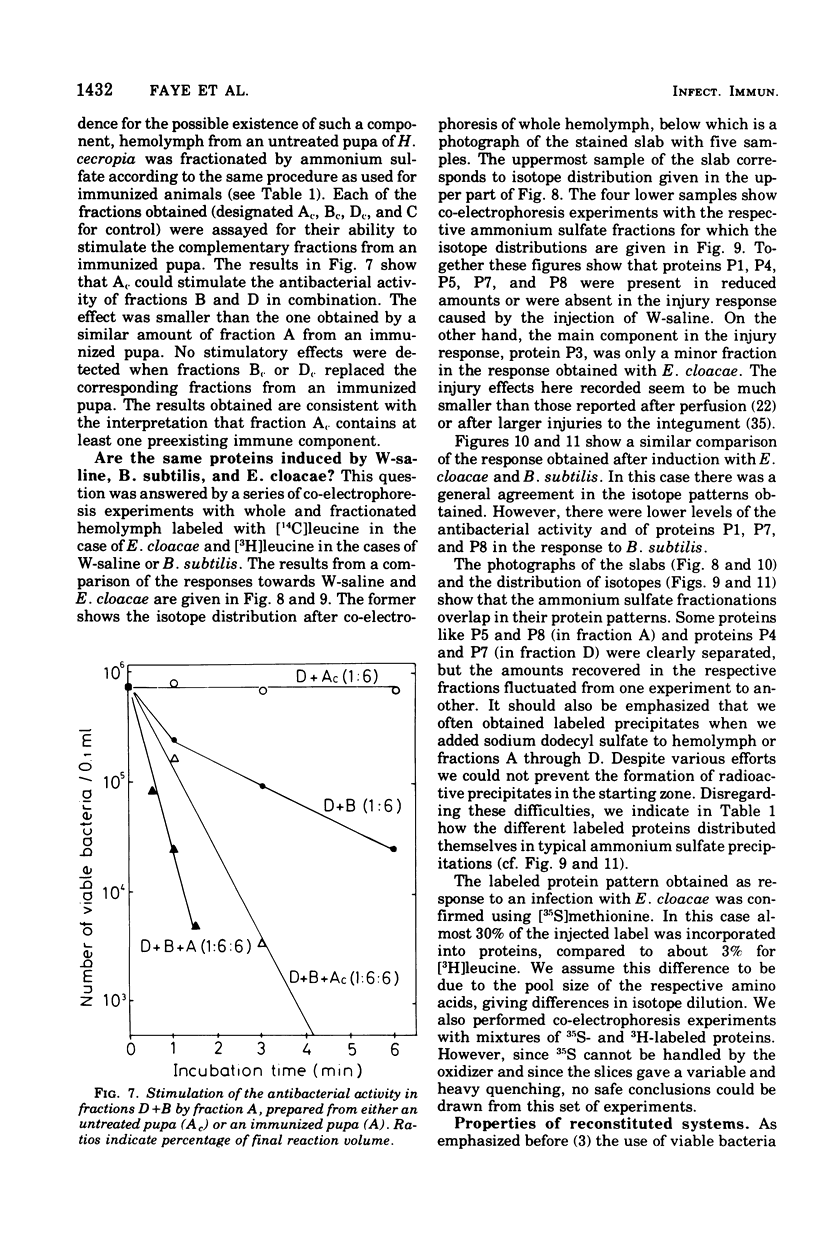
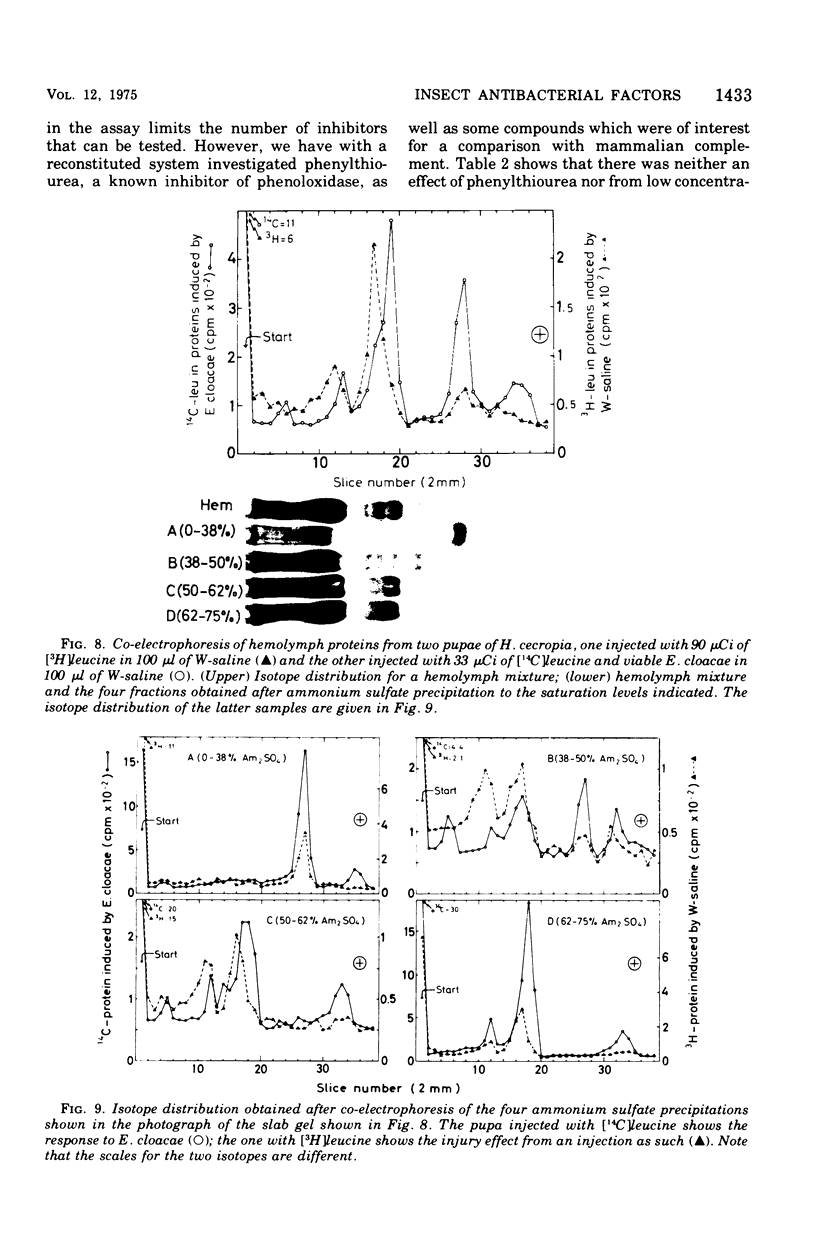
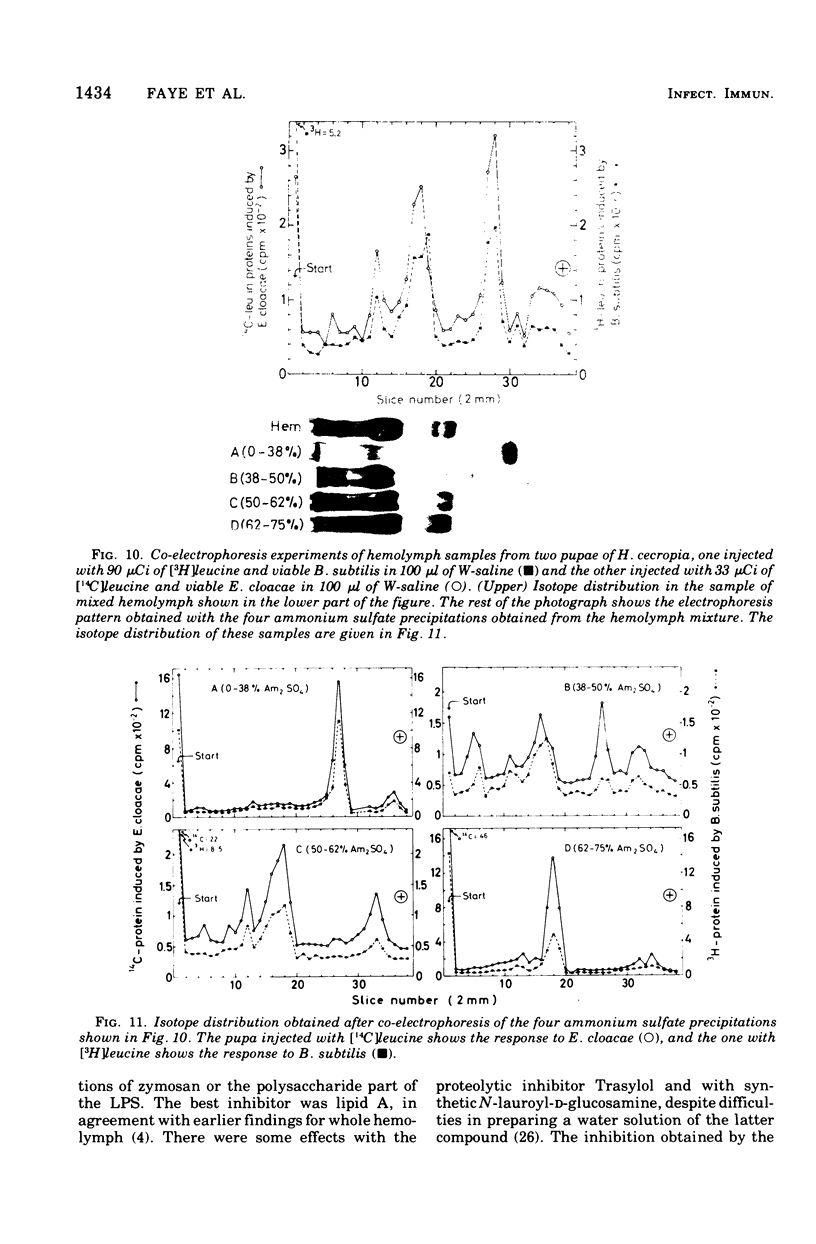
We have previously shown that pupae of the giant silkmoth Samia cynthia have a humoral antibacterial activity, which was induced by viable, nonpathogenic gram-negative bacteria (H.G. Boman et al., 1974). We show here that this activity was formed simultaneously with a selective incorporation of amino acids into eight polypeptide chains characterized by their electrophoretic behavior. If actinomycin D or cycloheximide were given at an early time, no antibacterial activity was found. If the inhibitors were given at the time of maximum activity, there was no effect with actinomycin D but a rapid decrease of the activity in the case of cycloheximide. The results imply that the messenger ribonucleic acid was stable, but that at least one protein component was turning over. Hemolymph from immunized pupae of another giant silkmoth, Hyalophora cecropia, was fractionated by ammonium sulfate precipitation. This procedure, together with the isotope distribution after co-electrophoresis in polyarylamide gels, was used for comparing the response to injury and to different infections. Almost identical polypeptide patterns were obtained as a response to an infection with either viable Enterobacter cloacae or Bacillus subtilis. These patterns differed both qualitatively and quantitatively from the injury effect created by an injection as such. There was only a low antibacterial activity in each of the four fractions obtained by ammonium sulfate precipitation. However, a combination of three fractions restored a high killing activity. Fractionation of hemolymph from untreated pupae provided evidence for at least one preexisting factor which stimulated the killing of Escherichia coli. The osmotic pressure of the bacteria contributed to the antibacterial activity towards E. coli, but not towards B. subtitlis. The killing of E. coli was inhibited by liped A and, to a lesser extent, by an inhibitor of proteolytic enzymes. The similarities and differences with the mammalian complement system are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G., Monner D. A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):455–464. doi: 10.1128/jb.121.2.455-464.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Nilsson-Faye I., Paul K., Rasmuson T., Jr Insect immunity. I. Characteristics of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infect Immun. 1974 Jul;10(1):136–145. doi: 10.1128/iai.10.1.136-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwich J. S. An assessment of the ability of individual moieties of Pseudomonas aeruginosa endotoxin to induce immunity in larvae of Galleria mellonella. J Invertebr Pathol. 1971 Mar;17(2):299–300. doi: 10.1016/0022-2011(71)90113-3. [DOI] [PubMed] [Google Scholar]
- Day N. K., Gewurz H., Johannsen R., Finstad J., Good R. A. Complement and complement-like activity in lower vertebrates and invertebrates. J Exp Med. 1970 Nov;132(5):941–950. doi: 10.1084/jem.132.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Jeso F. Ammonium sulfate concentration conversion nomograph for 0 degrees. J Biol Chem. 1968 Apr 25;243(8):2022–2023. [PubMed] [Google Scholar]
- Donaldson D. M., Roberts R. R., Larsen H. S., Tew J. G. Interrelationship between serum beta-lysin, lysozyme, and the antibody-complement system in killing Escherichia coli. Infect Immun. 1974 Sep;10(3):657–666. doi: 10.1128/iai.10.3.657-666.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz P., Vey A. Humoral encapsulation in Diptera (Insecta): defence reactions of Chironomus larvae against fungi. Parasitology. 1974 Apr;68(2):193–205. [PubMed] [Google Scholar]
- Hildemann W. H. Some new concepts in immunological phylogeny. Nature. 1974 Jul 12;250(462):116–129. doi: 10.1038/250116a0. [DOI] [PubMed] [Google Scholar]
- Johannsen R., Anderson R. S., Good R. A., Day N. K. A comparative study of the bactericidal activity of horseshoe crab (Limulus polyphemus) hemolymph and vertebrate serum. J Invertebr Pathol. 1973 Nov;22(3):372–376. doi: 10.1016/0022-2011(73)90167-5. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C. mRNA stability and cellular differentiation. Acta Endocrinol Suppl (Copenh) 1972;168:319–345. doi: 10.1530/acta.0.071s319. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Muller-Eberhard H. J. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Chemistry and reaction mechanisms of complement. Adv Immunol. 1968;8:1–80. doi: 10.1016/s0065-2776(08)60464-2. [DOI] [PubMed] [Google Scholar]
- Powning R. F., Davidson W. J. Studies on insect bacteriolytic enzymes. I. Lysozyme in haemolymph of Galleria mellonella and Bombyx mori. Comp Biochem Physiol B. 1973 Jul 15;45(3):669–686. [PubMed] [Google Scholar]
- Pye A. E. Microbial activation of prophenoloxidase from immune insect larvae. Nature. 1974 Oct 18;251(5476):610–613. doi: 10.1038/251610a0. [DOI] [PubMed] [Google Scholar]
- Ratcliffe N. A., Rowley A. F. In vitro phagocytosis of bacteria by insect blood cells. Nature. 1974 Nov 29;252(5482):391–392. doi: 10.1038/252391a0. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Asselineau J., Mergenhagen S. E., Nowotny A. A synthetic glycolipid with B-cell mitogenic activity. J Exp Med. 1974 Nov 1;140(5):1404–1409. doi: 10.1084/jem.140.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Rhoads R. E., Palacios R., Sullivan D. Ovalbumin mRNA, complementary DNA and hormone regulation in chick oviduct. Acta Endocrinol Suppl (Copenh) 1973;180:357–379. doi: 10.1530/acta.0.074s357. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weevers R. D. A lepidopteran saline: effects of inorganic cation concentrations on sensory, reflex and motor responses in a herbivorous insect. J Exp Biol. 1966 Feb;44(1):163–175. doi: 10.1242/jeb.44.1.163. [DOI] [PubMed] [Google Scholar]