Abstract
Purpose
To describe viral retinitis following intravitreal and periocular corticosteroid administration.
Methods
Retrospective case series and comprehensive literature review.
Results
We analyzed 5 unreported and 25 previously published cases of viral retinitis following local corticosteroid administration. Causes of retinitis included 23 CMV (76.7%), 5 HSV (16.7%), and 1 each VZV and unspecified (3.3%). Two of 22 tested patients (9.1%) were HIV positive. Twenty-one of 30 (70.0%) cases followed one or more intravitreal injections of triamcinolone acetonide (TA), 4 (13.3%) after one or more posterior sub-Tenon injections of TA, 3 (10.0%) after placement of a 0.59-mg fluocinolone acetonide implant (Retisert), and 1 (3.3%) each after an anterior subconjunctival injection of TA (together with IVTA), an anterior chamber injection, and an anterior sub-Tenon injection. Mean time from most recent corticosteroid administration to development of retinitis was 4.2 months (median 3.8; range 0.25–13.0). Twelve patients (40.0%) had type II diabetes mellitus. Treatments used included systemic antiviral agents (26/30, 86.7%), intravitreal antiviral injections (20/30, 66.7%), and ganciclovir intravitreal implants (4/30, 13.3%).
Conclusions
Viral retinitis may develop or reactivate following intraocular or periocular corticosteroid administration. Average time to development of retinitis was 4 months, and CMV was the most frequently observed agent. Diabetes was a frequent co-morbidity and several patients with uveitis who developed retinitis were also receiving systemic immunosuppressive therapy.
Keywords: Acute retinal necrosis, corticosteroid, cytomegalovirus, herpes virus, injection, retinitis
Herpetic retinitis is an uncommon, yet vision-threatening infection. In immunocompetent patients, necrotizing retinitis is caused most often by either varicella zoster virus (VZV) or herpes simplex virus (HSV).1,2 In immunocompromised patients, such as those with the acquired immune deficiency syndrome (AIDS), cytomegalovirus (CMV) is an important cause of retinitis.3–5 Regardless of cause, prompt diagnosis and treatment are required to prevent vision loss.
While uncommon, the development of active viral retinitis following intraocular or periocular corticosteroid injection has been described. In 2002, Dalessandro and colleagues reported reactivation of CMV retinitis in a 45-year-old HIV-positive man 2 months following a sub-Tenon injection of 40mg of triamcinolone acetonide for immune recovery uveitis.6 Since that report, 19 studies describing 24 additional patients have appeared in the literature (Table 1).7–25 The present report presents 5 new cases and includes a comprehensive review of the occurrence and management of viral retinitis following intraocular and periocular corticosteroid administration.
Table 1.
Summary of Previously Reported and Current Cases of Viral Retinitis Following Intraocular and Periocular Corticorsteroid Injection.
| Author (Year) | Age (years) | Gender | HIV status | Indication for corticosteroid | Dose / Route | Time from corticosteroid dosing to retinitis (months) |
Established
Diagnosis (Method) |
Zone
Involved (1=macula, 2=midperiphery, 3=outer periphery) |
Vision when retintitis first diagnosed |
Treatment | Duration
of Follow-Up (months) |
Vision at last visit | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previously Published Cases | Dalessandro et al, (2002)6 | 45 | M | + | Immune recovery uveitis | 40mg PST TA | 2.0 | CMV retinitis (NR) | 2 | 20/100 | Intravenous gancyclovir | 18 | “recovered habitual visual acuity” |
|
| |||||||||||||
| Saidel et al, (2005)7 | 75 | M | - | DME | 4mg IVTA | 4.0 | CMV retinitis (PCR) | 1 | 20/400 | Intravitreal gancyclovir, vancomycin, ceftazidime, Then valganciclovir, topical prednisolone, Repeated Intravitreal gancyclovir | 6 | 20/400 | |
|
| |||||||||||||
| Aggermann et al, (2006)8 | 69 | M | NR | CME/CRVO due to Factor V Leiden | 4mg IVTA | 0.75 | HSV retinitis (serology) | 1 | NLP | Intravitreal antibiotics, intravitreal corticosteroids, PPV, PK, Then systemic antiviral therapy | 24 | NLP | |
|
| |||||||||||||
| Toh et al, (2006)9 | 62 | F | NR | CNVM / AMD | 4mg IVTA | 6.0 | HSV retinitis (serology) | 2 | 6/18 | Intravenous acyclovir, Topical timolol, latanoprost, Oral acyclovir | 3 | 6/36 | |
|
| |||||||||||||
| Delyfer et al, (2007)10 | 77 | M | - | CNVM / AMD | 20mg IVTA | 4.0 | CMV retinitis (serology+PCR) | 2 | CF at 1.8m | Intravitreal, intravenous, valganciclovir | 6 | 20/200 | |
| 59 | M | - | CRVO / DME | 8mg IVTA three times over 3 month interval | 3 (after 3rd IVTA) | CMV retinitis (PCR) | 1 | 20/200 | Intravenous ganciclovir, valganciclovir | 3 | 20/400 | ||
|
| |||||||||||||
| Furukawa et al, (2007)11 | 54 | F | - | DME | 10mg IVTA | 4.0 | CMV retinitis (serology+PCR) | 2 | 1 | Intravenous panipenem, vancomycin/panipenem, Intravenous ganciclovir, Intravitreous foscarnet, Vitrectomy and silicone oil tamponade | 14 | 0.5 | |
|
| |||||||||||||
| Hsu et al, (2007)12 | 77 | M | - | DME | 4mg IVTA | 1.5 | CMV retinitis (PCR) | 2 | 3/200 | Valgancyclovir | 1 | 20/400 | |
|
| |||||||||||||
| Ufret-Vincenty et al, (2007)13 | 65 | M | NR | Uveitic CME / Behcet's disease | FA implant | 53 (after 1st implant), 5 (after 2nd implant) | CMV retinitis (“clinical diagnosis”) | 2 | 20/50 | Intravitreal foscarnet, ganciclovir implant | 5 | 20/40 | |
|
| |||||||||||||
| Park et al, (2008)14 | 77 | F | - | CRVO / CME due to HTN | 4mg IVTA | 4.0 | CMV retinitis (PCR) | 2 | LP | Intravenous acyclovir, intravitreous ganciclovir | 4 | HM | |
|
| |||||||||||||
| Sekiryu et al, (2008)15 | 63 | M | NR | BRVO / DME | 4mg IVTA | 7.0 | CMV retinitis (PCR) | 2 | 0.1 | Intravenous acyclovir, Intravenous gancyclovir, valgancyclovir | 1 | 0.6 | |
|
| |||||||||||||
| Ramaiya et al, (2010)16 | 22 | M | - | Idiopathic panuveitis | FA Implant | 13.0 | HSV retinitis (PCR) | 2 | HM | Intravitreal foscarnet, Intravenous acyclovir, Oral valcyclovir | NR | HM | |
|
| |||||||||||||
| Babiuch et al, (2010)17 | 77 | M | NR | Idiopathic iritis | 40mg PST TA | 0.25 | CMV retinitis (serology+PCR) | 2 | 20/40 | Vitrectomy, endolaser, Intravitreal ganciclovir, Ganciclovir implant | NR | NR | |
|
| |||||||||||||
| Chang et al, (2010)18 | 75 | F | - | Post-cataract inflammation | 1.5mg anterior chamber TA | 0.5 | HSV retinitis (serology) | 2 | 20/4C | Intravenous acyclovir, topical prednisolone and atropine, Oral prednisone and oral valacyclovir Vitrectomy, laser retinopexy and gas tamponade | 12 | 20/50 | |
|
| |||||||||||||
| Shah et al, (2010)19 | 62 | M | NR | BRVO / DME | 20mg IVTA x2 | 6.5 | CMV retinitis (PCR) | 2 | 20/400 | Valacyclovir, Vitrectomy, endolaser and silicone oil, Then Valganciclovir | NR | NR | |
| 43 | M | + | CME from IRU, history of CMV retintis and HIV recently started on HAART | 20mg IVTA | 3.3 | CMV retnitis (prior history/clinical appearance) | 2 | 20/40 | Valgancyclovir | NR | NR | ||
| 73 | F | NR | ERM / CME | 20mg IVTA | 25 | VZV retinitis (history of VZV keratouveitis and response to treatment) | 2 | 20/400 | Intravitreal foscarnet and valacyclovir | NR | NR | ||
|
| |||||||||||||
| Toyokawa et al, (2010)20 | 83 | M | - | CNVM / AMD | 20mg PST TA | 3.0 | CMV retinitis (PCR) | 2 | 0.3 | Oral valacylovir, oral prednisone vitrectomy, intravitreal acyclovir | 5 | 0.1 | |
|
| |||||||||||||
| Tugal-Tutkun et al (2010)21 | 30 | M | Behcet's Panuveitis | IVTA dose NR | 3.5 | CMV retinitis (serology+PCR) | 1,2,3 | 20/200 | Intravitreal ganciclovir x 2, intravenous ganciclvir x 5 wks, azathioprine changed to interferon alpha 2a | 8 | 20/60 | ||
|
| |||||||||||||
| Vertes et al, (2010)22 | 78 | F | - | BRVO / CME | 4mg IVTA | 3.0 | CMV retinitis (serology+PCR; | 2 | 20/4C | Intravenous ganciclovir, Oral ganciclovir and acetazolamide, topical dorzolamide/timolol, prednisolone intravitreous ganciclovir, Then vitrectomy, endolaser | 8 | 20/25 | |
|
| |||||||||||||
| Han et al, (2011)23 | 56 | M | - | DME | 4mg IVTA | 5.0 | unspecified viral retinitis (serology+clinical appearance) | 2 | 20/200 | Intravenous acyclovir, oral valacyclovir, oral aspirin and oral/topical steroids, barrier laser, vitrectomy, ERM removal combined with cataract operation | 12 | 20/40 | |
|
| |||||||||||||
| Zaborowski (2013)24 | 56 | F | - | Idiopathic panuveitis / Uveitic CME | 4mg IVTA | 6.0 | CMV retinitis (PCR; | 2 | CF | Azathioprine discontinued, Intravitreal ganciclovir twice weekly for three weeks (2 mg) | 2 | NR | |
|
| |||||||||||||
| Gupta (2013)2 | 70 | F | - | DME | IVTA dose NR | 4.0 | CMV retinitis (PCR) | 2 | CF | Systemic acyclovir, topical steroid therapy, intravitreal foscarnet, valacyclovir, intravitreal foscarnet x 2, ganciclovir implant | 32 | CF | |
| 60 | M | - | DME | IVTA NR | 6.0 | CMV retinitis (PCR) | NR | 20/400 | Intravitreal foscarnet, systemic valganciclovir, gancilcovir implant | NR | 20/300 | ||
| 84 | F | - | BRVO | IVTA NR | 6.0 | CMV retinitis (PCR) | 2 | 20/150 | Intravitreal foscarnet, systemic valaclovir, systemic valganciclovir | 19 | HM | ||
|
| |||||||||||||
| Current Cases | (1) | 66 | M | - | VKH with steroid-induced cataracts and ocular hypertension, IVTA given during cataract surgery | 4mg IVTA & ASCTA | 1.8 | CMV retinitis (PCR) | 2 | 20/200 | Intravitreal ganciclovir, oral valganciclovir, methotrexate and low dose oral prednisone | 2 | 20/70 |
|
| |||||||||||||
| (2) | 37 | F | - | Bilateral idiopathic posterior uveitis complicated by CME / retinal vasculitis | FA Implant | 13.0 | CMV retinitis (PCR) | 1 | 20/80 | Intravitreal foscarnet, Oral valganciclovir | 2 | 20/100 | |
|
| |||||||||||||
| (3) | 63 | M | - | Granulomatous uveitis with CME | 40mg IVTA x 2 | 3.0 | CMV retinitis (PCR) | 2 | 20/60 | Intravenous ganciclovir, Oral prednisone, PPV | 84 | 20/200 | |
|
| |||||||||||||
| (4) | 72 | M | NR | BRVO | 4mg IVTA | 1.3 | CMV retinitis (PCR) | 2 | 20/60 | Intravitreal ganciclovir | 12 | CF | |
|
| |||||||||||||
| (5) | 37 | F | - | Chronic idiopathic iridocyclitis | 10mg AST TA | 2.0 | HSV retinitis (PCR) | 2 | 20/30 | Acyclovir, Intravitreal ganciclovir | 36 | 20/200 | |
|
| |||||||||||||
| Summary | Mean: 62.6 yrs | Male:Femal e: 19:11 | HIV +: 2/22 (9.1%) | RVO: 8/30 (26.7%) | 1.5-4mg: 12/26 (46.2%) | Mean: 4.2 months | CMV retinitis: 23/30 (76.7%) | Zone 1 : 5/29(17.2%) | ≥ 20/40: 6/30(20.0%) | Systemic alone: 10/30 (33.3%) | Mean: 13.7 months | ≥ 20/40: 5/24 (20.8%) | |
| Median: 65.5 yrs | %Male: 63.3 | DME: 6/30 (20.0%) | 8-20 mg: 8/26 (30.8%) | Median: 3.8 months | HSV retinitis: 5/30 (16.7%) | Zone 2: 23/29 (79.3%) | < 20/40 & >20/200: 8/30 26.7%) | IVT alone: 4/30 (13.3%) | Median: 8.0 months | < 20/40 & >20/200: 5/24 (20.8%) | |||
| Range: 22.0-84.0 yrs | Uveitic CME: 4/30 (13.3%) | 40mg: 3/26(11.5%) | Range: 0.25-13.0 months | VZV retinitis: 1/30(3.3%) | Zone 1+2+3: 1/29 (3.5%) | ≤20/200: 16/30(53.3%) | Both systemic and IVT: 16/30 (53.4% | Range: 1 to 84 months | ≤20/200: 14/24 (58.4%) | ||||
| CNVM due to AMD: 3/30 (10.0%) IRU: 2/30 (6.7%) | FA Implant: 3/26 (11.5%) | Unspecified: 1/30(3.3%) | |||||||||||
| Other: 7/30 (23.3%) | Range of Doses: 1.5-40mg | ||||||||||||
Among the 30 total cases, 12 (40.0%) had Type II Diabetes Mellitus and 2(6.7%) had malignancies (Metastatic Ovarian Cancer and Lymphoma)
ASC TA = Anterior Sub-Conjunctival Triamcinolone Acetonide, AMD = Age-related Macular Degeneration, BRVO = Branch Retinal Vein Occlusion, CRVO = Central Retinal Vein Occlusion, CME = Cystoid Macular Edema, CMV = CytoMegaloVirus, CNVM = Choroidal NeoVascular Membrane, DME= diabetic
Methods
Cases were solicited by email from the members of the American Uveitis Society and the Francis I. Proctor Foundation. A retrospective chart review was performed on 5 previously undescribed patients who developed viral retinitis following intraocular or periocular corticosteroid administration. Case reports for these 5 patients are presented. A MEDLINE search using the terms “retinitis AND corticosteroid” was conducted to identify relevant literature published after 1950. Additional studies were identified by reviewing the references in the relevant articles selected from the aforementioned search. The demographic and clinical features of both the newly described and previously reported cases are summarized and discussed.
Results: Previously Unreported Case Descriptions (Table 1 and Figure 1)
Figure 1.
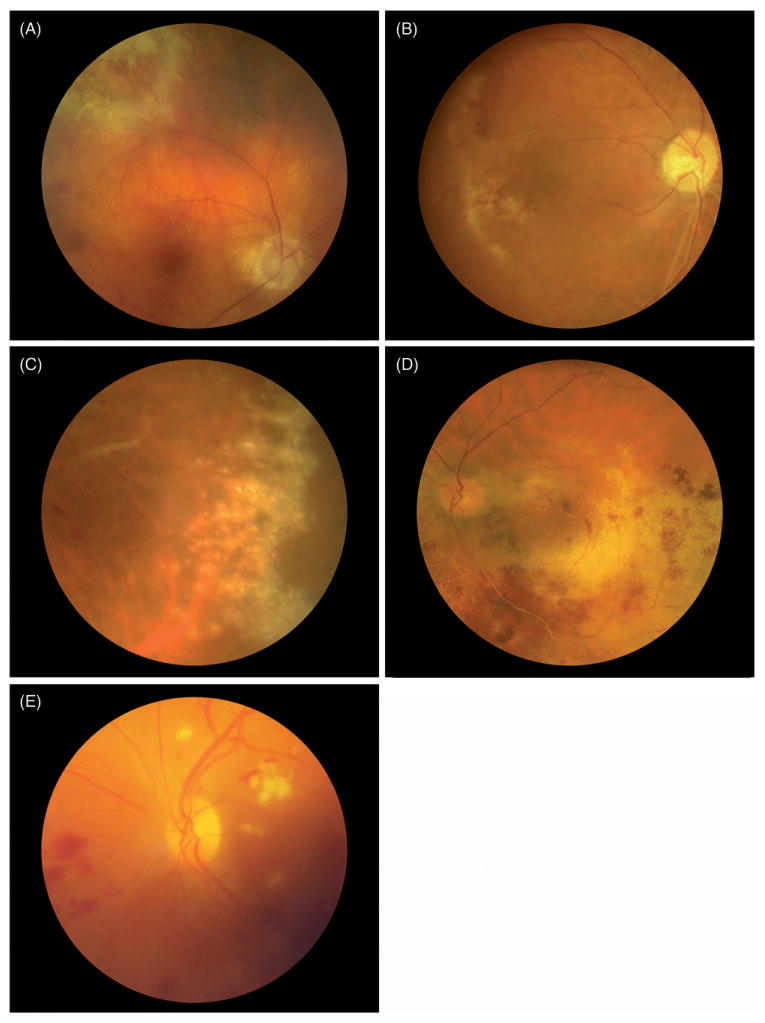
Color fundus photographs of the 5 previously unreported cases of viral retinitis following intraocular or periocular corticosteroid injection. Cases 1–4 were cytomegalovirus (CMV) retinitis. Case 5 was diagnosed clinically as acute retinal necrosis, although the causative virus was not identified. The retinitis resolved in all cases in response to antiviral therapy.
Case 1
A 65-year-old Asian man with Vogt-Koyanagi-Harada (VKH) disease on infliximab, methotrexate, and prednisone received both an anterior subcon-junctival and an intravitreal triamcinolone acetonide injection (4 mg) following an uneventful cataract surgery on his right eye. Seven weeks following surgery he presented with decreased vision to 20/200 in the operated eye. Posterior segment examination revealed an area of active retinitis. An aqueous chamber polymerase chain reaction (PCR) sample was positive for CMV deoxynucleic acid (DNA). The patient's infliximab was discontinued and he was treated with both intravitreal ganciclovir and oral valganciclovir. Over the following 2 months the patient's retinitis resolved and his vision improved to 20/40. His uveitis and retinitis remained inactive on methotrexate and low-dose oral prednisone, and off antiviral agents. Vision at last visit more than 1 year following the development of CMV retinitis was stable at 20/40.
Case 2
A 37-year-old Caucasian woman with a history of idiopathic bilateral posterior uveitis and persistent CME treated with infliximab and mycophenolate mofetil underwent fluocinolone acetonide implant placements in both eyes. Follow-up examinations were performed every 2–4 months following placement. Approximately 12 months after insertion, the patient presented with decreased vision from 20/40 to 20/80 in the right eye and necrotizing retinitis was seen in the macula. Aqueous PCR was positive for CMV DNA. Infliximab and mycophenolate mofetil were discontinued and the patient was treated with both intravitreal foscarnet and oral valganciclovir. Over the following 2 months the patient's retinitis resolved. Although the patient's CMV retinitis remained inactive, she subsequently developed hypotony, and visual acuity in the affected eye at last visit was 20/80.
Case 3
A 63-year-old Caucasian man with unilateral granulomatous panuveitis and persistent CME in the left eye presented with vision of 10/125 and was treated with two consecutive posterior sub-Tenon injections of 40 mg triamcinolone acetonide. Vision initially improved to 20/25, but 2 months later decreased to 20/40. Examination of the posterior segment revealed vitritis, sheathing of the retinal arteries, and numerous punctate, yellowish retinal infiltrates. Aqueous PCR was positive for CMV DNA. The patient was treated with intravenous ganciclovir and oral prednisone, but the retinal lesions persisted and pars plana vitrectomy (PPV) was performed. Retinal detachment developed 10 months after the vitrectomy and vision at the patient's last visit 7 years later was 20/200.
Case 4
A 72-year-old Caucasian man received a 4-mg intravitreal injection of triamcinolone acetonide for a branch retinal vein occlusion (BRVO) in the left eye. Approximately 1 month later, the patient returned with decreased vision in the left eye to 20/60. Fundus examination revealed necrotizing retinitis in the macula and midperiphery. A vitreous PCR was positive for CMV DNA. The patient was treated with four weekly intravitreal injections of ganciclovir and while the retinitis responded to therapy, the vision remained limited to counting fingers in the affected eye.
Case 5
A 37-year-old Caucasian woman with chronic idiopathic iridocyclitis in her left eye received an anterior sub-Tenon injection of triamcinolone acetonide (10 mg) for refractory inflammation. Two months later she developed active retinitis in the left eye. A presumed diagnosis of acute retinal necrosis (ARN) was made, and she was placed on 800mg acyclovir 5 times daily and given an intravitreal injection of ganciclovir. The retinitis became inactive with therapy, but there was marked retinal scarring with epiretinal membrane formation and she subsequently developed a nasal BRVO a month later with decreased vision to 20/200. Her visual acuity 3 years later remained stable at 20/200 in the affected eye.
Discussion
We provided clinical descriptions of 5 previously unpublished cases of viral retinitis following local corticosteroid administration. The mean age of patients was 55 years (median: 63 years, range 37–72 years). Three out of 5 patients (60.0%) were male. Indications for the corticosteroid included cataract surgery in a patient with uveitis (1), uveitic CME (2), BRVO (1), and refractory uveitic inflammation (1). There was one 4-mg intravitreal triamcinolone acetonide (IVTA) injection, 1 fluocinolone acetonide implant, 2 cases of sub-Tenon TA injections (1 anterior and 1 posterior), and 1 case that received both a 4-mg IVTA injection and a 4-mg anterior subconjunctival TA injection. The mean time from corticosteroid dosing to onset of viral retinitis was 4.2 months (median: 2 months, range: 1.3–13 month). Four cases were CMV retinitis comfirmed by aqueous PCR, and 1 case was diagnosed clinically as ARN, but did not have confirmatory PCR. At last follow-up visit (range: 2–84 months), none of the 5 cases had visual outcome better than or equal to 20/40, and 3 of the cases had visual acuity at last visit worse than or equal to 20/200. The retinitis involved the macula in 2 cases (cases 2 and 4), and the visual acuity at last visit was complicated by hypotony (20/80) and BRAO (CF), respectively. Case 1 did not involve the macula and had an outcome visual acuity at last visit of 20/70. Case 3 was complicated by a subsequent retinal detachment visual acuity at last visit of 20/200. Finally, case 5 was complicated by a nasal BRVO with marked retinal scarring and epiretinal membrane formation, with a visual acuity at last visit of 20/200. Three out of the 5 cases (60.0%) were treated with intravitreal and oral antivirals, 1 (20.0%) with intravenous antivirals alone, and 1 (20.0%) with intravitreal antivirals alone.
Twenty previously published reports, including 25 patients with viral retinitis following local corticosteroid administration, were identified through our review of the literature.7–25 Taken together with our case series, we identified a total of 30 cases (Table 1). The mean age of all 30 patients was 62.6 years (median 65.5 years; range: 22-84 years) with a slight male predominance (63.3%). Two patients had a diagnosis of HIV (2/22, 9.1%), and type 2 diabetes mellitus was present in 12/30 patients (40.0%). Of the 12 diabetic patients, 7 (58.3%) were treated with local corticosteroids for reasons other than diabetic macular edema. Overall, the most common indication for corticosteroid use was retinal vein occlusion (8/30, 26.7%), followed by persistent inflammation (5/30, 16.7%), diabetic macular edema (6/30, 20.0%), uveitic CME (4/30, 13.3%), choroidal neovascularization due to age-related macular degeneration (3/30, 10.0%), and immune recovery uveitis (2/30 6.7%). Both cases of immune recovery uveitis had a history of CMV retinitis that reactivated. Cytomegalovirus was the most common etiologic agent (23/30, 76.7%), followed by herpes simplex virus (5/30, 16.7%) and varicella zoster virus (1/30, 3.3%). The viral cause was not identified in 1 case (1/30, 3.3%). Doses of corticosteroid used ranged from 1.5 to 40mg, with the majority of cases involving doses between 1.5 and 20mg (20/26, 77.0.%). Three of the cases involved the use of fluocinolone acetonide implant. The mean time from corticosteroid administration to diagnosis of retinitis was 3.9 months for intravitreal injections (median 4.0 months, range 1.3–7 months). For posterior sub-Tenon injections, the mean time was 1.8 months (median: 2 months, range 0.25–3 months), 2 months for the 1 case following anterior sub-Tenon injection, 0.5 months for the 1 case following anterior chamber TA, and 10.3 months for the fluocinolone acetonide implants (Retisert) (median: 13 months, range 5–13 months). A majority of the cases did not involve the macula (23/29, 79.3%). Visual acuity at the time of diagnosis varied, with 6/30 (20.0%) patients having vision better than or equal to 20/40 while 16/ 30 (53.3%) had vision worse than or equal to 20/200. Although half of patients were treated with both systemic and intravitreal antivirals (14/30, 46.6%), others were treated either with systemic antivirals alone (10/30, 33.3%), or with intravitreal antivirals alone (4/30, 13.3%). Mean follow-up time was 13.7 months (median: 8.0 months, range: 1–84 months). Visual acuity at last visit tended to be poor, with half (58.4%) of all cases having a best-corrected vision worse than or equal to 20/200. Only 5/24 (20.8%) cases had a visual acuity at last visit better than or equal to 20/40.
Viral retinitis occurred in two-thirds of eyes following one or more intravitreal corticosteroid injections (21/30, 70.0%). Eighteen out of 21 eyes (85.7%) had one previous injection of intravitreal corticosteroid, 2/21 (9.5%) had two previous injections, and 1/21 (4.8%) had three previous injections. The mean time from last intravitreal injection to identification of viral retinitis was 3.9 months (median 4.0 months, range 0.75–7 months). In no instances did the authors describe removal of intravitreal corticosteroid or the corticosteroid releasing implant following the development of retinitis.
Nine out of 30 patients (30.0%) were administered intravitreal or periocular corticosteroids as part of the management of their uveitis. The mean age was 50.3 years (median 56 years; range 22–77 years) and 2/3 were male. Six out of 9 (66.7%) patients had idiopathic uveitis, 2/9 (22.2%) had Behçet disease, and 1/9 (11.1%) had Vogt-Koyanagi-Harada disease. A third of the cases involved intravitreal corticosteroids injections (3/9, 33.3%), another third involved fluocinolone acetonide (Retisert) implants, 2 involved posterior sub-Tenon TA injections, and 1 case involved an anterior sub-Tenon injection. After the diagnosis of viral retinitis, there were variable modifications made to the patients' corticosteroid and/or non-corticosteroid immunomodulating therapy. In one case, azathioprine was discontinued and 10mg of oral prednisone was continued.24 In a second case, infliximab was discontinued and low-dose oral prednisone and methotrexate were continued. In a third case, both infliximab and mycophenolate mofetil were discontinued. In a fourth case, azathioprine was discontinued and high-dose oral prednisone and interferon-alpha were started.21 In the remaining 5 cases no changes were made to their systemic therapy except for the addition of an antiviral agent.The contribution of concurrent systemic corticosteroids and/or non-corticosteroid immunosuppressive agents, including TNF inhibitors, to the development of viral retinitis remains unclear, as viral infections have been reported to occur in patient on such therapies.26,27 Overall, 5 out of 9 patients (55.6%) were treated with both antivirals and systemic antivirals, 2/9 (22.2%) were treated with both intravitreal antivirals and an intraocular ganciclovir implant, and 1 each was treated with either an intravenous or intravitreal antiviral alone. In all 3 patients who received fluocinolone acetonide (Retisert) implants, none of the implants were removed after the development of retinitis. The mean time from injection/ implantation of corticosteroid to development of retinitis was 5.3 months (median 3.5 months, range 0.25–13 months).
Of the 23 patients who had reported CMV retinitis following pericoular/intravitreal ocular corticosteroid injections, all but 2 had no prior history of CMV retinitis. The 2 who did have prior CMV retinitis were both HIV positive and were treated with local corticosteroid injections to treat immune recovery uveitis with or without cystoid macular edema.19,28 During their initial CMV retinitis episode, both patients were placed on highly active antiretroviral therapy (HAART) and antivirals, with their CD4 counts improving to 126 and 324 cells/mm3, respectively. They then developed IRU and were given local corticosteroid injections (a 40-mg sub-Tenon TA and a 20-mg IVTA, respectively). The maintenance dose of ganciclovir was continued for the first patient and discontinued in the second as there were no active areas of CMV retinitis. Two and 3 months later, respectively, they presented with reactivation of their CMV retinitis. That they reactivated despite the fact that their CD4 counts were elevated on HAART demonstrated that CMV retinitis can reactivate following local corticosteroid administration—even in the setting of relative immune reconstitution.
Ten patients (10/27, 37.0%) who developed CMV retinitis following corticosteroid injection did so in the setting of type 2 diabetes mellitus. The mean age of diabetic patients was 69.3 years (median 72.0 years, range 54–83 years), 9 out of 10 (90.0%) patients were male, and 4 out of 10 (40.0%) had corticosteroid injections for diabetic macular edema. In 9 out of 10 (90.0%) patients with diabetes, the viral retinitis was identified as CMV retinitis, and in 1 case (10.0%) the causative agent could not be identified. Radwan and colleagues recently reported 2 cases and reviewed the literature on CMV retinitis in immunocompetent patients. Of note, 6 of 12 previously reported patients (50%) had diabetes mellitus. 28 Although the relationship between diabetes mellitus and the development of CMV retinitis is difficult to discern, both Shah and associates and Radwan and colleagues suggested that the presence of diabetic vasculopathy may have facilitated entry of CMV into the retina and thereby promoted the development of retinitis.19,28 Others have reported that circulating CMV levels tend to be higher in patients with diabetes mellitus.29
In summary, although uncommon, viral retinitis may develop or reactivate following periocular or intravitreal corticosteroid administration. In three-quarters of affected eyes, CMV was identified as the causative agent, and in two-thirds of cases corticosteroid was given as an intravitreal injection. The average time between the injection of corticosteroid and the development of retinitis was between 3 and 4 months. Systemic and/or intravitreal antiviral agents were effective at controlling the retinitis in all patients, although vision at the last visit was worse than 20/200 in 14 out of 24 eyes (58.4%) due either to the location of the retinitis and/or the development of rhegmatogenous retinal detachment (4/24, 16.7%). Approximately 40% (12/30) of patients had type 2 diabetes mellitus, a co-morbidity previously noted to affect up to 50% of otherwise immunocompetent patients who developed CMVretinitis.28 Several of the patients with uveitis who developed retinitis were also receiving systemic immunosuppressive therapy. Hence, definitive proof of a causal role for periocular or intraocular corticosteroids in the development of viral retinitis has yet to be provided.
Acknowledgments
Supported in part by the Pacific Vision Foundation and the Retina Foundation.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013 May;97(5):545–52. doi: 10.1136/bjophthalmol-2012-301983. [DOI] [PubMed] [Google Scholar]
- 2.Kanoff J, Sobrin L. New diagnosis and treatment paradigms in acute retinal necrosis. Int Ophthalmol Clin. 2011;51:25–31. doi: 10.1097/IIO.0b013e31822d6864. [DOI] [PubMed] [Google Scholar]
- 3.Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome–bench to bedside: LXVII Edward Jackson Memorial Lecture. American J Ophthalmol. 2011;151:198–216. e1. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorne JE, Holbrook JT, Jabs DA, et al. Effect of cytomegalovirus retinitis on the risk of visual acuity loss among patients with AIDS. Ophthalmology. 2007;114:591–598. doi: 10.1016/j.ophtha.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham ET., Jr Cytomegalovirus: ophthalmic perspectives on a pervasive pathogen. Expert Rev Ophthalmol. 2011;6:489–491. [Google Scholar]
- 6.Dalessandro L, Bottaro E. Reactivation of CMV retinitis after treatment with subtenon corticosteroids for immune recovery uveitis in a patient with AIDS. Scand J Infect Dis. 2002;34:780–782. doi: 10.1080/00365540260348644. [DOI] [PubMed] [Google Scholar]
- 7.Saidel M, Berreen J, Margolis T. Cytomegalovirus retinitis after intravitreous triamcinolone in an immunocompetent patient. Am J Ophthalmol. 2005;140:1141–1143. doi: 10.1016/j.ajo.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 8.Aggermann T, Stolba U, Brunner S, Binder S. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica. 2006;220:131–133. doi: 10.1159/000090579. [DOI] [PubMed] [Google Scholar]
- 9.Toh T, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Clin Exp Ophthalmol. 2006;34:380–382. doi: 10.1111/j.1442-9071.2006.01229.x. [DOI] [PubMed] [Google Scholar]
- 10.Delyfer MN, Rougier MB, Hubschman JP, et al. Cytomegalovirus retinitis following intravitreal injection of triamcinolone: report of two cases. Acta Ophthalmol Scand. 2007;85:681–683. doi: 10.1111/j.1600-0420.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa M. Cytomeglaovirus Retinitis after intravitreal triamcinolone acetonide treatment of a vitrectomized eye in an immunocompetent patient. Retin Cases Brief Rep. 2007;1(4):205–7. doi: 10.1097/ICB.0b013e31804d1e3f. [DOI] [PubMed] [Google Scholar]
- 12.Hsu J. Cytomegalovirus retinitis after treatment with intravitreal triamcinolone acetonide in an immunocompetent patient. Retinal Case Brief Rep. 2007;1:208–210. doi: 10.1097/ICB.0b013e31802ea61c. [DOI] [PubMed] [Google Scholar]
- 13.Ufret-Vincenty RL, Singh RP, Kaiser PK. Cytomegalovirus retinitis after fluocinolone acetonide (Retisert) implant. Am J Ophthalmol. 2007;143:334–335. doi: 10.1016/j.ajo.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Park YS, Byeon SH. Cytomegalovirus retinitis after intravitreous triamcinolone injection in a patient with central retinal vein occlusion. Korean J Ophthalmol. 2008;22:143–144. doi: 10.3341/kjo.2008.22.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekiryu T, Iida T, Kaneko H, Saito M. Cytomegalovirus retinitis after intravitreal triamcinolone acetonide in an immunocompetent patient. Jpn J Ophthalmol. 2008;52:414–416. doi: 10.1007/s10384-008-0576-0. [DOI] [PubMed] [Google Scholar]
- 16.Ramaiya KJ, Rao PK. Herpetic necrotizing retinitis following flucinolone acetonide intravitreal implant. Ocul Immunol Inflamm. 2011;19:72–74. doi: 10.3109/09273948.2010.520404. [DOI] [PubMed] [Google Scholar]
- 17.Babiuch AS, Ravage ZB, Merrill PT. Cytomegalovirus acute retinal necrosis in an immunocompetent patient after sub-Tenon triamcinolone injection. Retinal Cases Brief Rep. 2010;4:364–365. doi: 10.1097/ICB.0b013e3181b5ef2a. [DOI] [PubMed] [Google Scholar]
- 18.Chang S, Weissgold DJ, Singer JA, Sobrin L. Acute Retinal Necrosis following Intraocular Triamcinolone Acetonide Injection. Retin Cases Brief Rep. 2010;4(4):306–308. doi: 10.1097/ICB.0b013e3181b5ee58. Fall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149:433–440. e1. doi: 10.1016/j.ajo.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyokawa N, Kimura H, Kuroda S. Cytomegalovirus retinitis after subtenon TA and intravitreal injection of anti-vascular endothelial growth factor in an immunocompetent patient with age-related macular degeneration and diabetes mellitus. Jpn J Ophthalmol. 2010;54:166–168. doi: 10.1007/s10384-009-0784-2. [DOI] [PubMed] [Google Scholar]
- 21.Tugal-Tutkun I, Araz B, Cagatay A. CMV retinitis after intravitreal triamcinolone acetonide injection in a patient with Behçet's uveitis. Int Ophthalmol. 2010;30:591–593. doi: 10.1007/s10792-009-9332-9. [DOI] [PubMed] [Google Scholar]
- 22.Vertes D, Snyers B, De Potter P. Cytomegalovirus retinitis after low-dose intravitreous triamcinolone acetonide in an immunocompetent patient: a warning for the widespread use of intravitreous corticosteroids. Int Ophthalmol. 2010;30:595–597. doi: 10.1007/s10792-010-9404-x. [DOI] [PubMed] [Google Scholar]
- 23.Han JM, Ahn J, Park KH, Woo SJ. Presumed necrotizing viral retinitis after intravitreal triamcinolone injection: case report. Korean J Ophthalmol. 2011;25:451–454. doi: 10.3341/kjo.2011.25.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaborowski AG. Cytomegalovirus retinitis following intravitreal triamcinolone acetonide in a patient with chronic uveitis on systemic immunosuppression. Ocul Immunol Inflamm. 2013 Apr;21(2):148–9. doi: 10.3109/09273948.2012.737889. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Vemulakonda GA, Suhler EB, et al. Cytomegalovirus retinitis in the absence of AIDS. Can J Ophthalm. 2013;48:126–129. doi: 10.1016/j.jcjo.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Shale MJ, Seow CH, Coffin CS, et al. Review article: chronic viral infection in the anti-tumour necrosis factor therapy era in inflammatory bowel disease. Aliment Pharmacol Ther. 2009;31:20–34. doi: 10.1111/j.1365-2036.2009.04112.x. [DOI] [PubMed] [Google Scholar]
- 27.Che H, Lukas C, Morel J, Combe B. Risk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis: systemic review and meta-analysis. Joint Bone Spine. 2013 Aug 7; doi: 10.1016/j.jbspin.2013.07.009. pii: S1297-319X(13)00194-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Dalessandro L, Bottaro E. Reactivation of CMV retinitis after treatment with subtenon corticosteroids for immune recovery uveitis in a patient with AIDS. Scand J Infect Dis. 2002;34:780–782. doi: 10.1080/00365540260348644. [DOI] [PubMed] [Google Scholar]
- 29.Liang H, Liang Y, Chen H, et al. Role of cytomegalovirus infection in the pathogenesis of type 2 diabetes mellitus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:351–353. [PubMed] [Google Scholar]


