Abstract
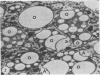
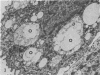
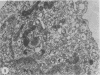
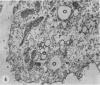
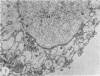
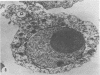
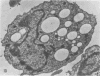
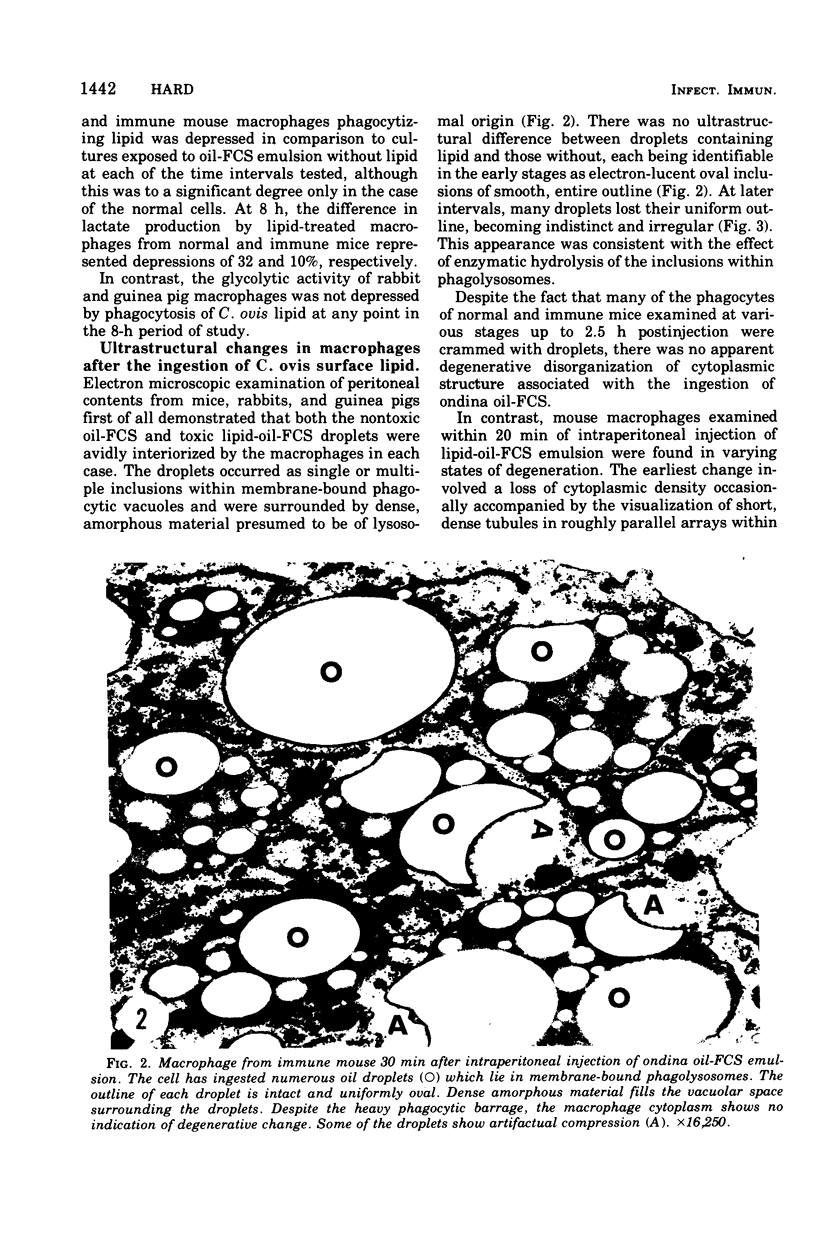
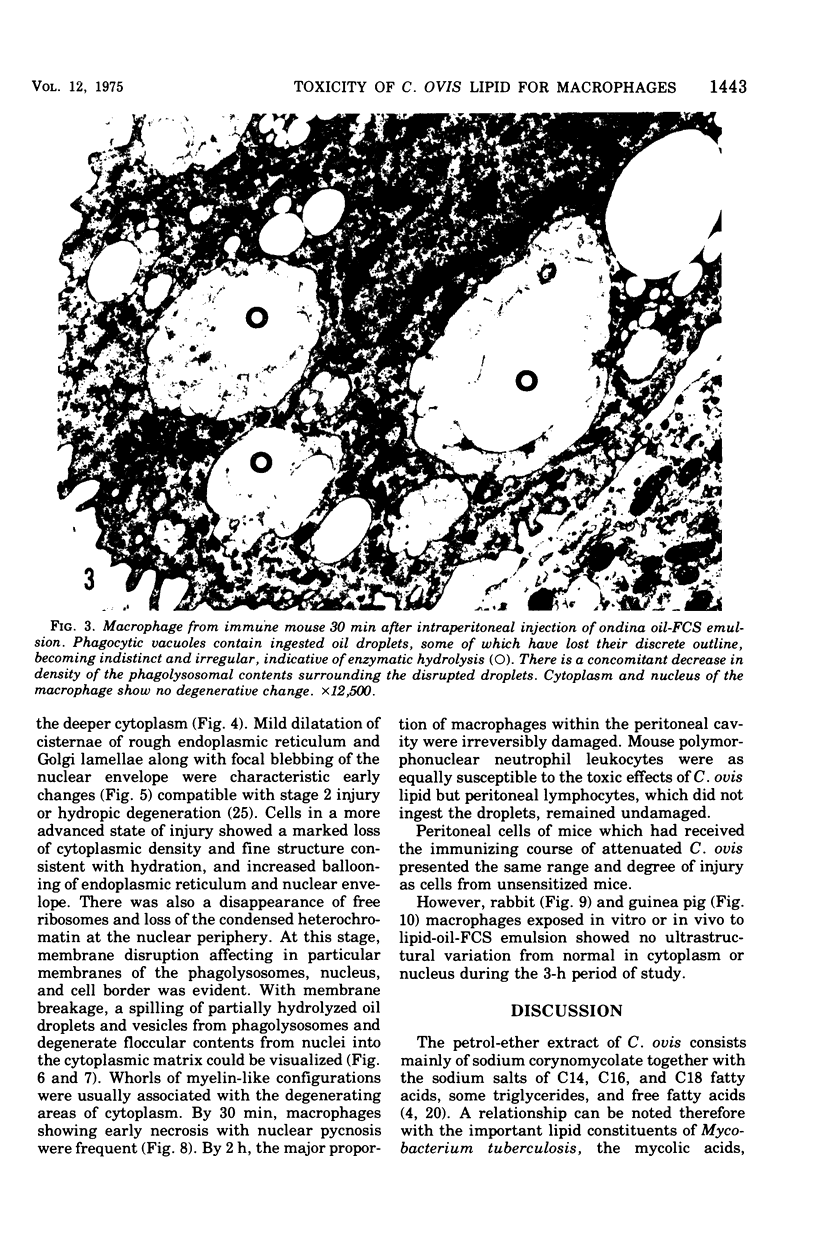
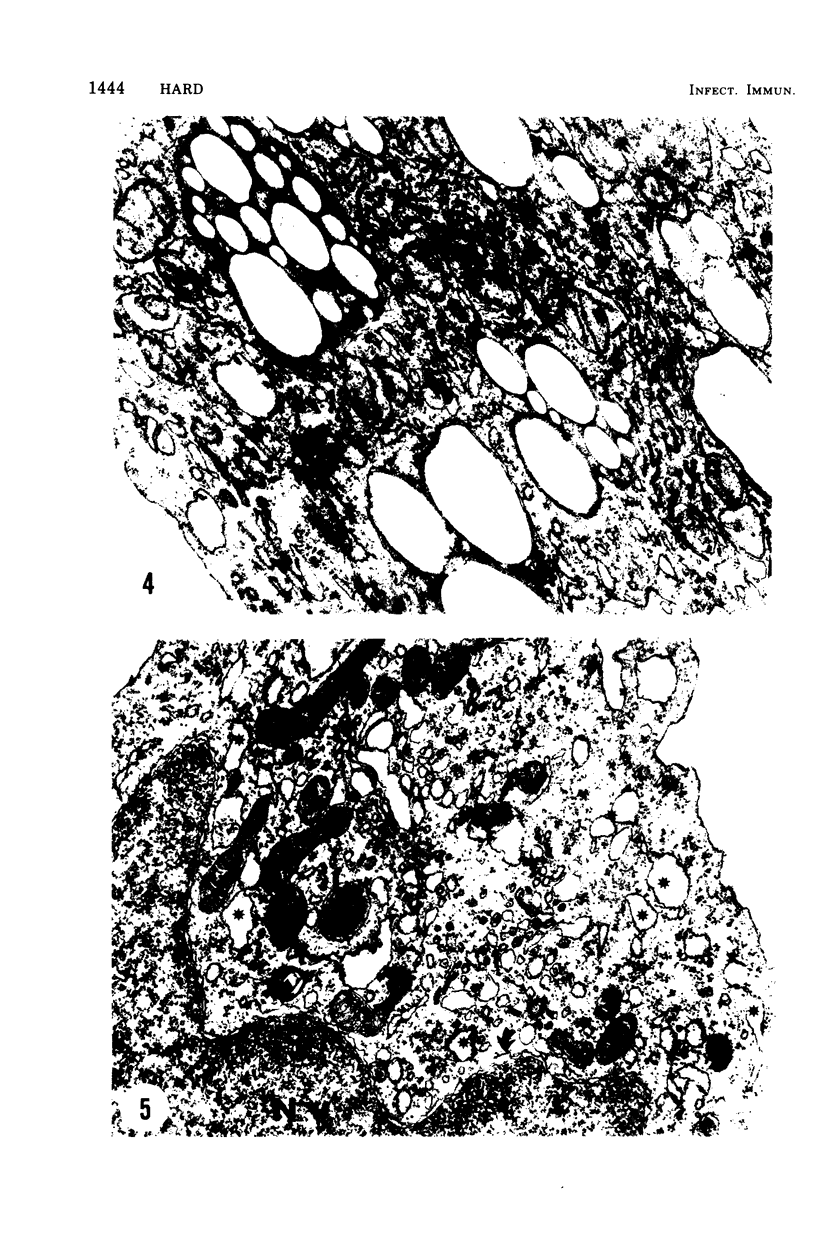
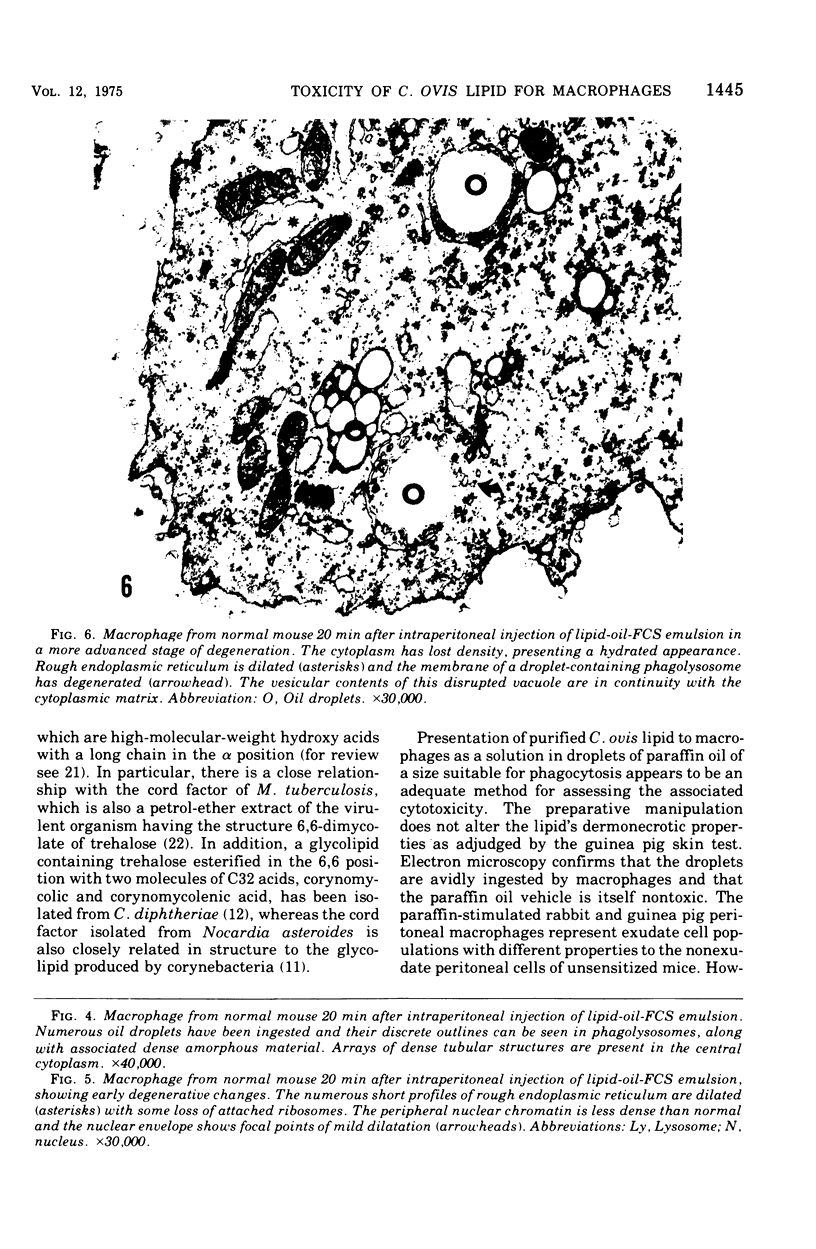
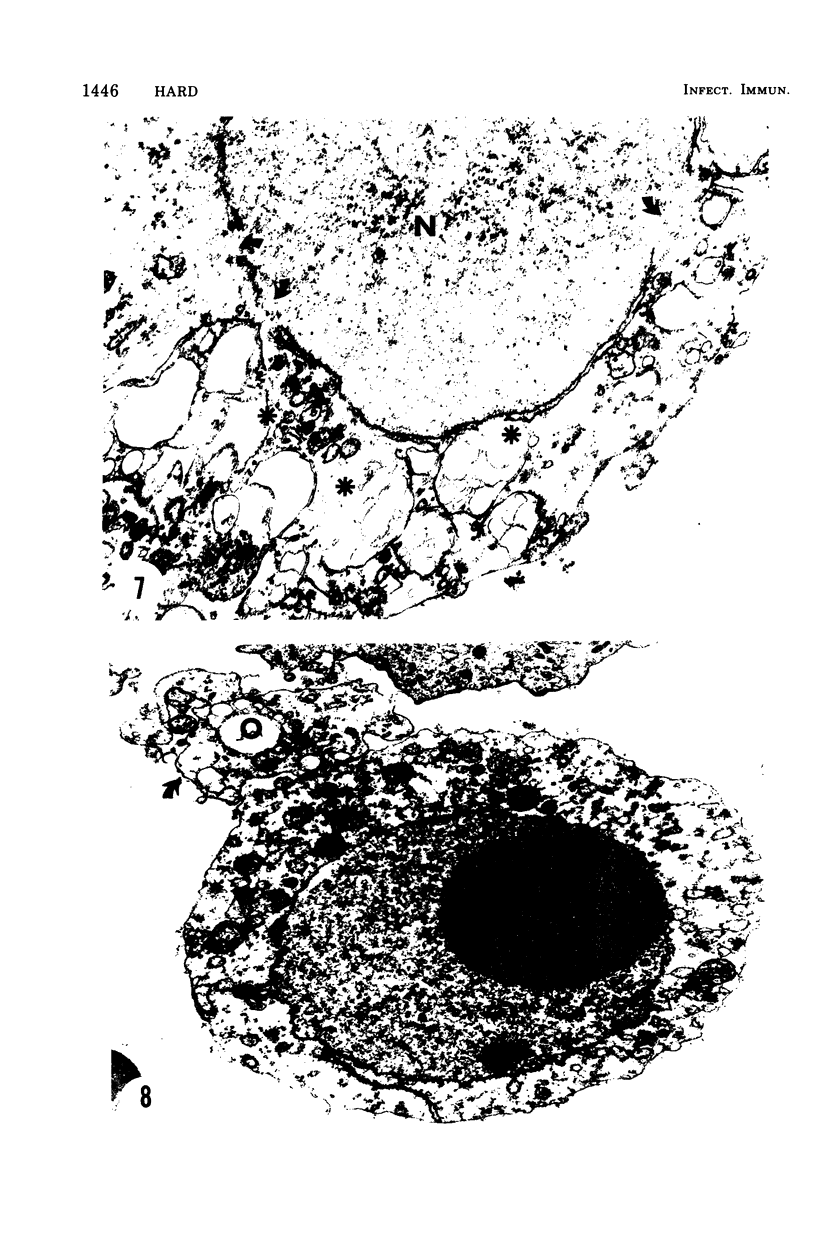
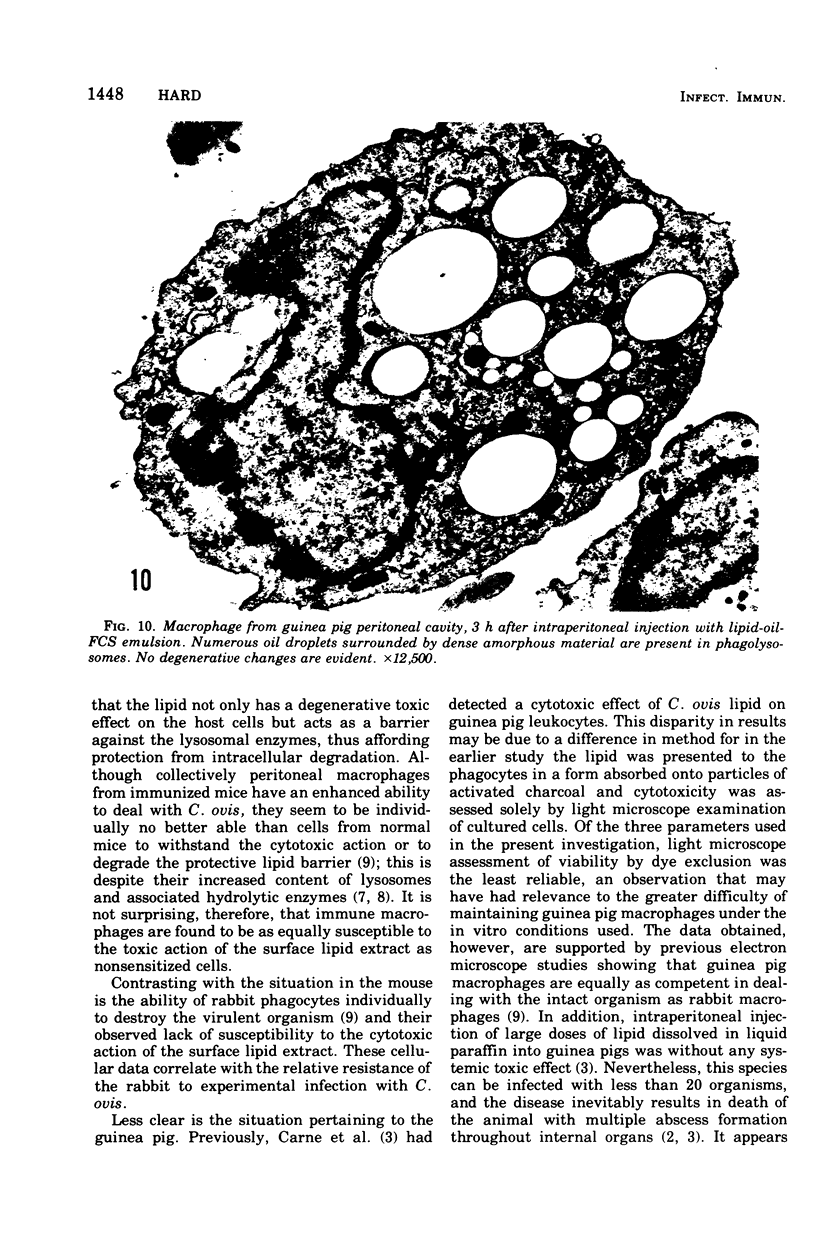
The postphagocytic effect on mouse, rabbit, and guinea pig peritoneal macrophages of a petrol-ether lipid extract from Corynebacterium ovis (C. pseudotuberculosis) representing the surface coat of the organism external to the cell wall was investigated by examing three parameters of cytotoxicity, viability assayed by dye exclusion, glycolytic activity, and ultrastructural morphology. The viability test demonstrated a lethal effect on normal and immune mouse macrophages but not on those of the rabbit or guinea pig. Measurement of glycolsis indicated a significant degree of cytotoxicity in normal mouse macrophages ingesting lipid, a nonsignificant depression of activity in cells from immune mice, and no alteration in the activities of rabbit and guinea pig macrophages. Electron microscopy demonstrated that C. ovis surface lipid caused acute lethal injury in normal and immune mouse macrophages. The early stages of degeneration were typified by dilatation of the cisternae of rough endoplasmic reticulum, Golgi lamellae, and nuclear envelope, proceeding to focal disruption of various cell membranes, particularly those of the lipidcontaining phagolysosomes and nucleus. In contrast, over the 3-h period of study, no cytotoxic change was evident in rabbit or guinea pig macrophages. The results add further support to previous observations that the surface lipid of C. ovis plays a major role in the pathogenesis of the organism in mice, but they do not explain the guinea pig's marked susceptibility to infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNS F. H., HORN H. D. Quantitative Bestimmung von L(+)-Milchsäure mit Milchsäuredehydrogenase. Biochim Biophys Acta. 1956 Aug;21(2):378–380. doi: 10.1016/0006-3002(56)90023-3. [DOI] [PubMed] [Google Scholar]
- CARNE H. R., KATER J. C., WICKHAM N. A toxic lipid from the surface of Corynebacterium ovis. Nature. 1956 Sep 29;178(4535):701–702. doi: 10.1038/178701a0. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Electron microscopic examination of Corynebacterium ovis. J Bacteriol. 1969 Mar;97(3):1480–1485. doi: 10.1128/jb.97.3.1480-1485.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard G. C. Electron microscopic study of the differentiation of mouse peritoneal macrophages stimulated by Corynebacterium ovis infection. Lab Invest. 1969 Oct;21(4):309–315. [PubMed] [Google Scholar]
- Hard G. C. Examination by electron microscopy of the interaction between peritoneal phagocytes and Corynebacterium ovis. J Med Microbiol. 1972 Nov;5(4):483–491. doi: 10.1099/00222615-5-4-483. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Immunity to experimental infection with Corynebacterium ovis in the mouse peritoneal cavity. Res Vet Sci. 1969 Nov;10(6):547–554. [PubMed] [Google Scholar]
- Hard G. C. Some biochemical aspects of the immune macrophage. Br J Exp Pathol. 1970 Feb;51(1):97–105. [PMC free article] [PubMed] [Google Scholar]
- JANOFF A., ZWEIFACH B. W. EFFECT OF ENDOTOXIN-TOLERANCE, CORTISONE, AND THOROTRAST ON RELEASE OF ENZYMES FOR SUBCELLULAR PARTICLES OF MOUSE LIVER. Proc Soc Exp Biol Med. 1963 Dec;114:695–698. doi: 10.3181/00379727-114-28773. [DOI] [PubMed] [Google Scholar]
- Jolly R. D. Experimental infection of convalescent mice with corynebacterium ovis. N Z Vet J. 1965 Dec;13(6):148–153. doi: 10.1080/00480169.1965.33619. [DOI] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- Kato M., Fukushi K. Studies of a biochemical lesion in experimental tuberculosis in mice. X. Mitochondrial swelling induced by cord factor in vivo and accompanying biochemical change. Am Rev Respir Dis. 1969 Jul;100(1):42–46. doi: 10.1164/arrd.1969.100.1.42. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. VII. Structural and functional damage in mouse liver mitochondria under the toxic action of cord factor. Am Rev Respir Dis. 1968 Aug;98(2):260–269. doi: 10.1164/arrd.1968.98.2.260. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. XI. Mitochondrial swelling induced by cord factor in vitro. Am Rev Respir Dis. 1969 Jul;100(1):47–53. doi: 10.1164/arrd.1969.100.1.47. [DOI] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Toubiana R. Sur quelques constituants lipidiques de Corynebacterium ovis. Eur J Biochem. 1967 Jul;2(1):37–43. doi: 10.1111/j.1432-1033.1967.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Lederer E. The mycobacterial cell wall. Pure Appl Chem. 1971;25(1):135–165. doi: 10.1351/pac197125010135. [DOI] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. New ultrastructural characteristics of cells fixed in a glutaraldehyde-osmium tetroxide mixture. Lab Invest. 1966 Jan;15(1 Pt 2):368–379. [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. I. The effects of endotoxin, endotoxin tolerance, and cortisone on the release of acid hydrolases from a granular fraction of rabbit liver. J Exp Med. 1962 Oct 1;116:433–450. doi: 10.1084/jem.116.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]