Abstract
In this paper we present two new assays of rat motor behavior which can be used to assess function linked to postural stability in each forelimb independently. Postural instability is a major deficit in Parkinson's disease that is resistant to levodopa therapy and contributes to the risk of falling. We applied both tests, one forelimb at a time, to normal rats as well as rats extensively depleted of dopamine by unilateral infusion of 6-hydroxydopamine (6-OHDA, given in the medial forebrain bundle) to produce a hemi-parkinsonian syndrome. The 6-OHDA rats showed severe postural instability in the impaired forelimb, but unexpectedly showed enhanced function in the non-impaired forelimb. The data suggest that the intact hemisphere may undergo rapid reorganization subsequent to unilateral dopamine depletion, which allows for compensatory function of the “intact” limb. Measurements of amphetamine-induced striatal c-fos expression, as well as behavior results gathered when animals were under the influence of apomorphine or haloperidol, indicate that this potential reorganization may require non-dopaminergic neural plasticity. The relevance of these findings for unilateral rat models of neurological disease is discussed.
Keywords: Parkinson's disease, 6-OHDA, cylinder test, adjusting-steps test, unilateral, postural instability
Introduction
In Parkinson's disease (PD), postural instability is very common and contributes to the danger of frequent falls (Jessop et al., 2006). Unfortunately, the impaired ability to make motor adjustments in response to involuntary changes in center of gravity is quite resistant to levodopa therapy (Horak et al., 1996; Jessop et al., 2006). Methods for detecting postural instability are typically included in standard clinical exams. For example, in the “pull” or “push” tests the patient stands in front of the neurologist and is gently pushed or pulled by the shoulders forward or backward, shifting their center of gravity. The capacity to make catch-up steps of appropriate size to maintain center of gravity is assessed. In severe cases the feet seem almost glued to the ground and the patient needs to be caught by the neurologist to prevent falling. A component of the deficit is that the patient responds poorly even when center of gravity is only slowly displaced over a considerable distance.
However, despite the importance of this symptom in PD, there has been no convincing animal model of postural instability. Postural instability may underlie many of the behavioral deficits observed in common animal models of PD. In the hemi-Parkinson rat or mouse, there is a decreased reliance on the impaired forelimb for movements involving a response to weight shift. The animals preferentially initiate movement with the non-impaired forelimb, particularly for lateral movements during vertical exploration of surfaces (Schallert et al., 1997). By lifting the animal's hindlimbs and one forelimb off the ground, each forelimb can be examined separately for impairments. In response to a rapidly imposed shift of weight on a smooth surface, parkinsonian rats often brace or drag the impaired forelimb instead of making adjusting steps, but step readily with the non-impaired forelimb to maintain center of gravity (Olsson et al., 1995; Schallert et al., 1979, 1992). In this report we show that on a rough surface, which prevents the bracing reaction, moving the animal forward induces stepping with either the impaired or the unimpaired forelimb to regain center of gravity. However, in the impaired forelimb the distance moved per step is greatly exaggerated, indicating a deficit in response to imposed weight shift, as in PD. In contrast, the distance moved per step by the non-impaired forelimb was unexpectedly shorter than that of control animals, indicating adaptive enhanced reactivity and suggesting that compensatory neural plasticity in the intact hemisphere may have occurred.
Materials and Methods
Animals
Male Sprague-Dawley rats (n=14; 8 lesioned animals and 6 shams) obtained from an in-house colony were used for the experiments. They were kept in pairs in polycarbonate cages with sawdust bedding, on a 12:12 light:dark cycle with food and water available ad libitum. The animals were aged 3-4 months at the time of surgery.
Surgeries
Animals were fasted overnight. Prior to surgery they were pre-tested on the limb-use asymmetry test (see below) and assigned to receive a unilateral lesion in the hemisphere opposite the limb they preferred to use in that task. On the day of surgery they were pretreated with 0.1 mg/kg atropine sulfate (s.c.) to dry respiratory secretions and 0.05 mg/kg buprenorphine (s.c.) for trans-operative analgesia, followed 10 min later by anesthesia induction with 40 mg/kg (i.p.) pentobarbital sodium, plus treatment with 20 mg/kg (i.p.) desipramine to reduce uptake of 6-OHDA by norepinephrine terminals. When necessary, anesthesia was boosted with injections of 80 mg/kg chloral hydrate (i.p.). Body temperature was measured rectally throughout the surgery and maintained between 36.5 and 38.5°C by use of a heating pad. Following anesthesia the rats were placed in a stereotaxic apparatus and their skull exposed and leveled in the dorsal-ventral plane. A small burr hole was drilled through the skull at −4.3 mm AP and ±1.5 mm ML relative to bregma. The needle of a 2 μl Hamilton gastight syringe was slowly lowered through the center of the burr hole to a depth of 8.0 mm below the dural surface, to target the medial forebrain bundle. Via this needle, a solution of 10 ug (free base weight) of 6-OHDA hydrobromide dissolved in 2 μl of artificial cerebrospinal fluid containing 0.05% (w/v) ascorbic acid was infused at a rate of 0.2 ul/min for 10 min. At the end of the 10 min infusion, the needle was left in place for an additional 2 min before being slowly retracted. The burr hole was sealed with bone wax and the scalp incision sutured, and the animals were then allowed to recover in a humidified incubator before being returned to their home cages. Sham-operated animals received the same treatment except that no solution was infused via the Hamilton needle, and the needle was retracted immediately following its lowering into the medial forebrain bundle.
Measurements of striatal c-fos expression
Following all behavioral testing (2-3 weeks post-lesion), animals were treated with 3 mg/kg (i.p.) d-amphetamine sulfate (Sigma) to induce striatal c-fos expression in dopamine-receptive neurons (Graybiel et al., 1990). Two hours later the animals were anesthetized with 1.25 g/kg urethane and the chest cavity was exposed. The animals were transcardially perfused with 200 ml of heparinized (20 U/ml) 0.1 M phosphate buffer, pH 7.4, followed by 400 ml of 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer as a fixative. The brains were removed and postfixed in the same fixative for 48 hours. They were then sliced into serial 100 μm coronal sections using a vibratome. Every third section through the striatum was collected and stained immunocytochemically for c-fos as follows. Endogenous peroxidase was first inactivated by treatment with 0.3% hydrogen peroxide in 10 mM phosphate-buffered saline (PBS) for 30 min. The slices were then rinsed and transferred to a blocking solution consisting of 2% normal goat serum, 0.1% bovine serum albumin, and 0.4% Triton-X 100 in PBS, in which they were agitated for 90 min. They were then incubated in a primary antibody solution consisting of a 1:5,000 dilution of c-fos rabbit polyclonal antibody (catalog no. SC-52, from Santa Cruz Biotechnology, Santa Cruz, CA) diluted in the aforementioned blocking solution for 48 hours at 4°C. Slices were next rinsed and incubated for 90 min in a secondary antibody solution of 1:200 goat anti-rabbit IgG (Sigma) in PBS containing 2% normal goat serum. Then, after another PBS rinse, slices were incubated for 90 min in a solution of 12 drops reagent A and 12 drops reagent B from a Vectastain Elite ABC kit, model PK-6100 (Vector Labs, Burlingame, CA), dissolved in 60 ml of PBS. Slices were then rinsed and developed with a solution of 0.7% (w/v) nickel ammonium sulfate and 0.05% (w/v) diaminobenzidine dissolved in Tris-buffered saline (TBS) containing 0.0013% freshly-added hydrogen peroxide. The reaction was monitored and stopped by repeated rinsing in TBS when c-fos-positive nuclei were easily visible under a microscope. Slices were then mounted on gelatin-coated slides, dehydrated, and coverslipped.
C-fos activity in the mounted slices was measured by automated counting of the darkly-stained c-fos-positive nuclei using NIH ImageJ software (http://rsb.info.nih.gov/ij/), version 1.33. Magnified digital images of a 1.9 mm-diameter circular region of the central striatum were captured using a microscope with 10X objective and Nikon Coolpix 4500 digital camera. Images were converted to 8-bit and thresholded with ImageJ such that the dark c-fos-positive nuclei were separated from the lighter background. Thresholded nuclei were counted using ImageJ's “Analyze Particles” feature and the total number of nuclei counted within the image frame for each striatum (left and right) in each slice was recorded. This counting was performed on every other c-fos-stained slice (i.e., on a 100 μm slice every 600 um), beginning with the most anterior slice containing a joined corpus callosum and working posterior until nuclei had been counted from four slices. Results of this count are expressed as the total number of nuclei counted within a given hemisphere from the four slices.
Behavioral testing
Behavioral testing was performed by experienced testers blind to the treatment condition during the dark portion of the light cycle. Methods for each behavioral test used are detailed below. All tests (except for amphetamine-induced rotation) were performed twice before the lesioning surgery as a baseline. Rats were also tested in the postural instability test at 1, 2, 3, 4, 7, 10, and 14 days post-lesion; in the forelimb placing and limb- use asymmetry (“cylinder”) tests on day 14; and in the rotation test (with sacrifice immediately afterwards) on approximately day 18 post-lesion. Performance in the PIT test under the influence of either haloperidol or apomorphine was assessed on the 8th and 9th post-operative days, with half of each experimental group receiving haloperidol on day 8 and apomorphine on day 9, and the other half vice-versa.
Postural instability test (PIT)
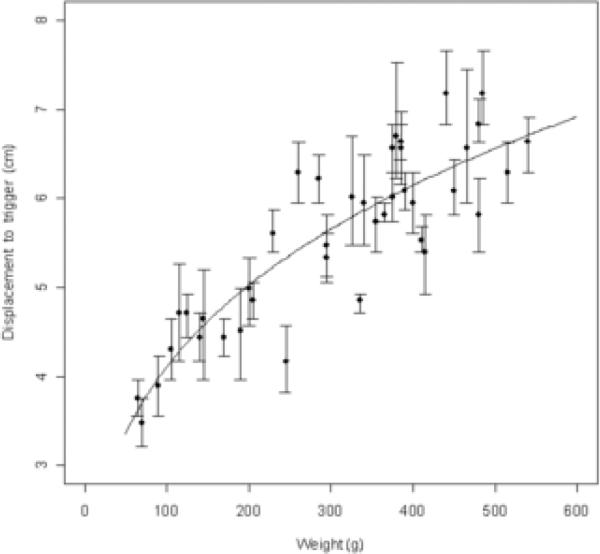
Rats were held almost vertically upside-down (in a “wheelbarrow”-like position) over a sandpaper-covered surface alongside a ruler (see Figure 1), by a tester who was blind to the experimental condition of the rats. This rough surface material induced stepping, rather than dragging or bracing, in response to imposed weight shifts. Viewed from above, the tip of the rat's nose was aligned with the zero line of the ruler, and one forelimb was gently restrained against the animal's torso by the experimenter while the animal was moved forward over the single planted forelimb until making a “catch-up” step to regain its center of gravity. The new position of the nose tip indicated the displacement of the body needed to trigger a catch-up step in the unrestrained supporting forelimb. We examined each forelimb independently while slowly shifting the center of gravity during weight support, in hemi-parkinson and normal rats, and quantified the size of the adjusting response used to regain center of gravity. We performed three trials on each forelimb on a given day of testing. This is a new test that is a refinement of an earlier test variously referred to as the stepping test, adjusting-steps test, or bracing test (Schallert et al., 1979, 1992; Olsson et al., 1995; Schallert and Tillerson, 2000; Woodlee et al., 2004) which, in contrast to the present test, measured number of steps taken across a set distance of displacement (in 6-OHDA rats these tests showed that the number of contralateral forelimb steps were reduced compared to the number of ipsilateral steps, due to bracing reactions on a smooth surface). Earlier pilot work with the PIT test in our lab and others (J. Mithyantha and P. A. Garris, unpublished data) also measured subsequent multiple steps (i.e., continuing to move the rat forward after the first catch-up step until an additional step or two had been made), as well as sideways stepping (measuring lateral displacement required in either direction to trigger a step), but found that these measures were not more sensitive or qualitatively different in dopamine depleted rats than the easier-to-perform version of the test described above. In testing a large group of intact rats we also found the results of this test to be dependent upon body weight, size, and/or age, as presented in the results (see Figure 2), as expected based on the physics of maintaining center of gravity.
Figure 1.

Performing the postural instability test (PIT). Left: a rat at the zero-line before being moved forward. Center: a rat having made a catch-up step with the right limb after 9.5 cm of displacement. Right: a sideways view showing the angle at which the rat is held. In this case the left forelimb is being restrained against the rat's torso by the experimenter.
Figure 2.
Dependence of PIT test results on animal weight. The displacement needed to trigger a catch-up step in a group of 40 intact male Sprague-Dawley rats of various weights is shown. Larger animals require greater displacement. Points are the individual animals’ means (averaged across six trials—three on each of the two forelimbs), and error bars represent bootstrapped 95% confidence intervals for retesting within a given animal. Curve formula is Displacement = 1.092(Weight0.288); R2 = 0.787, p < .001.
Limb-use asymmetry (“cylinder”) test
In the limb-use asymmetry test (Schallert et al., 2000), rats are placed in a clear acrylic cylinder (30 cm tall by 20 cm diameter). The cylinder is high enough to prevent rats from jumping out, and wide enough to allow a small (1 cm) gap between the base of the tail and the cylinder wall when the rat is on all fours. Rats placed in the cylinder engage in exploratory behavior in which they rear and contact the wall of the cylinder with their forepaws. The number of wall contacts made using the ipsilateral (unimpaired), the contralateral (impaired), and both (simultaneously) limbs is recorded, and an asymmetry score calculated as the number of “ipsi” observations plus 1/2 the number of “both” observations, divided by the total number of observations (ipsi plus contra plus both). In this overall asymmetry percentage score, 50% indicates an animal that explores symmetrically with both limbs, higher scores (>50%) indicate a greater reliance on the ipsilateral limb, and lower scores (<50%) indicate a greater reliance on the contralateral limb (see Schallert and Woodlee, 2005 for more details and tips on performing the test). On each day of testing we recorded 20 limb uses; the time required to generate this number of observations varied from animal to animal.
In addition to this standard version of the test, we also measured “serial stepping” behavior in each forelimb as an additional measure of adaptation in the “intact” limb. Whenever the animal placed one forelimb independently onto the inner wall of the cylinder, it would sometimes subsequently proceed to make several rapid lateral weight-shifting steps with the same limb, without using the other limb. As can be seen in the results, we observed this behavior to be relatively rare in intact rats but common in the ipsilateral forelimb of lesioned animals. Thus, “ipsi-step” or “contra-step” was recorded for any forelimb placement made in this way following the initial independent forelimb use, as long as the chain of lateral steps was continuous and not broken by long pauses or the animal pushing back to a standing rear in which no forelimbs were in contact with the cylinder wall. In calculating the asymmetry percentage score (as described above), these “step” behaviors were treated as ordinary ipsi- or contra-limb use events. However, in the results these behaviors are also analyzed independently as percentage “step” behaviors out of the total number of independent forelimb uses for each forelimb.
Vibrissae-evoked forelimb placing test
As described elsewhere (Barth and Stanfield, 1994; Woodlee et al., 2005), rats were held aloft and the left or right vibrissae were brushed against the edge of a tabletop to trigger a forelimb placing response in the forelimb ipsilateral to the stimulated vibrissae. Normal animals readily place their forelimb on the table's surface in response to vibrissae stimulation, but this response may be lost following certain types of lesions (Woodlee et al., 2005). The test is scored as percent successful placing out of ten trials.
Behavioral testing with dopaminergic drugs
On the 8th and 9th days post-lesion, rats were tested 15 min following an injection of either the dopamine antagonist haloperidol (Sigma; 3 mg/kg i.p., dissolved in 0.3% w/v tartaric acid) or agonist (R)-apomorphine (MP Biomedicals; 0.5 mg/kg s.c. freshly dissolved in saline and injected immediately), to test the contributions of the dopamine system to the effects seen in the PIT test.
Amphetamine-induced rotation
The number of turns clockwise and counter-clockwise that a rat made in a large circular bowl were counted for 5 min, beginning 20 min after an i.p. injection of 3 mg/kg d-amphetamine sulfate (Sigma; dissolved in saline). This was the same amphetamine injection that was used for inducing striatal c-fos expression (see above), and animals were subsequently sacrificed two hours post-injection. We used rotation together with results from the limb-use asymmetry test as an index of lesion severity and completely excluded from this study two 6-OHDA-infused animals that displayed neither robust rotation to amphetamine nor a forelimb use asymmetry in the cylinder test. We considered these animals to be either partial depletions or “stereotaxic misses”, as often occurs with this surgical technique due to the small size of the targeted medial forebrain bundle, and they are not part of the n of 8 lesioned rats.
Statistics
Statistical analyses were performed using R version 2.6.1, available free online at http://www.r-project.org. Data in the plots are expressed as mean values ± 95% confidence intervals as determined using bootstrapping methods with 10,000 replicates per data point. When appropriate, bootstrapped versions of various tests of significance were also applied to the data as detailed in the results section below. In these cases, p-values were determined by comparing the calculated statistic against an empirical sampling distribution generated from 10,000 bootstrap replicates, rather than using idealized (i.e., normality-assuming) distributions. (For more information on bootstrap methods and their application, see Davison and Hinkley, 1997; Efron and Tibshirani, 1993.)
Results
PIT test
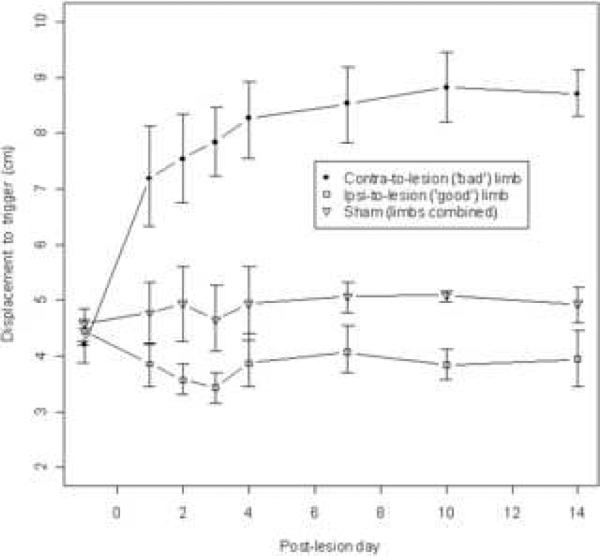
Data from the PIT test are plotted in Figures 2 and 3. Figure 2 shows the dependence of test results on the weight of the animal, as tested in a separate group of unmanipulated male Sprague-Dawley rats (n=40). Larger animals required a greater shift in the center of gravity over the planted forelimb in order to trigger a catch-up step. We also examined the effect as a function of animal age, but the correlation with weight was slightly better so only the latter is presented here (best curve fit: Displacement = 1.092(Weight0.288), R2 = 0.787, p < .001). Figure 3 shows the results of PIT testing following unilateral 6-OHDA lesion (n=8). As early as day 1 post-lesion, and continuing throughout our observation period, the reaction to body mass displacement was impaired in the contralateral limb relative to that of sham-lesioned controls (n=12 forelimbs from 6 animals). When the center of mass was sufficiently shifted, the contralateral limb always responded by stepping, but the distance required to elicit this response was consistently greater than the unimpaired forelimbs in every animal in both the control and 6-OHDA groups. Compared to either limb in the sham control group, the ipsilateral limb in 6-OHDA treated rats always showed increased reactivity to the experimenter-imposed weight shift. A repeated-measures ANOVA revealed a significant interaction between the effects of limb condition and post-operative time (F(14,133) = 20.5, p<.001), as well as main effects for both factors individually (Limb: F(2,19) = 86.6, p<.001; and Time: F(7,133) = 20.2, p<.001). Post-hoc t-tests were performed to compare the performance of the ipsilateral limbs of lesioned animals to the limbs of sham animals at each postoperative time point and indicated statistically significant differences at the p<.05 level on all post-operative days, but not at the pre-surgery time point.
Figure 3.
PIT test results following unilateral 6-OHDA or sham lesion. The ipsilateral limb of lesioned animals (n=8) becomes more reactive to experimenter-imposed shifts of the animal's center of gravity, while the contralateral limb (n=8) becomes less reactive relative to sham-operated animals (n=12 limbs [6 animals]). Data are means ± bootstrapped 95% confidence intervals. T-tests showed that ipsilateral limb performance differed significantly from sham limb performance with p < .05 at all post-op time points (but not at the pre-op baseline).
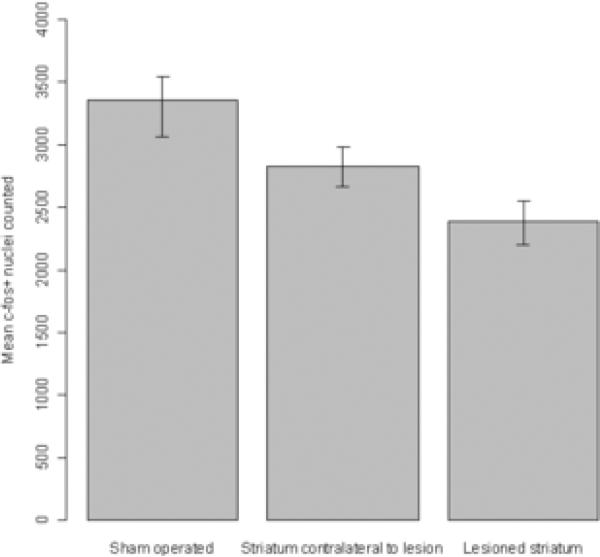
Amphetamine-induced striatal c-fos expression
The results of counts of c-fos-positive nuclei in the striatum of rats that had been treated with d-amphetamine prior to sacrifice are presented in Figure 4. Amphetamine induced a robust expression of c-fos in sham-lesioned animals. In the dopaminedenervated (ipsilateral) striatum this expression was reduced to 72% of the control (sham-operated) level, while the striatum contralateral to the lesion displayed a reduction to 86% of the sham-operated level. Student's t-tests showed significant differences among all hemisphere conditions: ipsilateral vs. contralateral, t = 3.32, p < .01; ipsilateral vs. sham, t = 5.99, p < .001; contralateral vs. sham, t = 3.41, p < .05.
Figure 4.
Amphetamine-induced striatal c-fos expression. C-fos induction in response to amphetamine challenge was reduced in both hemispheres of unilaterally lesioned animals (n=8) relative to sham controls (n=12 hemispheres). Data are means ± bootstrapped 95% confidence intervals. T-tests showed all group comparisons to be significantly different with p < .05.
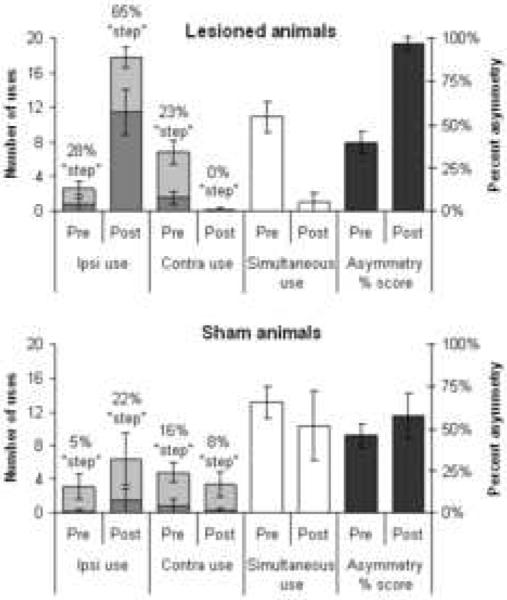
Limb use asymmetry test
Results from the limb-use asymmetry test are shown in Figure 5 for both the 6-OHDA and sham-operated sets of animals. The limb use behavior of sham-operated animals did not differ greatly between the pre-operative and 2 weeks post-operative testing points shown in the figure. The 6-OHDA animals, by contrast, showed a much greater reliance on independent use of the ipsilateral forelimb following the lesion, at the expense of both contralateral limb use and simultaneous limb use. This effect is summarized by the limb-use asymmetry score, which in lesioned animals rose from 40% at baseline to 97% post-lesion (using paired-samples t-tests to compare pre- vs. post-surgery conditions: 6-OHDA lesions, t = 22.5, p < .001; sham-operates, t = 2.59, p = 0.049). In addition, the percent of ipsilateral limb uses that were part of serial lateral stepping behavior increased dramatically after surgery (paired t-tests comparing percent ipsilateral stepping behavior pre- vs. post-surgery: 6-OHDA lesions, t = 3.85, p < .01 ; sham-operates, t = 1.52, p = 0.19).
Figure 5.
Limb-use asymmetry (“cylinder”) test. These data show the number of ipsilateral limb, contralateral limb, or simultaneous limb use events recorded in the cylinder test both pre- and post-operatively (use the axis on the left for these), as well as the calculated percent asymmetry scores (use the axis on the right) in 6-OHDA versus sham-operated animals. For independent uses of the contra or ipsi limbs, the data are further broken down to show the percentage of such limb uses that were part of “serial-stepping” behaviors as described in the text (denoted by the portion of the bars that is darker-shaded). Data are means ± bootstrapped 95% confidence intervals. The difference in proportion of serial-stepping behaviors between pre- and post-surgery tests differed significantly at p < .01 for the lesioned animals, but not for sham-operates.
Placing
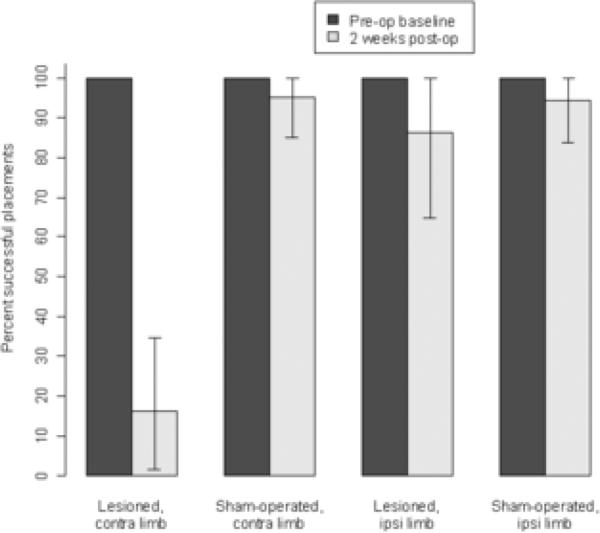
Results from the vibrissae-elicited forelimb placing test are plotted in Figure 6. Pre-operatively, both limbs of all rats placed with 100% success. Post-operatively only small decrements of placing ability were seen in the ipsilateral limb of lesioned animals or either limb of sham animals. However, placing ability in the contralateral limb of lesioned animals declined such that only 16% of placing attempts were successful after surgery.
Figure 6.
Vibrissae-evoked forelimb placing test. Data show the percent successful forelimb placing resulting from ten trials of vibrissae stimulation. The only significant anomaly in placing behavior was seen in the contra-to-lesion limb following 6-OHDA lesion. Data are means ± bootstrapped 95% confidence intervals.
PIT test with dopaminergic drugs
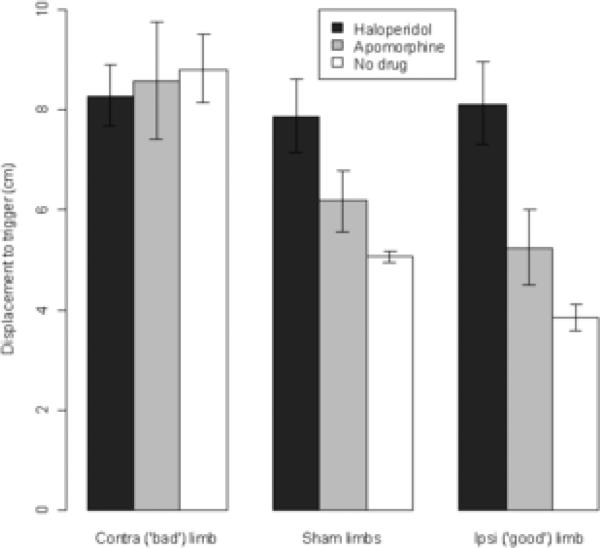
Results from performing the PIT test under the influence of systemically administered apomorphine or haloperidol are charted in Figure 7. The administration of the dopamine antagonist haloperidol caused both limbs of animals, regardless of experimental group, to react more slowly to displacement (i.e., haloperidol caused all limbs to perform like the contralateral limbs of undrugged but lesioned animals). Surprisingly, administration of the dopamine agonist apomorphine actually slightly increased the displacement needed to trigger a catch-up step in sham animals or in the ipsilateral limbs of lesioned animals, while not having an effect on the contralateral (impaired) limb of lesioned animals (paired t-tests comparing no-drug vs. apomorphine conditions: sham limbs, t = 3.30, p < .01; limbs ipsilateral to lesion, t = 3.92, p < .01).
Figure 7.
PIT testing under dopaminergic drugs. These data show the amount of displacement needed to trigger a catch-up step in the PIT test following systemic administration of either apomorphine or haloperidol. Data are means ± bootstrapped 95% confidence intervals. Performance under apomorphine differed significantly from the no-drug condition in both sham limbs and ipsilateral limbs, with p < .01.
Rotation
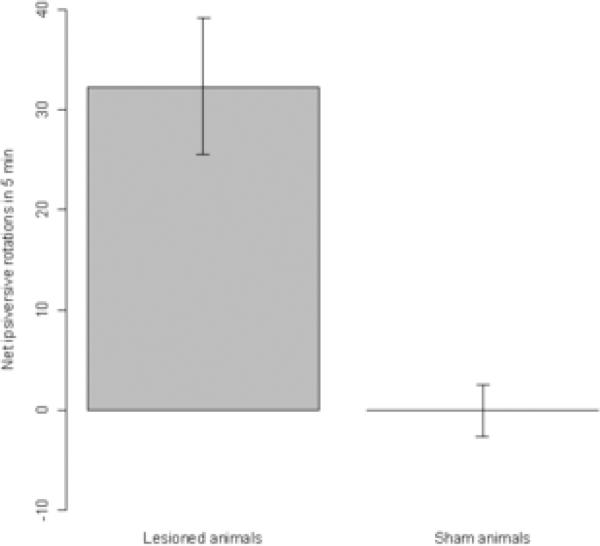
Rotational behavior of the animals following an injection of 3 mg/kg d-amphetamine sulfate is summarized in Figure 8. As expected, lesioned animals displayed robust ipsiversive rotation in response to amphetamine administration, while sham-operated animals showed virtually no consistent rotation. These rotational responses, in conjunction with performance in the limb-use asymmetry test, were used to verify that lesions were successful in the lesioned group.
Figure 8.
Rotational behavior in response to amphetamine injection. These data show the net number of ipsiversive rotations observed over a 5-minute time period, between 20-25 minutes following an injection of 3 mg/kg d-amphetamine sulfate. Data are means ± bootstrapped 95% confidence intervals.
Discussion
We observed a severe decrease in the reactive capacity of the forelimb contralateral to dopamine depletion in response to an involuntary shift of center of mass, which was not ameliorated by a dopamine agonist. This may model, at least in part, the postural instability in PD (Jessop et al., 2006). Marked changes in the motor behavior of the ipsilateral (unimpaired) forelimb were also found. These changes included an increased reactivity to experimenter-imposed displacements of the animal's center of gravity and an increased propensity to use the ipsilateral limb for lateral weight-shifting steps during rearing behaviors. Re-examination of data in the literature led us to find that the speed with which rats will react to remove a small sticky piece of tape placed on the wrist of their ipsilateral forelimbs is also enhanced when compared to unlesioned animals (Schallert et al., 1982, 1983). In addition, under some conditions 6-OHDA rats can display a reduced latency to orient towards an ipsilateral perioral stimulus when compared to unlesioned animals (Schallert and Hall, 1988). However, changes ipsilateral to 6-OHDA lesions do not always appear to be “super-normal”: it has been shown in testing skilled reaching for food pellets that 6-OHDA-lesioned rats display subtle impairments in the “good” limb which differ qualitatively from the type observed in the contralateral limb (Vergara-Aragon et al., 2003).
Changes in motor behavior ipsilateral to a brain lesion are not confined only to unilateral models of PD. Evidence supports changes in both behavior and the capacity to acquire new skills in the intact limb following unilateral injury to the motor cortex, used as a model of stroke (Bury and Jones, 2002; Hsu and Jones, 2006). Research in this area indicates that the presence of a motor cortical lesion promotes increased brain plasticity especially in the motor cortex contralateral to the lesion which, when coupled with the increased reliance on the non-impaired forelimb displayed by rats after such an injury, may allow the animal to adapt to the loss of function in its impaired limb (Jones and Schallert, 1992, 1994). Our behavioral evidence presented here suggests that similar phenomena may occur following lesions to the nigrostriatal pathway. The anatomical and/or neurochemical underpinnings of these changes remain to be assessed, though Miklyaeva et al. (2007) found an increase in dendritic arborization in the motor cortex contralateral to a dopamine depletion which may be linked to this. However, the compensatory behavioral changes were observed in the present study early after neurotoxin exposure, which perhaps is more consistent with rapid neural adaptations or with loss of inter-hemispheric inhibitory influences.
We measured c-fos expression in the striatum in response to challenge with d-amphetamine, a technique used to provide an index of dopaminergic signaling throughput dependent upon pre-synaptic release of dopamine by amphetamine. In so doing we did not find an upregulation of c-fos expression in the striatum contralateral to the lesion, as would be expected if increased dopamine innervation of or signaling within that striatum was the mechanism behind the ipsilateral behavioral changes we observed. In fact, we observed a slight decrease in c-fos signal in the unlesioned hemisphere following 6-OHDA, suggesting that whatever reorganization is responsible for the enhanced responsiveness of the ipsilateral side might be non-dopaminergic. Supporting this is our finding that administration of the dopamine agonist apomorphine did not relieve the contralateral postural stability deficit in the 6-OHDA animals, nor did it enhance the responsiveness of sham-operated animals’ forelimbs. Possibly changes in corticostriatal connectivity or in striatal dendritic morphology, or changes in areas outside of the striatum, are responsible for the ipsilateral motor effects. Extensive postoperative experience using the ipsilateral (“crutch”) limb for compensation during weight shifting movements might serve to solidify the adaptive reactive capacity and ensure long-term postural stability. Indeed, if increased reliance on the ipsilateral forelimb following unilateral motor cortical injury can trigger plastic changes in the intact cortex, perhaps similar changes in behavioral demand following nigrostriatal injury could have effects on the motor cortex as well (Hsu and Jones, 2006).
On the other hand, evidence does support some changes in the nigrostriatal dopamine system contralateral to 6-OHDA lesion, which might be linked to the behavioral phenomena we observed in a way that is not revealed by measurement of c-fos expression. The finding of increased levels of extracellular dopamine in striatal dialysates contralateral to 6-OHDA (Robinson and Whishaw, 1988), which until the present study has not had a potential behavioral correlate, coupled with more recent evidence of bilateral upregulation of D2 receptor density following unilateral 6-OHDA lesions (Ferre and Fuxe, 1992; Nikolaus et al., 2003; Waszczak et al., 2006) suggest that the “intact” striatum may have the capacity for increased dopaminergic signaling, possibly via the D2 pathway. Evidence also exists showing an upregulation in post-mortem striatal dopamine content (as measured by HPLC) in striata contralateral to a severe 6-OHDA lesion relative to the striata of sham-operated controls (Warenycia and McKenzie, 1987), though this is not consistently found. Finally, bilateral electrophysiological anomalies have been observed in the striatum, substantia nigra, and subthalamic nucleus of animals following unilateral 6-OHDA infusions (Warenycia and McKenzie, 1987; Breit et al., 2006; White-Cipriano and Waszczak, 2006). In this paper we found that systemic administration of the dopamine antagonist haloperidol did lead to a deficit in the PIT test, indicating that although nondopaminergic changes might be responsible for enhanced reactivity on the unimpaired side following unilateral 6-OHDA lesion, intact dopamine systems are nevertheless important for normal execution of this task.
The presence of changes in the ipsilateral forelimb following a unilateral lesion indicates the need for careful behavioral analysis in such lesion models to partition out the contributions of possible changes in the unlesioned hemisphere. Though behavioral tests providing an asymmetry score (such as the limb-use asymmetry test) are convenient and allow for within-animal control, they must also be combined with tests which can evaluate function on each side of the body independently in order to distinguish between the contributions of the ipsilateral and contralateral sides to an asymmetry score. Even reaching tests, in which each forelimb is examined separately, do not allow one to evaluate the function of the non-impaired limb relative to that of control animals. During reaching behavior the opposite limb is required for adequate postural support; if it is impaired, this can influence reaching success and qualitative measures of reaching dynamics (Vergara-Aragon et al., 2003).
Of course one of the most straightforward ways to deal with the dilemma presented by potential changes in the contralateral hemisphere is to always include sham-lesioned or unlesioned control animals in studies that involve unilateral lesions. Indeed, more frequent use of such controls is likely to lead to discoveries about the ways in which the unlesioned hemisphere can plastically adapt to brain injury, and about mechanisms of brain plasticity in general.
Acknowledgments
We gratefully acknowledge the assistance of Dr. DeAnna Adkins and Dr. Theresa Jones's lab in developing our c-fos immunohistochemical technique; of Jahnavi Mithyantha and Dr. Paul Garris in early refinement of the PIT test; and of our undergraduate assistants Ted Lin, Jennifer Gench and Jennifer Whiddon for behavioral testing, tissue processing, and editorial assistance. This work was supported by an NSF graduate research fellowship to MTW and NIH and DARPA grants NS-19608 and USAMRMC 03281055 (respectively).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barth TM, Stanfield BB. Homotopic, but not heterotopic, fetal cortical transplants can result in functional sparing following neonatal damage to the frontal cortex in rats. Cereb Cortex. 1994;4:271–278. doi: 10.1093/cercor/4.3.271. [DOI] [PubMed] [Google Scholar]
- Breit S, Martin A, Lessmann L, Gasser T, Schulz JB. Bilateral changes of subthalamic nucleus and substantia nigra neuronal activity in the unilateral 6-OHDA rat model.. Society for Neuroscience conference; Atlanta, GA. 2006. Program no. 654.4. [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap Methods and their Application. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall.; Boca Raton, FL: 1993. [Google Scholar]
- Ferre S, Fuxe K. Dopamine denervation leads to an increase in the intramembrane interaction between adenosine A2 and dopamine D2 receptors in the neostriatum. Brain Res. 1992;594:124–130. doi: 10.1016/0006-8993(92)91036-e. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Jessop RT, Horowicz C, Dibble LE. Motor learning and Parkinson disease: Refinement of movement velocity and endpoint excursion in a limits of stability balance task. Neurorehabil Neural Repair. 2006;20:459–467. doi: 10.1177/1545968306287107. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklyaeva EI, Whishaw IQ, Kolb B. A Golgi analysis of cortical pyramidal cells in the unilateral Parkinson rat: Absence of change in the affected hemisphere versus hypertrophy in the intact hemisphere. Restor Neurol Neurosci. 2007;25:91–99. [PubMed] [Google Scholar]
- Nikolaus S, Larisch R, Beu M, Forutan F, Vosberg H, Muller-Gartner HW. Bilateral increase in striatal dopamine D2 receptor density in the 6-hydroxydopamine-lesioned rat: a serial in vivo investigation with small animal PET. Eur J Nucl Med Mol Imaging. 2003;30:390–395. doi: 10.1007/s00259-002-1056-2. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: A microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Schallert T, DeRyck M, Whishaw IQ, Ramirez VD, Teitelbaum P. Excessive bracing reactions and their control by atropine and L-DOPA in an animal analog of Parkinsonism. Exp Neurol. 1979;64:33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Hall S. ‘Disengage’ sensorimotor deficit following apparent recovery from unilateral dopamine depletion. Behav Brain Res. 1988;30:15–24. doi: 10.1016/0166-4328(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–238. [PubMed] [Google Scholar]
- Schallert T, Norton D, Jones TA. A clinically relevant unilateral rat model of Parkinsonian akinesia. J Neural Transplantation and Plasticity. 1992;3:332–333. [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sanberg PR, editors. Central Nervous System Diseases: Innovative models of CNS diseases from molecule to therapy. Humana Press; Totowa, NJ: 2000. pp. 131–151. [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Wilcox RE, Vaughn DM. Posture-independent sensorimotor analysis of inter-hemispheric receptor asymmetries in neostriatum. Pharmacol Biochem Behav. 1983;18:753–759. doi: 10.1016/0091-3057(83)90019-9. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT. Motor systems: orienting and placing. In: Whishaw IQ, Kolb B, editors. The Behaviour of the Laboratory Rat: A handbook with tests. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the “good” side of the hemi-Parkinson rat improved with rehabilitation. J Neurosci. 2003;23:579–586. doi: 10.1523/JNEUROSCI.23-02-00579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenycia MW, McKenzie GM. Activation of striatal neurons by dexamphetamine is antagonized by degeneration of striatal dopaminergic terminals. J Neural Transm. 1987;70:217–232. doi: 10.1007/BF01253599. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, White-Cipriano P, Dickerson WM. Changes in dopamine receptor expression in the striatum of unilateral 6-hydroxydopamine (6-OHDA) lesioned rats are reflected both ipsilateral and contralateral to the lesion.. Society for Neuroscience conference; Atlanta, GA. 2006. Program no. 450.21. [Google Scholar]
- White-Cipriano P, Waszczak BL. Unilateral 6-hydroxydopamine (6-OHDA) lesions induce electrophysiological changes in both the ipsilateral and contralateral substantia nigra (SN).. Society for Neuroscience conference; Atlanta, GA.. 2006. Program no. 352.18. [Google Scholar]
- Woodlee MT, Mithyantha J, Kane JR, Chang J, Garris PA, Schallert T. Functional reorganization of the intact hemisphere following unilateral nigrostriatal dopamine depletion: Implications for parkinsonian models.. Society for Neuroscience conference; San Diego, CA.. 2004. Program no. 562.16. [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]