Abstract
RNA modifications impact numerous cellular processes including pre-mRNA splicing and protein synthesis. The elucidation of the mechanisms by which these modifications impact cellular processes necessitates the ability to both detect and quantify the presence of these modifications within RNA. Here, we present a detailed procedure that allows for both detecting and quantifying RNA base modifications. This procedure involves a number of techniques, including oligonucleotide-affinity selection, site-specific cleavage and radiolabeling, nuclease digestion, and thin layer chromatography.
Keywords: RNA modifications, pseudouridine, 2′-O-methylation, RNase H, U2 snRNA, site-specific radiolabeling
1. Introduction
Posttranscriptionally modified ribonucleotides were first identified in the hydrolysates of RNA more than a half a century ago (1, 2). It is now accepted that virtually all species of RNA contain posttranscriptional modifications. However, despite the fact that over 50 years have passed since the first identification of non-canonical ribonucleotides, the function of many remains undefined (see Chapter 1).
A prerequisite to elucidating the functions of posttranscriptional modifications is the knowledge of their existence in exact location (detection) as well as the amount present (quantitation). Prior to the early 1990s, detection of RNA base modifications was both time consuming and laborious, requiring a combination of techniques including in vivo radiolabeling, nuclease digestion, and chromatography (or fingerprinting) (3-7). The advent of primer extension/reverse transcription-based approaches greatly facilitated research regarding posttranscriptional modifications. These methods are primarily based on the fact that certain modified nucleotides will result in a premature stop during a primer-extension reaction. For instance, the chemical derivatization of pseudouridine with N-cyclohexyl-N′-(2-morpholinoethyl)-carbodiimid-methop-toluolsulfonate (CMC) blocks reverse transcription one nucleotide prior to the CMC-modified pseudouridine (8, 9). Similarly, the presence of a 2′-O-methylated nucleotide results in a premature stop when primer extension is carried out at low dNTP concentrations (10).
Though these experimental techniques ease the burden of detecting specific posttranscriptional modifications, there are several caveats to these approaches. For instance, with the exception of 2′-O-methylation (the sugar ring modification), most base modifications require chemical derivatization to induce premature stops during primer extension. As a variety of base modifications exist within RNA, identification of the specific chemical modifier and reaction conditions for all known modifications is a formidable task (11). Furthermore, while these primer extension-based methods are suitable for detection, they are not quantitative. Other disadvantages of these approaches include the dependency on visual observation of premature stops by gel electrophoresis, which is not very sensitive and may lead to errors in the identification of posttranscriptional modifications.
More advanced techniques based on mass spectroscopy have also been developed (12). However, these techniques are not practical to utilize in the common laboratory as they require expensive and specialized equipments (i.e., mass spectrometers and high-pressure liquid chromatography systems). In addition, they are not amenable for all modifications. Recently, a promising ligation-based approach has been described which takes advantage of T4 DNA ligase’s ability to discriminate modified nucleotides (13, 14). While this technique has the potential to be utilized in high-throughput screening for modified ribonucleotides, it is currently not optimized for all modifications.
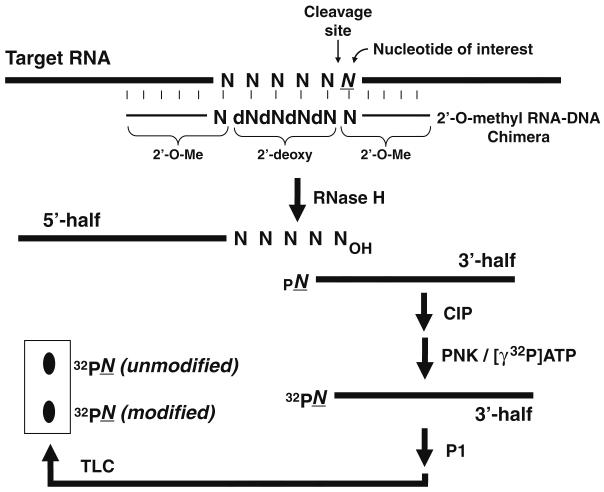
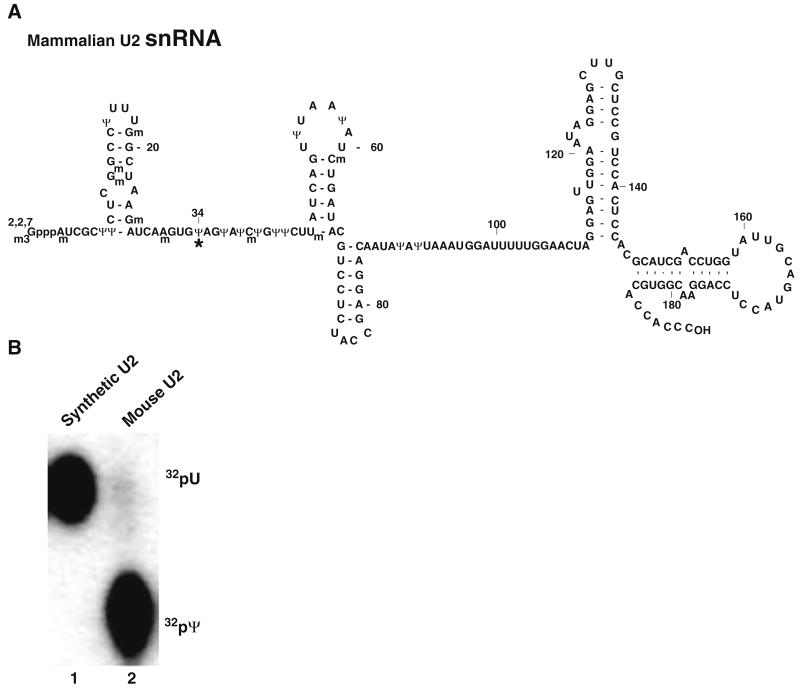
Here we describe an approach that when coupled with the primer extension-based approaches provides an extremely effective way of detecting and quantifying modified nucleotides in a variety of RNAs. Based on the fact that RNase H cleavage occurs only at sites where the 2′-OH of RNA is not modified, cleavage can be directed to a specific nucleotide of interest through the use of 2′-O-methyl RNA–DNA chimeric oligonucleotides (15-18) or even DNAzymes (11, 19). Following radiolabeling of the cleaved RNA, the RNA can be digested to single nucleotides by ribonucleases. The digested nucleotides can then be separated by thin layer chromatography (TLC), and the nucleotide of interest can be visualized by autoradiography (Fig. 2.1). We have successfully implemented this approach in the detection and quantitation of numerous base modifications including pseudouridylation and 5-fluorouridylation (11, 18, 20). As an example of application, below we apply the method to quantify the pseudouridylation at position 34 within mouse brain U2 snRNA. Our analysis indicates that the uridine at this position is nearly 100% converted to pseudouridine (Fig. 2.2).
Fig. 2.1.
The method for detecting and quantifying base modification is schematized (adapted from Zhao and Yu 1 with some modification). The thick lines and thin lines represent target RNA and 2′-O-methyl RNA–DNA chimera, respectively. See text for detailed description.
Fig. 2.2.
(a) The primary sequence and the secondary structure of mammalian U2 snRNA are shown. The modified nucleotides, including 2′-O-methylated residuals (Nm) and pseudouridines (Ψ), are indicated. The asterisk indicates the pseudouridine at position 34, which is quantified in (b). (b) Using RNase H cleavage directed by a 2′-O-methyl RNA–DNA chimera targeting the phosphodiester bond between positions 33 and 34, both synthetic mammalian U2 and mouse brain U2 were cleaved. The 3′ RNA fragments were gel purified, and the 5′ phosphate of both fragments was further replaced with 32P through dephosphorylation and consequent rephosphorylation. Both fragments were then treated with nuclease P1 to completion, and the resulting mononucleotides were subjected to TLC analysis. Lane 1, in vitro synthesized mammalian U2; lane 2, mouse brain U2. The spots corresponding to uridylate and pseudouridylate are indicated.
2. Materials
2.1. Purification of U2 snRNA from Mouse Brain
1. Trizol reagent.
2. Dounce tissue grinder.
3. Biotinylated antisense U2 2′-O-methyl oligonucleotide complimentary to nucleotides 158–177 of mouse U2 snRNA [UmCmCmUmGmGmAmGmGmUmAmCmUmGmCm AmAmUmAmCmBBB, where B stands for biotin–TEG (triethylene glycol)].
4. NET-2-MgCl2 buffer: 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 0.05% (v/v) NP-40, and 2 mM MgCl2.
5. Preblocking mix: 100 μg/mL glycogen and 100 μg/mL tRNA in 50 mM WB50.
6. WB50: 20 mM Tris–HCl (pH 7.6), 0.01% NP-40, 50 mM NaCl, 1.5% NaN3.
7. WB250: 20 mM Tris–HCl (pH 7.6), 0.05% NP-40, 250 mM NaCl, 0.1% NaN3.
8. Streptavidin agarose beads.
9. Dissociation buffer: 10 mM Tris–HCl (pH 7.5), 0.1% sodium dodecyl sulfate (SDS), and 0.5 mM ethylenedi-aminetetraacetic acid (EDTA).
10. Formamide loading buffer: 95% formamide, 10 mM EDTA, 0.1% xylene cyanol FF, 0.1% bromophenol blue.
11. PCA: Tris–HCl (pH 7.5)—buffered phenol/chloroform/isoamyl alcohol (50:49:1).
12. G50 buffer: 20 mM Tris–HCl (pH 7.5), 300 mM sodium acetate, 2 mM EDTA, 0.25% SDS.
13. Glycogen: 10 mg/mL.
14. Polyacrylamide gel solution (40%).
15. Urea.
16. TBE (10×; Omnipur, EMD chemicals).
17. Ammonium persulfate.
18. TEMED.
19. Chloroform.
20. Isopropanol.
21. Ethanol.
22. Autoclaved distilled water.
23. Electrophoresis apparatus.
2.2. RNase H Site-Specific Cleavage Directed by 2′-O-Methyl RNA–DNA Chimera
1. 2′-O-Methyl RNA–DNA chimera: For the purposes of this protocol the sequence of the chimera used is specific for cleavage between positions 33 and 34 of mouse U2 snRNA: UmAmdCdAdCdTUmGmAm UmCmUmUmAm GmCmCm.
2. RNase H buffer (2×): 40 mM Tris–HCl (pH 7.5), 20 mM MgCl2, 200 mM KCl, 50 mM DTT, 10% sucrose.
3. RNasin (20 units/μL).
4. RNase H.
5. Formamide loading buffer: 95% formamide, 10 mM EDTA, 0.1% xylene cyanol FF, 0.1% bromophenol blue.
6. PCA: Tris–HCl (pH 7.5)—buffered phenol/chloroform/isoamyl alcohol (50:49:1).
7. G50 buffer: 20 mM Tris–HCl (pH 7.5), 300 mM sodium acetate, 2 mM EDTA, 0.25% SDS.
8. Glycogen: 10 mg/mL.
9. Polyacrylamide gel solution (40%).
10. Urea.
11. TBE (10×).
12. Ammonium persulfate.
13. TEMED.
14. Chloroform.
15. Isopropanol.
16. Ethanol.
17. Autoclaved distilled water.
18. Electrophoresis apparatus.
2.3. Radiolabeling of the Cleaved U2 snRNA (3′ Half)
1. Dephosphorylation buffer (10×).
2. Calf intestinal alkaline phosphatase (CIAP).
3. Sodium acetate solution: 3 M NaAc, pH 5.2.
4. G50 buffer: 20 mM Tris–HCl (pH 7.5), 300 mM sodium acetate, 2 mM EDTA, 0.25% SDS.
5. PCA: Tris–HCl (pH 7.5)—buffered phenol/ chloroform/isoamyl alcohol (50:49:1).
6. Ethanol.
7. Glycogen: 10 mg/mL.
8. Polynucleotide kinase (PNK).
9. PNK buffer (10×).
10. [γ-32P]ATP (6,000 Ci/mmol; DuPont NEN).
11. Polyacrylamide gel solution (40%).
12. Formamide loading buffer: 95% formamide, 10 mM EDTA, 0.1% xylene cyanol FF, 0.1% bromophenol blue.
13. Urea.
14. TBE (10×).
15. Ammonium persulfate (APS).
16. TEMED.
17. Electrophoresis apparatus.
2.4. Detection and Quantification of U2 snRNA Pseudouridylation by TLC
1. Nuclease P1.
2. TLC PEI membrane.
3. TLC buffer: 70% isopropanol, 15% HCl, 15% dH2O.
4. Sodium acetate.
5. PhosphorImager.
3. Methods
3.1. Purification of U2 snRNA from Mouse Brain
1. In a 15-mL tube, mince 200 mg of mouse brain into small pieces using scissors and resuspend the tissue with 4 mL Trizol reagent.
2. Using a pre-chilled Dounce tissue grinder, homogenize the tissue by passing them through the grinder for about 30 times (see Note 1). The entire homogenization procedure should be carried out on ice.
3. Extract the homogenized sample by adding 800 μL of chloroform to the sample and mix by vortexing. Centrifuge at 13,000×g for 5 min at 4°C.
4. Collect the aqueous phase in a new 15-mL tube and add 2 mL of isopropanol. Store the tube at −80°C.
5. In a 1.5-mL microfuge tube, add 200 μL of streptavidin agarose beads (100 μL total bed volume) and microfuge the tube for 10 s at 5,000×g. Add 250 μL of preblocking mix and rotate the tube on a rotator for 20 min at 4°C.
6. Wash the beads by adding 1 mL of cold WB50 and microfuge for 1 min at 5,000×g. Remove the supernatant and resuspend the beads with 1 mL of WB50. Repeat this step two more times without resuspending the beads in WB50 the last time.
7. Resuspend the beads in 1 mL WB250 and microfuge for 1 min at 5,000×g. Repeat this step one more time.
8. Resuspend the beads in 400 μL of WB250 and aliquot 50 μL (~10 μL total bed volume) in a 1.5-mL microfuge tube.
9. Obtain the total mouse RNA tube from step 4 and microfuge at 13,000×g for 15 min at 4°C and discard the supernatant.
10. Resuspend the pellet with 50 μL of NET-2-MgCl2 buffer and transfer to a 1.5-mL microfuge tube.
11. Add 200 pmol of the biotinylated antisense U2 oligonucleotide to the tube and vortex briefly. In parallel, set up an identical binding reaction with an irrelevant biotinylated oligonucleotide as a control.
12. Heat the mixture for 2 min at 95°C and then incubate for 10 min at 65°C and 30 min at 30°C.
13. Obtain the resuspended beads from step 8 and microfuge the tube for 10 s at 5,000×g. Remove the supernatant (bead volume is ~10 μL).
14. Resuspend the beads with 50 μL of the RNA/oligo mix and bring up the volume to 300 μL with NET-2-MgCl2 buffer. Gently nutate the tube at 4°C for 1.5 h.
15. Microfuge the tube briefly at 4°C for 30 s at 5,000×g and discard the supernatant.
16. Wash the beads five times at 4°C, each time with 1 mL of WB250 (microfuge for 1 min at 5,000×g each time).
17. Add 250 μL of dissociation buffer to the pelleted beads and incubate at 85°C for 15 min.
18. Microfuge the tube for 30 s at 5,000×g and collect the supernatant in a new 1.5-mL microfuge tube.
19. Add 250 μL of G50 buffer to the supernatant (up to 500 μL) and then add 500 μL of PCA. Vortex vigorously for 30 s and microfuge for 5 min at 13,000×g. Collect the aqueous phase in a new 1.5-mL microfuge tube.
20. Add 1 μL of glycogen and 1 mL of 100% ethanol to the aqueous phase. Vortex briefly and then place the tube on dry ice for 10 min. Microfuge at 13,000×g for 15 min to precipitate the RNA. Remove and discard the supernatant promptly.
21. Resuspend the RNA pellet in 2 μL of autoclaved distilled water, mix well with 4 μL of formamide loading buffer, heat at 95°C for 3 min, chill on ice immediately.
22. Meanwhile, make an 8% polyacrylamide–7 M urea gel by mixing 23.75 g of urea in 5 mL of 10× TBE, 10 mL polyacrylamide gel solution. Bring the volume to 50 mL with autoclaved distilled water and stir on a hot plate until the urea has completely dissolved. Add 0.5 mL of 10% APS and 30 μL TEMED, mix briefly, and pour into pre-taped gel plates (~25 cm wide and ~40 cm height).
23. Load the RNA sample (from step 21) on the 8% polyacrylamide–7 M urea gel. Electrophorese for ~1 h at 30 W.
24. Place the gel on an intensifying screen and visualize the RNA under 254 nm UV light and excise the RNA with a razor. Place the gel slice in a 1.5-mL microfuge tube.
25. Add 450 μL of G50 buffer to the tube and place on dry ice for 5 min. Transfer to room temperature for elution overnight (16 h).
26. Microfuge the tube containing the gel slice at 13,000×g for 5 min. Transfer the supernatant to a new 1.5-mL microfuge tube. Extract the supernatant with 500 μL of PCA and precipitate the gel-purified RNA with 1 mL of 100% ethanol (using 1 μL of glycogen as carrier) as previously described.
27. Resuspend the pellet with 10 μL of autoclaved distilled water and quantify the concentration using UV/VIS spectroscopy.
3.2. RNase H Site-Specific Cleavage Directed by 2′-O-Methyl RNA–DNA Chimeras
1. In a 1.5-mL microfuge tube, mix 1 μL (~1.2 pmol) of U2 snRNA with 5 pmol of the 2-O-methyl RNA–DNA chimera in 4 μL of water.
2. Heat the mixture at 95°C for 3 min and slowly cool down to room temperature. Microfuge the tube briefly.
3. Meanwhile, place 1 μL (20 units) of RNasin, 1 μL (2 units) of RNase H, and 7 μL of 2× RNase H buffer in a new 1.5-mL microfuge tube (see Notes 2 and 3). Keep the tube on ice.
4. Transfer the RNase H mixture to the tube containing the hybridized U2 snRNA. Mix gently by pipetting.
5. Incubate the resulting mixture at 37°C for 1 h.
6. Recover the two cleaved RNA fragments (5′ and 3′ halves) via PCA extraction and ethanol precipitation as in steps 19 and 20.
7. Resuspend the recovered RNA pellet in 2 μL of autoclaved distilled water, mix well with 4 μL of formamide loading buffer, heat at 95°C for 3 min, chill on ice immediately.
8. Meanwhile, prepare an 8% polyacrylamide–7 M urea gel as in step 22.
9. Load the sample on the 8% polyacrylamide–7 M urea gel. Electrophorese for ~1 h at 30 W.
10. Place the gel on an intensifying screen and visualize the two 5′- and 3′-RNA halves under 254 nm UV light (see Note 4) and excise the band corresponding to the 3′ half with a razor. Place the gel slice in a 1.5-mL microfuge tubes.
11. Add 450 μL of G50 buffer to the tube and place on dry ice for 5 min. Transfer to room temperature for elution overnight (16 h).
12. Microfuge the tube containing the gel slice at 13,000×g for 5 min. Transfer the supernatant to a new 1.5-mL microfuge tube. Extract the supernatant with 500 μL of PCA and precipitate with 1 mL of 100% ethanol (using 1 μL of glycogen as carrier) as previously described.
13. Resuspend the pellet with 10 μL of autoclaved distilled water and quantify the concentration using UV/VIS spectroscopy.
3.3. Dephosphorylation and Rephosphorylation of the 3′ Half of U2 snRNA
1. Dephosphorylate 0.15 pmol of the 3′ half of U2 snRNA in a 10 μL reaction containing 1× dephosphorylation buffer, and 1 unit of CIAP for 45 min at 50°C (see Note 5).
2. Following the reaction, add 250 μL autoclaved dH2O and extract the sample with 300 μL of PCA. Add 25 μL of sodium acetate solution and precipitate with 1 mL of 100% ethanol (using 1 μL of glycogen) as previously described.
3. Rephosphorylate the recovered RNA at its 5′ terminus for 30 min at 37°C in a 10 μL reaction containing 1× phosphorylation buffer, 0.15 pmol of 3′ half U2 snRNA, 150 μCi of [γ-32P]ATP, and 10 units of T4 PNK.
4. Add 250 μL of G50 buffer, PCA extract once, and then ethanol precipitate the RNA as previously described.
5. Resuspend the 5′-radiolabeled 3′ half of U2 snRNA in 2 μL autoclaved distilled water. Add 4 μL of formamide loading buffer, heat at 95°C for 3 min, chill on ice immediately.
6. Meanwhile, prepare an 8% polyacrylamide–7 M urea gel as described in Section 3.1, step 22.
7. Load the sample on a 8% polyacrylamide–7 M urea gel. Electrophorese for ~1 h at 30 W.
8. Locate the band by autoradiography and excise the band. Place gel slice in a 1.5-mL microfuge tube.
9. Add 450 μL of G50 buffer to the tube and place on dry ice for 5 min. Transfer to room temperature for elution overnight (16 h).
10. Microfuge the tube containing the gel slice at 13,000×g for 5 min. Transfer the supernatant to a new 1.5-mL microfuge tube. Extract the supernatant with 500 μL of PCA and precipitate with 1 mL of 100% ethanol (using 1 μL of glycogen) as previously described. (Do not resuspend pellet here.)
3.4. Detection and Quantification of U2 snRNA Pseudouridylation
1. Resuspend the 5′-radiolabeled 3′ half of U2 snRNA with nuclease P1 (200 μg/mL) in 3 μL of 20 mM sodium acetate (pH 5.2) for 1 h at 37°C.
2. Dot 1,000 cpm of the digested nucleotide mixture on cellulose TLC PEI membrane approximately 1 cm from one of the edges.
3. Place the edge of the TLC PEI membrane closest to the mixture in TLC buffer and wait until the front of the buffer is three-fourth the length of the membrane.
4. Visualize labeled uridylate and pseudouridylate by autoradiography (see Fig. 2.1) and the ratio of uridylate to pseudouridylate can be determined using a PhosphorImager (see Note 6).
The used chimeric oligonucleotide should be specific for cleavage at the desired positions. In our case, the chimera is designed to guide the cleavage between positions 33 and 34 (pseudouridylation at position 34 is targeted). RNA-cleaving ribozymes or RNA-cleaving DNAzymes can also be used to direct site-specific cleavage (11, 19, 21). The use of a DNAzyme would conveniently leave a 5′-OH on the downstream target fragment, thus making dephosphorylation unnecessary (11, 19).
Acknowledgments
We would like to thank the members of the Yu laboratory for discussion and inspiration. Our work was supported by grant GM62937 (to Yi-Tao Yu) from the National Institute of Health. John Karijolich was supported by a NIH Institutional Ruth L. Kirschstein National Research Service Award GM068411.
Footnotes
Number of passes could vary in order to reach complete homogenization depending on the tissue and the cell type.
Units of RNase H added may vary according to supplier.
RNase H from different suppliers may have different cleavage specificities (22). RNase H purchased through Amersham cleaves RNA specifically at the site 3′ to the nucleotide that base pairs with the 5′ -most deoxynucleotide of the chimera (22).
Based on the fact that RNase H cleaves RNA only at sites where the 2′ position of the sugar is not modified (2′-OH), cleavage at 2′-O-methylated residues is completely blocked. Thus, the degree of resistance to RNase H cleavage quantitatively reflects the level of 2′-O-methylation (18). Accordingly, this method can also be used to quantify 2′-O-methylation. To this end, end labeling (for example, 3′-end labeling with 32pCp and RNA ligase) of RNA is desirable, because this will allow an accurate measurement/quantification of the level of cleavage.
Shrimp alkaline phosphatase (SAP), which is sensitive to heat (65°C), may be a better choice for the dephosphorylation reaction. Heat inactivation of SAP after the dephosphorylation reaction could allow the omission of PCA extraction and ethanol precipitation, which would otherwise be needed to remove active phosphatase (see CIAP-catalyzed dephosphorylation reaction above).
References
- 1.Cohn WE, Volkin E. Nucleoside-5′-phosphates from ribonucleic acid. Nature. 1951;167:483–484. [Google Scholar]
- 2.Grosjean H. Modification and Editing of RNA: Historical Overview and Important Facts to Remember. Springer-Verlag; Berlin Heidelberg: 2005. [Google Scholar]
- 3.Gupta RC, Randerath K. Rapid print–readout technique for sequencing of RNA’s containing modified nucleotides. Nucl Acids Res. 1979;6:3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maden BE. Identification of the locations of the methyl groups in 18 S ribosomal RNA from Xenopus laevis and man. J Mol Biol. 1986;189:681–699. doi: 10.1016/0022-2836(86)90498-5. [DOI] [PubMed] [Google Scholar]
- 5.Maden BE. Locations of methyl groups in 28 S rRNA of Xenopus laevis and man. Clustering in the conserved core of molecule. J Mol Biol. 1988;201:289–314. doi: 10.1016/0022-2836(88)90139-8. [DOI] [PubMed] [Google Scholar]
- 6.Kuchino Y, Mizushima H, Nishimura S. Nucleotide Sequence Analysis and Identification of Modified Nucleotides of tRNA. CRC Press; Boca Raton: 1990. [Google Scholar]
- 7.Reddy R, Henning D, Epstein P, Busch H. Primary and secondary structure of U2 snRNA. Nucl Acids Res. 1981;9:5645–5658. doi: 10.1093/nar/9.21.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakin A, Ofengand J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyl transferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993;32:9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- 9.Bakin AV, Ofengand J. Mapping of pseudouridine residues in RNA to nucleotide resolution. Methods Mol Biol. 1998;77:297–309. doi: 10.1385/0-89603-397-X:297. [DOI] [PubMed] [Google Scholar]
- 10.Maden BE, Corbett ME, Heeney PA, Pugh K, Ajuh PM. Classical and novel approaches to the detection and localization of the numerous modified nucleotides in eukaryotic ribosomal RNA. Biochimie. 1995;77:22–29. doi: 10.1016/0300-9084(96)88100-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Yu YT. Detection and quantitation of RNA base modifications. RNA. 2004;10:996–1002. doi: 10.1261/rna.7110804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crain PF. Detection and Structure Analysis of Modified Nucleosides in RNA by Mass Spectrometry. American Society of Microbiology Press; Washington, DC: 1998. [Google Scholar]
- 13.Saikia M, Dai Q, Decatur WA, Fournier MJ, Piccirilli JA, Pan T. A systematic, ligation-based approach to study RNA modifications. RNA. 2006;12:2025–2033. doi: 10.1261/rna.208906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Q, Fong R, Saikia M, Stephenson D, Yu YT, Pan T, Piccirilli JA. Identification of recognition residues for ligation-based detection and quantitation of pseudouridine and N6-methyladenosine. Nucl Acids Res. 2007;35:6322–6329. doi: 10.1093/nar/gkm657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu YT. Construction of 4-thiouridine site-specifically substituted RNAs for cross-linking studies. Methods. 1999;18:13–21. doi: 10.1006/meth.1999.0752. [DOI] [PubMed] [Google Scholar]
- 16.Yu YT. Site-specific 4-thiouridine incorporation into RNA molecules. Methods Enzymol. 2000;318:71–88. doi: 10.1016/s0076-6879(00)18045-0. [DOI] [PubMed] [Google Scholar]
- 17.Lapham J, Crothers DM. Site-specific cleavage of transcript RNA. Methods Enzymol. 2000;317:132–139. doi: 10.1016/s0076-6879(00)17011-9. [DOI] [PubMed] [Google Scholar]
- 18.Yu YT, Shu MD, Steitz JA. A new method for detecting sites of 2′-O-methylation in RNA molecules. RNA. 1997;3:324–331. [PMC free article] [PubMed] [Google Scholar]
- 19.Hengesbach M, Meusburger M, Lyko F, Helm M. Use of DNAzymes for site-specific analysis of ribonucleotide modifications. RNA. 2008;14:180–187. doi: 10.1261/rna.742708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu YT, Steitz JA. A new strategy for introducing photoactivatable 4-thiouridine ((4S)U) into specific positions in a long RNA molecule. RNA. 1997;3:807–810. [PMC free article] [PubMed] [Google Scholar]
- 22.Lapham J, Yu YT, Shu MD, Steitz JA, Crothers DM. The position of site-directed cleavage of RNA using RNase H and 2′-O-Methyl oligonucleotides is dependent on the enzyme source. RNA. 1997;3:950–951. [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silberklang M, Prochiantz A, Haenni AL, Rajbhandary UL. Studies on the sequence of the 3′-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977;72:465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuchino Y, Hanyu N, Nishimura S. Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol. 1987;155:379–396. doi: 10.1016/0076-6879(87)55026-1. [DOI] [PubMed] [Google Scholar]
- 25.Keith G. Mobilities of modified ribonucleotides on two-dimensional cellulose thin-layer chromatography. Biochimie. 1995;77:142–144. doi: 10.1016/0300-9084(96)88118-1. [DOI] [PubMed] [Google Scholar]
- 26.Grosjean H, Keith G, Droogmans L. Detection and quantification of modified nucleotides in RNA using thin-layer chromatography. Methods Mol Biol. 2004;265:357–391. doi: 10.1385/1-59259-775-0:357. [DOI] [PubMed] [Google Scholar]
- 27.Grosjean H, Motorin Y, Morin A. RNA-Modifying and RNA-Editing Enzymes: Methods for Their Identification. American Society of Microbiology Press; Washington, DC: 1998. [Google Scholar]