Abstract
In nature, vector borne flaviviruses are persistently cycled between either the tick or mosquito vector and small mammals such as rodents, skunks, and swine. These viruses account for considerable human morbidity and mortality worldwide. Increasing and substantial evidence of viral persistence in humans, which includes the isolation of RNA by RT PCR and infectious virus by culture, continues to be reported. Viral persistence can also be established in vitro in various human, animal, arachnid, and insect cell lines in culture. Although some research has focused on the potential roles of defective virus particles, evasion of the immune response through the manipulation of autophagy and/or apoptosis, the precise mechanism of flavivirus persistence is still not well understood. We propose additional research for further understanding of how viral persistence is established in different systems. Avenues for additional studies include determining whether the multifunctional flavivirus protein NS5 has a role in viral persistence, the development of relevant animal models of viral persistence, and investigating the host responses that allow vector borne flavivirus replication without detrimental effects on infected cells. Such studies might shed more light on the viral–host relationships and could be used to unravel the mechanisms for establishment of persistence.
Keywords: vector borne flaviviruses, arboviruses, viral persistence
Persistent infections by vector borne flaviviruses are an important, but inadequately studied topic.
Graphical Abstract Figure.
Persistent infections by vector borne flaviviruses are an important, but inadequately studied topic.
Introduction
Defining mechanisms of viral persistence will be critical for understanding vector borne flavivirus infections. These viral infections account for considerable human morbidity and mortality worldwide. Furthermore, incidence is increasing and infections are being appreciated in previously nonendemic geographic locations. Prominent vector borne flaviviruses (VBFVs) associated with significant human infections include both tick borne and mosquito borne agents. The tick borne flaviviruses (TBFVs) are exemplified by the tick borne encephalitis virus (TBEV) sero complex group, Omsk hemorrhagic fever virus, Kyasanur forest disease virus, Alkhurma virus, Powassan virus (POWV), and deer tick virus (DTV), the latter two occurring in the United States (Holbrook et al., 2005; Brackney et al., 2008b; Ebel, 2010). The mosquito borne flaviviruses (MBFVs) are perhaps better known and include yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and dengue virus (DENV) serotypes 1–4. Infections with all of these viruses can lead to severe disease, prolonged debilitating neurological sequelae, hemorrhagic fever, and/or death in some cases (Solomon et al., 1998; Glass et al., 2002; Haglund & Gunther, 2003; Madden, 2003; Van Gerpen, 2003; Carson et al., 2006).
Viral persistence is a hallmark of the ecology of VBFVs. Both TBFVs and MBFVs are cycled between arthropod and vertebrate hosts (Figs 1 and 2), and in many cases, they are maintained without deleterious effects on the hosts. In nature, TBFVs, such as POWV and TBEV, alternately infect small vertebrates such as rodents, hares, some carnivores, and a range of hard bodied (ixodid) ticks, although recent evidence suggests that the soft bodied Ornithodoros (argasid) ticks can also support TBFV (Rajagopalan et al., 1969; Charrel et al., 2007). Similarly, MBFVs, such as WNV and JEV, primarily alternate in nature between small mammals, birds, and mosquitoes (Fig. 1). In addition, there is evidence that MBFV and TBFV persistence also occurs in humans, and persistence in cell culture is well documented (Poidinger et al., 1991; Lancaster et al., 1998; Bugrysheva et al., 2001; Farfan Ale et al., 2009; Murray et al., 2010).
Figure 1.
Flavivirus maintenance and transmission cycle in ticks and vertebrate hosts. Ticks are crucial for viral persistence as they remain infected once they acquire viral infection. Infected ticks are capable of transmitting TBFVs to other ticks when they feed in close proximity on the same animal, as well as the different stages of the tick life cycle. TBFVs persist also in a cycle between small mammals (e.g. rodents) and the ticks that feed on them. Large mammals and humans tend to be incidental, dead end hosts.
Figure 2.
A representation of a mosquito borne flavivirus amplification and transmission cycle. WNV is cycled between the mosquito and avian hosts that play an amplification and maintenance role. Swine are important amplifying hosts for JEV, and mosquitoes that acquire blood meals on infected pigs can become infected and transmit the virus. Similar to TBFVs, MBFV infection in humans and large animals, such as horses, is accidental.
Although the hosts for the VBFVs are highly varied, the genomic and structural organization of the viruses themselves is remarkably similar. All flaviviruses are spherical, enveloped particles that contain a genome of (+) ssRNA measuring approximately 11 kb. The genome also functions as mRNA and encodes a single polyprotein that is cleaved into 10 proteins (Table 1). Reasonable progress has been made toward understanding how these viruses replicate (Chambers et al., 1990; Mandl, 2005; Miorin et al., 2008; Tuplin et al., 2011; Pierson & Diamond, 2012; Brinton, 2013; Pierson & Kielian, 2013). We present a simplified overview of the general aspects of a VBFV replication cycle in Fig. 3. The individual proteins are three structural proteins: capsid (C), precursor membrane/membrane (prM/M), and envelope (E), and seven nonstructural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (Chambers et al., 1990; Ryan et al., 1998; Bollati et al., 2010). The defined functions of these proteins are presented in Table 1. However, the precise function of some of the proteins remains to be elucidated. Furthermore, it should be noted that the bulk of the studies have been carried out in MBFV systems, and it is possible that the functions may not be identical in TBFV.
Table 1.
The flavivirus proteins, untranslated regions (UTRs), and their known functions
| Flavivirus protein | Nucleotide weight, aa | Location in cell | Defined functions of viral proteins and untranslated regions of genome | ||
| Mosquito borne flavivirus (MBFV) | Tick borne flavivirus (TBFV) | ||||
| 5′ UTR | 1–132 | The 5′ UTR contains conserved RNA stem loops (SL), cis elements located upstream and downstream of the AUG region (UAR and DAR, respectively), and complementary sequence (CS) sponsoring cyclization of the genome by interacting with the 3′ UTR to form a ‘panhandle’ (Alvarez et al., 2005; Villordo & Gamarnik, 2009; Friebe & Harris, 2010; Friebe et al., 2011). The ‘panhandle’ structure is essential for certain steps in the flavivirus replication cycle such as replication/translation and viral assembly (Gritsun & Gould, 2007b). SL1 2 or (SL A and SL B) is located within the 5′ UTR, whereas the SL3 4 is extended and situated in the N terminal of the open reading frame ORF (Alvarez et al., 2005; Lodeiro et al., 2009; Villordo & Gamarnik, 2009; Friebe & Harris, 2010; Friebe et al., 2011). The SL1 structure is essential for viral replication and acts as a promoter which is targeted initially by NS5 and then delivered to the 3′ end via cyclization (Filomatori et al., 2006; Zhang et al., 2008; Lodeiro et al., 2009) | Although there is low nucleotide conservation between flaviviruses and different CS homology (Hahn et al., 1987), the 5′ UTRs of TBFVs share the same genomic organization as the MBFVs (Kofler et al., 2006) | ||
| Structural proteins | Capsid (C) | 133–468, 11 kDa, 114 aa | Cytosol/nucleus | The capsid protein is a dimeric alpha helical (Jones et al., 2003) protein and assembles into an icosahedral structure, measuring 30 nm in diameter, which initiates encapsidation of the associated genomic RNA in virus induced membrane invaginations of the ER (Welsch et al., 2009). The C protein is also a pro apoptotic factor in various cell lines (Yang et al., 2002, 2008; Oh et al., 2006; Netsawang et al., 2010; Morchang et al., 2011). C of JEV was reported to limit viral neurovirulence (Mori et al., 2005) | The C encoding nucleotide sequence of TBEV contains conserved RNA structures that function as replication enhancer elements (Tuplin et al., 2011) |
| Precursor membrane (prM) | 469–972, 26 kDa, 165 aa | ER lumen | During virion assembly, the prM forms a heterodimer with the E protein and acts as a chaperone for correct E protein folding (Kuhn et al., 2002; Lorenz et al., 2002; Zhang et al., 2003). In the final step of virion maturation in the trans Golgi network prior, to viral release, the precursor is cleaved by furin and only the C terminal region (M) is retained in the viral membrane (Heinz et al., 1994; Elshuber & Mandl, 2005; Lindenbach et al., 2007). prM protein of JEV also limits viral neurovirulence (Kim et al., 2008) | ||
| Membrane (M) | 748–972, 8 kDa, 90 aa | ||||
| Envelope (E) | 973–2460, 53 kDa, 495 aa | ER lumen | The E glycoprotein consists of a soluble ectodomain, a stem region, and a transmembrane domain (Bressanelli et al., 2004). The glycoprotein forms ‘head to tail’ homodimers that completely cover the surface of the mature icosahedral virions. It is also responsible for receptor binding, which leads to virus internalization by clathrin mediated endocytosis (Lindenbach et al., 2007). The E protein contains three β sheets domains (DI–DIII): DII includes the membrane fusion peptide (Beasley & Barrett, 2002; Bressanelli et al., 2004; Sukupolvi Petty et al., 2007); DIII is associated with receptor binding and is also a target for several neutralizing monoclonal antibodies. Crystal structures of the E protein from several flaviviruses all show similar secondary structure and domain organization (Modis et al., 2003, 2005; Zhang et al., 2004; Kanai et al., 2006; Nybakken et al., 2006). The E protein is important in determining neuroinvasiveness and neurovirulence for several MBFVs (Cecilia & Gould, 1991; Hiramatsu et al., 1996; Ni & Barrett, 1996; Sanchez & Ruiz, 1996; Chambers et al., 1998; Beasley et al., 2004; Lee et al., 2004, 2006; Bordignon et al., 2007). For WNV, JEV, and DENV, mutations in the E protein (located in the lateral surface of DIII and the base of DII) affect fusion and receptor binding with target cells (Lee et al., 2004) | The E protein was first described in 1995 and later has been extensively defined and crystalized for many other flaviviruses (Rey et al., 1995). TBFV E protein is important for determining neurovirulence and neuroinvasiveness (Holzmann et al., 1990; McMinn, 1997; Beasley et al., 2005; Mandl, 2005; Engel et al., 2010) |
|
| Nonstructural Proteins | NS1 | 2461–3516/46–55 kDa/325 aa | ER lumen/cytosol/secreted | Low resolution structural studies found the NS1 structure to be an open barrel configuration with D3 symmetry measuring 10 nm by 7.5 nm and with a central cavity approximately 4.5 nm in diameter (Gutsche et al., 2011; Edeling et al., 2014). All flavivirus NS1 genes share a conserved degree of homology. NS1 is found at different cellular locations such as vesicular compartments associated with the cell membrane or on the cell surface and is also secreted as an extracellular hexamer composed of dimer subunits. In the viral replication cycle, the NS1 glycoprotein has role in early RNA replication where its association with NS4A is critical for formation of the replicase complex (RC; Westaway et al., 1997; Lindenbach et al., 2007). Each subunit forms a homodimer located in the ER lumen, and it has been shown to colocalize with viral dsRNA. Secreted and cell surface associated NS1 is immunogenic and induces an antibody response that can serve as an important diagnosis marker (Falgout et al., 1990; Avirutnan et al., 2006; Sun et al., 2007). NS1 is also implicated in immune evasion functions as it activates human complement and induces host vascular leakage by specific inhibition of the classical and lectin pathways of complement activation. This occurs through a direct interaction with complement components C4 and C1s (Avirutnan et al., 2010). DENV, WNV, and YFV NS1 are shown to limit C4b deposition and C3 convertase activity by enhancing cleavage of C4 through the recruitment of the complement specific protease C1s (Avirutnan et al., 2010). This effect might explain some of the clinical manifestations of dengue hemorrhagic fever and dengue shock syndrome |
|
| NS2A | 3517–4206, 19 kDa, 218 aa | Cytosol | The NS2A protein is highly hydrophobic and is involved in viral RNA replication and capsid assembly as it binds strongly to the 3′ UTR, NS3, and NS5 in the perinuclear regions of cells (Lindenbach et al., 2007; Welsch et al., 2009). Bioinformatic analyses on JEV subgroup revealed an internal RNA sequence and structure located at N terminal of NS2A that might be important for ribosomal frame shift resulting a NS1of different size (NS1′) (Firth & Atkins, 2009; Firth et al., 2010; Melian et al., 2010). It is also suggested that the size different might be caused by different glycosylation pattern or differently cleavage in NS2A (Blitvich et al., 1995, 1999). Mutations within this pseudo knot abolish the NS1′, suggesting a role in viral neuroinvasiveness and attenuation in mice (Liu et al., 2006; Melian et al., 2010) | ||
| NS2B | 4207–4599, 14 kDa, 130 aa | ER lumen/membrane bound | NS2B is a hydrophobic protein and acts as a cofactor for NS3. Together, they form a serine protease complex essential for processing the virus polyprotein (Chambers et al., 1991; Falgout et al., 1991; D'Arcy et al., 2006; Erbel et al., 2006) | ||
| NS3 | 4600–6462/69 kDa, 618 aa | Cytosol | The NS3 protein structure is made up of two segregated globular domains (Assenberg et al., 2009). NS3 is a highly conserved and multifunctional protein: The protein has protease activity in one domain, RNA stimulated NTPase activity and RNA triphosphate activity (RTPase) in the other domain (Chambers et al., 1991; Valle & Falgout, 1998; Lescar et al., 2008). NS3 protein is an essential member of the RC and is activated in association with NS5 to bind the genomic RNA 3′ SL prior to replication (Chen et al., 1997; Mackenzie et al., 1998; Lindenbach et al., 2007). Beside its essential role in the viral replication cycle, NS3 can also induce apoptosis (Shafee & AbuBakar, 2003; Ramanathan et al., 2006; Yang et al., 2009; Yiang et al., 2013). NS3 is involved in the inhibition of the transcriptional factor AP1 signaling pathway, which also directly affects apoptosis (Lin et al., 2006). Studies on neurovirulence associated with DENV 1 NS3 have shown that mutations in NS3 (Leu480Ser) enhance the ability of replication in CNS causing leptomeningitis and encephalitis in mice (Bordignon et al., 2007). This mutation was also shown to induce cell death in DENV 1 infected cells (Duarte dos Santos et al., 2000). The NS3 protein can be targeted by antiviral drugs such as ivermectin, ribavirin as inhibitors of the ATPase activity of helicases (Mastrangelo et al., 2012) |
The protease domain of LGTV NS3 associates with caspase 8 and induces apoptosis (Prikhod'ko et al., 2002) | |
| NS4A | 6463–6840, 16 kDa, 286 aa | ER lumen/membrane bound | The mature NS4A is a hydrophobic transmembrane protein and associates with most of the RC factors (NS1, NS2A, NS3, NS5, and dsRNA) (Lindenbach et al., 2007). Its interaction with NS1 is essential for viral RNA replication as NS4A acts as a docking site (Bartenschlager et al., 1995; Miller et al., 2007). NS4A interacts with the polypyrimidine tract binding protein (PTB), with the latter being implicated in translation, transcription, and viral processes for various viruses (Bieleski et al., 2004; Florez et al., 2005; Shi & Lai, 2005) including the role in regulation of HCV replication (Tischendorf et al., 2004; Domitrovich et al., 2005; Aizaki et al., 2006; Chang & Luo, 2006). NS4A PTB plays an indirect role in stabilization of the viral genome dsRNA intermediates by binding to host cell PTB protein in a similar manner as stabilizing WNV RNA synthesis by the elongation factor 1 alpha (Davis et al., 2007; Jiang et al., 2009). NS4A together with NS4B and NS3 is also responsible for inducing the rearrangement of the host ER membrane leading to the formation of virus induced membranous spherules and vesicles encasing the dsRNA and RC; this is thought to minimize exposure of the replicating RNA to innate immune sensors such as retinoic acid inducible gene I, RIG I (Mackenzie et al., 1998; Roosendaal et al., 2006; Miller et al., 2007). The mature NS4A can also induce the PI3K dependent autophagy signaling, which in turn lead to protection from camptothecin and staurosporine induced cell death (McLean et al., 2011). The role of NS4A in the up regulation of the autophagy and ER rearrangement makes the protein a potential target for antiviral drug development |
||
| 2K | 6841–6909, 2 kDa, 23 aa | Complete cleavage of the VBFV polyprotein generates an ER spanning 2K peptide located between NS4A and NS4B (Lin et al., 1993; Roosendaal et al., 2006; Miller et al., 2007). The 2K peptide functions as a signal peptide for NS4B and might play a role in enhancing viral replication by conferring resistance to lycorine, a suppressor of flavivirus RNA synthesis (Zou et al., 2009) | |||
| NS4B | 6910–7665, 27 kDa, 112 aa | ER lumen/membrane bound | Several studies have shown colocalization of NS4B/dsRNA with NS3, suggesting that NS4B plays a role in RC formation or function (Westaway et al., 2003; Miller et al., 2006). NS4B appears also to be involved in ER rearrangement and modification during viral replication (Westaway et al., 1997). The NS4B protein of WNV, JEV, and DENV inhibits type I interferon (IFN α/β) response by blocking the phosphorylation of STAT1 (Muñoz Jordán et al., 2003, 2005; Lin et al., 2004; Liu et al., 2005; Evans & Seeger, 2007). The same is true for KUNV and WNV strain NY99, which blocks the activation of STAT1/STAT2 and its translocation to the nucleus (Liu et al., 2005). These events disrupt the host immune responses by blocking the IFN alpha/beta/gamma pathways | ||
| NS5 | 7666–10 374, 100 kDa, 900 aa | Cytosol/ER/nucleus | NS5 is the largest and most conserved among the VBFV proteins. NS5 primarily functions as the RNA dependent RNA polymerase (RdRp) through its C terminal domain (Lindenbach et al., 2007). The NS5 has been crystalized and contains an N terminal domain with S adenosyl methionine methyltransferase activity (MTase), and possibly guanylyltransferase (GTase) for RNA capping (Egloff et al., 2002; Malet et al., 2007; Yap et al., 2007). NS5 is also involved in activating other viral proteins, such as NS3 NTPase and RTPase activity in a dose dependent manner. DENV NS5 contains both nuclear localization signal (NLS) and nuclear export signal (NES): These facilitate its importation into the nucleus by both importin β1 and the importin α/β complex, and an additional function of the signals could be enhancement of virus replication and hyperphosphorylation of NS5 (Johansson et al., 2001; Brooks et al., 2002). For WNV, NS5 is exported from the nucleus in a chromosome region maintenance 1 (CRM1) dependent manner (Pryor et al., 2007; Rawlinson et al., 2009). NS5 is involved in different cellular pathways and has a crucial role in the escape from, or blocking, the interferon alpha/beta (IFN alpha/beta) signaling pathway. DENV NS5 inhibits host TYK2 and STAT2 phosphorylation and prevents the activation of JAK STAT signaling pathway (Mazzon et al., 2009) | In addition to the viral RdRp functions of NS5 described for the MBFVs, the TBFV NS5 was the first to be implicated in disrupting innate immune signaling. To suppress critical host responses, LGTV NS5 interacts with IFNAR2 and IFNGR2, two IFN receptor subunits, and antagonizes IFN dependent responses by suppressing JAK STAT signal transduction (Best et al., 2005; Park et al., 2007). Cellular responses targeted at restricting viral replication occur via lysosomal degradation of NS5, NS2B, and NS3. The NS5 of LGTV and TBEV interacts with TRIM79 alpha, an IFN inducible protein, and induces the degradation (Taylor et al., 2011). The cellular antiviral state is also compromised by TBEV NS5 interaction with the scaffold protein Scribble (Werme et al., 2008). This complex blocks the phosphorylation of STAT1 and disrupts the JAK STATsignaling pathway | |
| 3′ UTR | 10 375–11 141 | The extreme 3′ terminal region contains secondary structures and RNA elements important for cyclization of the genome and virus viability. The MBFV 3′ UTR can be divided into three regions (Markoff, 2003; Liu et al., 2009): a variable region directly upstream of the stop codon; a second region that is relatively conserved in nucleotide sequences containing hairpin motifs; and a highly conserved core element contains stable SL structures and cyclization motif (Villordo & Gamarnik, 2009) | The 3′ UTR of TBFVs exhibits significant heterogeneity in length and sequence even among closely related strains. The TBFV 3′UTR can also be divided into a variable region part and an extremely conserved core element (Gritsun et al., 1997), where the length variability of the variable region has been suggested to be related to the number of laboratory passages (Mandl et al., 1998; Mandl, 2005). A hypothesis interprets the biological importance of the variable region that influences the replication/translation dynamics in mammalian and tick cells (Elvang et al., 2011). The conserved core element contains highly essential sequential and structural motifs defined as SL1 5 for viral maintenance (Mandl et al., 1998; Pletnev, 2001). The pentanucleotide (GAGAG) motif exposed on the SL4 is strictly conserved among all the flavivirus genomes (Khromykh et al., 2003). Other important elements present in the conserved region include the homopurine box, a homopyrimidine box, and cyclization sequences (Mandl, 2005; Gritsun & Gould, 2007a,b) | ||
Nucleotide numbers are related to the strain Neudoerfl of TBEV.
Shared function is presumed for both mosquito and tick borne flaviviruses. In many cases, no specific studies have been carried out in TBFV.
Figure 3.
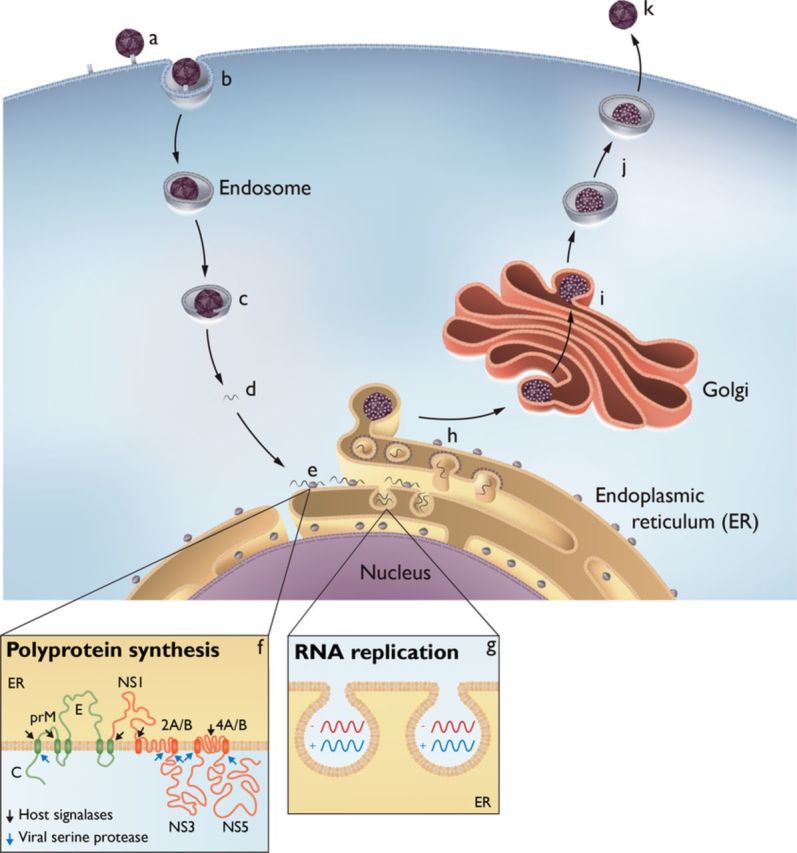
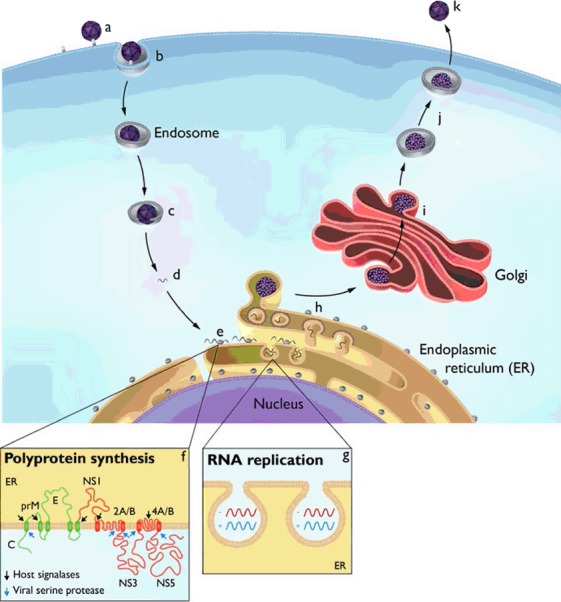
A simplified overview of the replication cycle for a vector borne flavivirus. Inbound virions bind to the cell membrane via poorly characterized receptors (a), are captured in vesicles by a clathrin mediated pathway (b), and delivered to endosomes, where a pH dependent fusion of the particles with the endosome membrane occurs (c). Subsequent to uncoating, the single stranded, positive sense RNA genome (d) migrates to the endoplasmic reticulum (ER) and is translated (e) as a polyprotein traversing the ER membrane several times (f). The polyprotein is cleaved into the viral proteins by viral and cellular proteases, although prM and E remain covalently attached. Through the agency of several viral nonstructural proteins and cellular proteins, there is a proliferation of ER derived membranes and the formation of spherules that maintain a pore like connection to the cytoplasm (g); the viral genome is replicated within these spherules by the viral proteins comprising the replication complex. By an as yet uncharacterized mechanism, progeny genomes are delivered to adjacent ER membranes where the capsid protein mediates assembly and inclusion of prM E into immature virions (h). The immature virions transit the Golgi membrane system (i), and as a mild pH change occurs, the cellular enzyme furin cleaves the prM E linkage (j), allowing the virus particle to assume its final mature stage prior to release from the cell (k). Defined roles for the individual viral proteins are enumerated in Table 1.
Recognizing that the VBFVs cycle between vertebrate and arthropod hosts and that both viral and host factors are likely to be involved, it is highly probable that viral persistence is exceedingly complex. The purpose of this review is to evaluate the current literature on flavivirus persistence as well as to suggest ideas for additional research into this interesting and important area.
Flavivirus infection and persistence in humans
Humans are inadvertent targets of VBFV infection (Figs 1 and 2), and infection is often associated with debilitating, acute neurological syndromes or hemorrhagic fever. However, there are several lines of evidence suggesting flavivirus persistence in humans (Murray et al., 2010; Gibney et al., 2011; Baty et al., 2012). The establishment of flavivirus persistence in humans seems to be mainly associated with encephalitic flaviviruses (Diamond, 2003). However, YFV and DENV persistence has been described in eukaryotic cells in culture and in successive generations of Aedes mosquitoes (Lodge et al., 1987; Takasaki et al., 2001; Farfan Ale et al., 2009). YFV and DENV commonly cause a hemorrhagic illness, jaundice, and dengue shock syndrome, and encephalitis is atypical and extremely rare (Tomori, 2004; Gulati & Maheshwari, 2007; Varatharaj, 2010), but persistence cannot be excluded.
Human infection with the encephalitic viruses almost always occurs following a bite from infected ticks or mosquitoes (Figs 1 and 2). Generally, illness tends to be biphasic. The first phase is characterized by a flu like illness with symptoms such as headache, arthralgia, and malaise. The second phase may present with neurological symptoms, such as mild meningitis to severe encephalitis. Survivors of encephalitis may develop persistent neurological sequelae, suggesting neurological tissue damage and/or viral persistence. Neurological tissue damage, such as loss of neurons, has been reported for WNV infection (Guarner et al., 2004). Long term morbidity following TBEV infection is known to occur frequently (Haglund et al., 1996; Haglund & Gunther, 2003). A study conducted in Sweden reported chronic postencephalitic syndrome in 36% of patients who had been infected with TBEV (Haglund et al., 1996). In up to 60% of patients who develop encephalitis due to WNV infection, neurological and/or neuropsychological symptoms continue to be reported for up to 7 years following the infection (Murray et al., 2010; Sadek et al., 2010).
The most definitive evidence for viral persistence would be the isolation of viable virus or the demonstration of viral antigens or RNA long after the acute illness. Several reports describe the isolation of infectious VBFVs from persistently infected humans. For example, the Siberian TBEV strain Za was isolated from an individual who had harbored the virus for 10 years (Gritsun et al., 2003). He later died following 2 years of a progressive form of TBEV encephalitis (Gritsun et al., 2003). In addition, isolation of JEV from the cerebrospinal fluid was possible for more than 3 weeks following infection in 19% of individuals that had developed encephalitis (Ravi et al., 1993). JEV persistence was also demonstrated in peripheral mononuclear cells in infected children in northern India (Sharma et al., 1991). In these children, virus could be isolated by culture 8–9 months after acute infection (Sharma et al., 1991). Furthermore, WNV has been transmitted to patients who had received blood transfusions or organ transplants from asymptomatic donors (Pealer et al., 2003; Montgomery et al., 2006; CDC, 2009), suggesting viral persistence in the donors as well as stored blood for transfusion.
Long term presence of viral nucleic acid is another indicator for persistent infection; however, study results are inconsistent. Viral RNA can be readily sought using molecular biology techniques, such as RT PCR and transcription mediated amplification (TMA) (Sánchez Seco et al., 2005; Muñoz Jordán et al., 2009; Patel et al., 2013). Using TMA, WNV RNA was detectable in the blood of donors for up to 104 days following the index donation (Busch et al., 2008; Prince et al., 2008). Using RT PCR, WNV RNA could also be detected in urine of persistently infected individuals for up to 6.5 years following the acute phase of infection (Murray et al., 2010), although virus could not be isolated from the RNA positive urine samples. In contrast, WNV RNA could not be detected after 6 years in urine in another study reported a year later (Gibney et al., 2011). Baty and colleagues also failed to detect WNV RNA by RT PCR, but were able to detect viral RNA using TMA (Baty et al., 2012). Similarly for JEV, viral RNA could not be detected by RT PCR in the individuals who had anti JEV IgM antibodies (Zhao et al., 2013). In the study by Gibney et al. (2011), WNV persistence could not be excluded. Even though the results in these studies are somewhat variable, it seems reasonable to conclude that the presence of viral RNA can also be taken as an indicator of persistent infection.
Viral serology may be an additional surrogate measure for VBFV persistence. In general, infection or vaccination leads to a sterilizing immunity, but long term persistence of anti VBFV IgM antibodies has often been assumed to indicate continued exposure to viral antigens or virus particles (Ravi et al., 1993; Stiasny et al., 2012). An exception to this is, of course, DENV where antiviral antibody plays a key role in pathogenesis (Martina et al., 2009; Pierson, 2010). IgM antibodies induced by flaviviruses, such as TBEV and WNV, are known to persist in serum and CSF for 12 months or more (Kapoora et al., 2004; Stiasny et al., 2012). Indeed, IgM antibodies against TBEV persisted for up to 32 months (Stiasny et al., 2012). Furthermore, anti WNV IgM antibodies persist in previously exposed blood donors for up 16 months (Busch et al., 2008; Prince et al., 2008). However, IgM antibody persistence does not necessarily correlate with infectious virus persistence. For example, persistent IgM antibodies against TBEV can be detected in some cases following vaccination without active viral infection (Rendi Wagner et al., 2004; Stiasny et al., 2012). For WNV infection, IgM antibodies against the nonstructural protein NS5 cannot be used to distinguish between recent/active infection from past infection (Prince et al., 2008). Thus, serology may be a less useful marker for viral persistence.
A major consideration in VBFV in humans would be identification of sites of viral persistence. As flavivirus persistence seems to be mainly associated with the neurotropic and encephalitogenic viruses, the central nervous system may well be the preferred site for viral persistence. Furthermore, this may account for why patients who recover from encephalitis often have prolonged neurological symptoms. However, other studies by Murray and colleagues postulated kidneys as a preferred site for the establishment of persistent flavivirus infections following the detection of WNV RNA in urine (Murray et al., 2010). Certainly, additional work is required to define the true incidence, the biology, and the pathogenic potential in humans of persistent VBFV infections.
The role of arthropods and arachnids in flavivirus persistence
Arthropod hosts play crucial roles in the biology of both TBFV and MBFV and contribute to persistent infection. In nature, ticks are the important arachnid reservoirs of TBFVs. The persistence of the TBFVs in arachnids is well established, and the role of ticks as long term reservoirs and vectors for the viruses is clear. Estimates suggest that ticks transmit about 25% of the known flaviviruses. TBFVs are capable of infecting about 14 tick species, but the ixodid ticks, Ixodes scapularis and Ixodes ricinus, account for nearly all transmission of TBEV to humans. Ixodes cookei may also harbor POWV and DTV, while the soft bodied Onithodoros tick transmits Alkhurma virus (Main et al., 1979; Charrel et al., 2007). To transmit virus, these ticks need only 15 min of attachment to the host (Crowder et al., 2013). The virus is present in the tick salivary glands, and saliva constituents enhance infectivity (Nuttall & Labuda, 2003; Girard et al., 2004). A recent publication revealed that a selected subset of salivary gland genes is expressed when infected ticks feed on naïve mice (McNally et al., 2012).
Ticks acquire the virus while feeding on infected rodents, typically by a process called ‘cofeeding’ (Fig. 1; Labuda et al., 1993a,b), and the virus persists throughout several life stages. Ticks at the larvae, nymph, and adult life stages can become infected by feeding on infected animals (horizontal transmission) or can transmit virus vertically across instars (transstadial transmission) and through the eggs (transovarial transmission; Fig. 1a; Labuda et al., 1993a, 1996; Nuttall & Labuda, 2003). Indeed, results of a carefully controlled study in our laboratory show that transstadial transmission seems to be very high (Mitzel et al., 2007). The blood meal remains in the midgut for long periods of time, allowing infection of the epithelial cells lining the midgut with subsequent translocation into the hemocoel (Nuttall & Labuda, 2003). Infection of the midgut cells may be facilitated by the heterophagous nature of ticks (i.e. blood meal digestion is principally an intracellular process). In the open circulatory system, tissues and organs are bathed in hemolymph, which acts as a medium for transporting nutrients, hormones, and immune effector molecules. Therefore, the hemolymph likely serves as a viral dissemination medium. TBE virus was found in the esophagus and subesophageal ganglion in Dermacentor marginatus larvae and in columnar epidermal cells of Dermacentor reticulatus nymphs (Nuttall & Labuda, 2003). In D. reticulatus nymphs, TBE virus was demonstrated in epidermal cells and in vacuoles in the region of Golgi complexes of salivary gland cells (Nosek et al., 1984). The precise mechanisms by which TBFVs traverse various tissues in the tick and reach the salivary glands remain to be fully elucidated.
Prevalence surveys indicate that 0.5–5% of ixodid ticks carry the virus in Europe, but prevalence of up to 40% has been reported in Russia (Ustinova et al., 1997). In north central USA, up to 4.9% of I. scapularis ticks are infected by Powassan or DTV (Brackney et al., 2008b; Dupuis et al., 2013). Interestingly, in a study carried out in Chicago, I. scapularis was found to infest 2.8% of wild birds, which figure prominently in the MBFV cycle (Fig. 1b; Hamer et al., 2011). However, identification of TBFV was not attempted in this study. In brief, TBFV persistence in ticks is well established, and the essential role of ticks in the biology of these viruses is not in doubt.
As is the case for TBFVs, the role of mosquitoes in the maintenance of the MBFV cycle is also well characterized. Mosquitoes are thought to account for transmitting approximately 50% of more than 70 known flaviviruses (Gould, 2001). Diverse mosquito species can be infected by MBFVs. However, the two major mosquito vectors are Aedes aegypti (e.g. transmission of YFV and DENV) and Culex species (e.g. transmission of WNV and JEV). Adult female mosquitoes become infected when they obtain blood meals from flavivirus infected animals, and virus replication in the mosquito has been well described (Girard et al., 2005; McGee et al., 2010; Colpitts et al., 2012). Girard et al. (2004) used immunohistochemistry to demonstrate that WNV infects epithelial cells of the Culex pipiens midgut and that viral antigen can be detected as early as day 2 postinfection. Viral antigen staining becomes more intense in the cells of the midgut over time until day 14 to 21 following infection (Girard et al., 2004). Using DENV 2, virus titer in the midgut peaks to about 9 × 103 pfu mL−1 by 10 days postinfection, but declines to about 7.4 × 102 pfu mL−1 by day 12 (Sánchez Vargas et al., 2009). Dissemination of virus into various tissues occurs at various time points, and the amount of antigen also varies. Similar to TBFVs in ticks, the hemolymph serves as a vehicle for viral dissemination. However, virus also spreads in a cell–cell fashion to the muscle of the posterior midgut from 6 to 27 days postinfection (Girard et al., 2004). Studies with DENV 2 showed that infected mosquitoes mount an RNA interference (RNAi) mediated antiviral response, but impairing the vector RNAi resulted in increased viral replication (Sánchez Vargas et al., 2009). This mechanism could be associated with observations of flavivirus related RNA that persists as DNA in mosquitoes (Crochu et al., 2004). As mosquitoes are such efficient vectors for the transmission of the MBFVs, these reports suggest that the viruses have evolved a mechanism of evading the host response to persist in the vector.
Mosquitoes also acquire and deliver virus horizontally during blood meals and are competent to transmit vertically to progeny by transovarial passage (Rosen et al., 1983). WNV can persist overwinter in mosquitoes that hibernate in cold months. Cool temperatures also facilitate persistence of flaviviruses in adult female mosquitoes. For example, St. Louis encephalitis virus (SLEV) survived for more than 100 days of winter in Culex quinquefasciatus (Kramer & Ebel, 2003). Similarly, JEV and WNV have been transmitted by mosquitoes that carried the viruses at cold temperatures when exposed to temperature increases equal to ambient levels (Kramer & Ebel, 2003). However, at low temperature, mosquitoes are less likely to acquire new infections (Colpitts et al., 2012). At 18 °C, low WNV dissemination to the legs of the C. pipiens was observed, and rapid viral dissemination occurred at higher temperatures (Colpitts et al., 2012). Furthermore, higher temperatures increase vector population growth rate and the rate of viral evolution in the mosquito (Girard et al., 2005).
The prevalence and maintenance of WNV across landscapes is mediated by environmental factors, such as local effects of agriculture on vector and host communities (Crowder et al., 2013). Results of investigations carried out in the states of Oregon and Washington showed that the prevalence of WNV in both C. pipiens and Culex tarsalis was similar at 14.5% or 13.5%, respectively (Crowder et al., 2013). However, a study conducted in Stratford, Connecticut, showed that the C. pipiens was the most dominant mosquito captured in this WNV focus area (Anderson et al., 2004). In the captured mosquitoes, more than 85% of WNV isolations were from the same species, whereas Culex salinarius accounted for between 5% and 12% (Anderson et al., 2004). In a similar study conducted in Mexico, C. quinquefasciatus was more common at 48.3% and MBFV RNA was detected in 70% of the pools (Farfan Ale et al., 2009). It is evident that mosquitoes are important as viral hosts and vectors. Furthermore, it can be concluded that the MBFVs have evolved mechanisms of evading the host innate responses to persist in the MBFVs.
Flavivirus infection and persistence in animals
The principal vertebrate hosts for VBFVs are small mammals, marsupials, and birds (Fig. 1), but the viruses can also infect reptiles (Mackenzie et al., 2002, 2004; Steinman et al., 2003; Jacobson et al., 2005; Root et al., 2005; Marschang, 2011). In most instances, larger animals, such as cervids, goats, and sheep, are incidental hosts. The 1999 outbreak of WNV in humans in New York is thought to be associated with WNV infection in birds (Strausbaugh et al., 2001). The mosquito borne Wesselsbron virus could also be cycled between mosquitoes and birds, but not much is known about the vertebrate host(s). Thus, in nature, numerous species are susceptible to VBFVs and might serve as reservoirs or secondary amplifying hosts. In this section, we will survey the literature and the three potential markers for viral persistence: isolation of virus, identification of viral RNA or protein, and viral serology.
Small mammals, particularly rodents, are the principal vertebrate hosts and reservoirs for TBFVs (Mansfield et al., 2009; Dobler et al., 2012). In Europe, the yellow necked mouse (Apodemus flavicollis) and bank vole (Myodes glareolus) are implicated as the most common hosts, and they develop sufficient viremia to infect ticks that feed on them (Weidmann et al., 2011; Dobler et al., 2012; Knap et al., 2012). In various wild rodent species captured in Brandenburg, Germany, an average TBEV infection rate of 15% was reported (Achazi et al., 2011). In the captured rodents, TBEV RNA was detected by RT PCR in the brains and spleens. In North America, deer mice (Peromyscus), squirrels, and the striped skunk (Mephitis mephitis) are important reservoirs of POWV and DTV (Main et al., 1979; Telford et al., 1997). Based on serological studies, a 6.2% DTV prevalence in the red backed voles (Myodes rutilus) was found in Siberia and Alaska (Deardorff et al., 2013). DTV in New Mexico was serologically prevalent in Peromyscus truei and Peromyscus maniculatus at 22.2% and 6.0%, respectively (Deardorff et al., 2013). Therefore, it seems likely that persistent infection of these small mammals occurs.
Deer may stabilize and maintain TBFVs at levels that are important for transmission (Pugliese et al., 2007; Carpi et al., 2009; McGee et al., 2010; Dobler et al., 2012). While deer are important sources of blood meals for ticks, they develop low virus titer and are therefore not competent in transmission of TBFVs. However, in a broader sense, deer are important in viral persistence because they help sustain tick populations (Cagnacci et al., 2012).
The situation for domesticated animals is also less clear than for rodents. Dogs, horses, and monkeys can be infected with TBFVs, but case reports in veterinary practice are relatively infrequent (Jaenson et al., 2012). Large domesticated animals, such as goats, sheep, and cattle, become viremic for a short while and develop antibodies following infection with TBEV, but do not show any specific clinical signs of illness (Mansfield et al., 2009; Klaus et al., 2012). However, transmission of TBEV via milk from these domesticated animals (Fig. 1) has been documented, suggesting that virus may persist following the acute viremia in at least some instances (Vereta et al., 1991; Caini et al., 2012; Hudopisk et al., 2013).
Birds and primates are considered to be the primary reservoirs of MBFVs. Other mammals are generally accidental hosts, but this may not always be the case, and when viremic, these domestic animals are able to infect mosquitoes.
For JEV, the major amplifying hosts are birds and pigs, which attain high levels of viremia. They provide a source of infection for the mosquito species that subsequently transmit JEV to humans (Hukkanen et al., 2006; van den Hurk et al., 2009). JEV control has been achieved through vaccination of pigs and humans in Korea, Japan, and Taiwan (Igarashi, 2002) while horses are considered dead end hosts of JEV infection due to a very low level of viremia.
In geographic regions where pig populations are low, swine may not be important for zoonotic transmission of JEV. Herons and egrets are important JEV amplifying hosts and source of infection for mosquito species that transmit JEV to humans (Nemeth et al., 2009). Wild caught pigeons were shown to have antibodies that persist for up to 15 months. It is not clear whether the antibody persistence is associated with viral persistence. Although various bird species do play a prominent role in JEV infection, evidence of birds as a source of persistent infection is less certain. In some instances, birds can acquire JEV viremia and then fail to develop or lose neutralizing antibodies.
Birds are also excellent amplifying hosts for WNV, and migratory birds can move the viruses to different areas because they become viremic for several days. Near 100% mortality is associated with natural or experimental WNV infection in birds, such as American crows (Corvus brachyrhynchos), blue jays (Cyanocitta cristata), and greater sage grouse (Centrocercus urophasianus; Steele et al., 2000; Komar et al., 2003; Clark et al., 2006), but some birds are able to carry the virus for a longer time before they develop antibodies or succumb to disease (McKenzie & Goulet, 2010). For instance, a combined 37% of house sparrows that were either naturally or experimentally infected with WNV tested positive for WNV RNA by RT PCR (Wheeler et al., 2012). In the same study, Wheeler et al. (2012) showed that 97% and 100% of WNV infected house sparrows and finches, respectively, seroconverted following exposure to WNV. Birds that develop neutralizing anti WNV antibodies are protected from reinfection (Nemeth et al., 2009), suggesting total virus clearance in seroconverted birds, and antibodies against WNV have been found in wild birds, suggesting exposure to WNV.
Early demonstration of WNV persistence was described in blue gray pigeons, from which virus was isolated up to 100 days postinfection, and viral antigen could be detected in liver tissue for up to 180 days (Ruiz et al., 2010). American robins (Tardus migratorius) are known to be competent reservoirs of WNV and SLEV (Kilpatrick et al., 2006). WNV persistence was also demonstrated by the detection of viral RNA in the presence of antibody for up to 36 weeks in spleens of naturally infected house sparrows and finches (Wheeler et al., 2012). Taken together, it seems that there is decent evidence for persistent infection by WNV in some bird species. However, many details about the biology of persistence remain to be studied.
Antibodies to various flaviviruses, such as SLEV, POWV, JEV, and WNV, have been identified in chelonians, snakes, and crocodiles in different geographic locations (Steinman et al., 2003; Marschang, 2011), and it is known that alligators and crocodiles can be infected with WNV. However, there are no reports suggesting that reptiles host persistent MBFV infection.
In summary, it is apparent that various animals and birds can sponsor persistent VBFV infection. Nevertheless, the precise role that these persistent infections play in larger scheme of viral maintenance merits additional study.
Experimental models for persistent flavivirus biology
There are several animal models of persistent mosquito borne flavivirus infection, including WNV and SLEV (Charlier et al., 2004; Kimura et al., 2010). As shown in Table 2, mice and hamsters have been the main study models. In a nonhuman primate study, WNV was isolated from central nervous system tissues more than 5 months post intra cerebral inoculation (Pogodina et al., 1983), suggesting that these species might also be relevant system.
Table 2.
Experimental animal models developed for various flaviviruses
| Vector | Flavivirus | Experimental models for VBFV persistence |
| Mosquito | WNV | C57BL/6 (B6) mice and C3H/HeN (C3H) mice (Appler et al., 2010; Pierson & Diamond, 2012): WNV RNA persisted in a pantropic manner in 12% of infected mice for up to 6 months. Infectious virus could be isolated in 12% of mice for up to 4 months. C3H mice survival rate was lower and 22% when compared to the survival rate of B6 mice, which was 78% |
| Macaque rhesus (Pogodina et al., 1981): Virus persisted in asymptomatic animals for 5½ months and could be isolated from cerebellum, cerebral subcortical ganglia, lymph nodes, and kidneys | ||
| Golden hamster (Mesocricetus auratus; Tesh et al., 2005; Tonry et al., 2005): Chronic renal infection with persistent shedding of virus for up to 8 months. Virus could be recovered by culture, and genotypic and phenotypic changes were identified | ||
| House sparrow (Passer domesticus; Nemeth et al., 2009): infectious virus persisted in tissues for up to 43 days, but was not detectable in sera after 6 days. WNV RNA persisted in tissues for up to 65 days | ||
| SLEV | Golden hamster (Siirin et al., 2007): Infected animals remained asymptomatic, but virus could be cocultivated in various organs for up to 185 days | |
| JEV | Swiss albino mice (Mathur et al., 1986a, b): Persistence was demonstrated by reactivation in 41% of congenitally infected pups. In adult mice, viral persistence was shown to last longer (16 weeks) in pregnant mice compared with 4 weeks in nonpregnant mice | |
| Tick | TBEV | Macaque rhesus (Pogodina, 1983; Pogodina et al., 1984, 1981): Monkeys recovered from encephalitis and virus persisted for at least 738 days. In asymptomatic animals, virus persisted for 302 days |
| LIV | Immunosuppressed guinea pigs (Zlotnik et al., 1971): LIV was lethal in young animals, but older animals acquire a nonapparent infection with viral replication in the brain and spleen | |
| POWV | Deer mouse (Peromyscus leucopus; Telford et al., 1997): not a well characterized model, but adult mice appear to survive infection |
Louping ill virus infects sheep.
Persistent WNV has also been studied experimentally in the golden hamster. The clinical outcomes of WNV infection in hamsters vary and depend on animal age and immune competence, viral dose, and route of infection. WNV infection can lead to asymptomatic illness, encephalitis, severe paralysis, and acute death (Xiao et al., 2001; Morrey et al., 2004; Tesh et al., 2005). Adult golden hamsters infected with WNV develop chronic infection, characterized by shedding of virus in urine for up to 8 months (Xiao et al., 2001; Ding et al., 2005; Tonry et al., 2005). The chronically infected animals exhibit antigens in tubular epithelial cells, interstitial cells, and macrophages of distal renal tubules (Tesh et al., 2005). Interestingly, naïve hamsters inoculated intraperitoneally with WNV containing urine from persistently infected hamster do not develop clinical disease, but the hamsters become viremic and develop antibody responses (Ding et al., 2005). This suggests possible viral genetic changes, which may facilitate persistent infection, although other explanations cannot be totally excluded.
Hamsters have also been used as experimental models to study the persistence of SLEV and are among the natural vertebrate hosts of the Banzi flavivirus, a member of the YFV group of flaviviruses (Grard et al., 2010). Golden hamsters infected with SLEV did not develop clinical signs of illness, but they developed viruria and antibodies against SLEV at 28 days postinfection (Siirin et al., 2007). Hamsters that become persistently infected with SLEV shed virus in their urine for up to 185 days postinfection. The shedding of virus in the urine of infected hamsters also suggests that the kidneys could be an organ preferred for viral persistence.
C57BL/6J mice maintained a persistent WNV infection in the face of robust neutralizing antibody levels for more than 6 months (Appler et al., 2010; Stewart et al., 2011). In this study, infectious virus was recovered in the skin, and viral RNA was identified in the skin, as well as the spinal cord and brain (Appler et al., 2010). In contrast, WNV persistence was uncommon in kidneys and rare in the heart (Appler et al., 2010). However, in another study in which C57BL/6J mice were infected with WNV derived from the urine of persistently infected hamsters, the kidney was found to be the preferred organ for viral persistence (Saxena et al., 2013). The hamster derived WNV was also found to be highly attenuated in both neuroinvasiveness and neurovirulence in infected mice (Saxena et al., 2013). These results also suggest that the virus acquires phenotypic changes to be able to persist.
While the inbred laboratory mouse models are patently useful for understanding some aspects of flavivirus persistence, very few natural rodent hosts have been established as experimental animal models. Evidence of exposure to WNV has been reported in rodent species, such as rats (Rattus), bank voles (M. glareolus), and deer mice (Peromyscus; Molnar et al., 1976; Root et al., 2005; Docherty et al., 2006; Gomez et al., 2008). However, infectious virus was only identified in the bank vole, whereas antibodies were detected in all of the species (Molnar et al., 1976; Root et al., 2005).
Although persistence in mammals obviously plays a significant role in TBFV infections, little attention has been devoted to experimental studies of this aspect of TBFV biology. While disease or therapy models have been established for TBFVs (Kreil et al., 1997; Holbrook et al., 2005; Hayasaka et al., 2010; Palus et al., 2013), none of these specifically investigated viral persistence. Experiments to determine the susceptibility of several wild and domesticated mammals to POWV showed that viremia lasted for 0–3 days in the goat, pig, skunk, red fox, and gray fox (Yiang et al., 2013). In another study, adult deer mice (Peromoyscus leucopus) survived challenge with POWV without apparent illness, but evidence of viral persistence was not sought (Telford et al., 1997). Development of suitable experimental models for persistent TBFV infection would clearly provide useful information about this aspect of these important pathogens.
Mechanisms of flavivirus persistence
The previous sections have looked at flavivirus persistence in humans, animals, and arthropod vectors, as well as some relevant animal models. In this section, we will survey information related to the initiation and maintenance of persistence.
When mammalian cell cultures are acutely infected with TBFV, a legion of general cellular defense and antiviral systems are triggered, as are specific factors designed to limit or restrict virus reproduction. Some of these include type I interferon (IFN α/IFN β), type III interferon (IFN λ), mitochondrial activated signaling, and the induction of inflammatory factors, such as interleukins (Tam & Messner, 1999; Madden, 2003; Van Gerpen, 2003). For example, the IFN induced tripartite motif protein, TRIM79α, has been shown to restrict TBEV replication by degrading NS5 (Taylor et al., 2011). The unimpeded deployment of these antiviral factors and systems would lead to cell death. Cell death is thought to be mediated primarily through apoptosis (Růžek et al., 2009), and programmed cell death induced by various TBFVs has been described in neurons, epithelial cells, hepatocytes, Kupffer cells, and neuroblastoma cells (Ramanathan et al., 2006).
Impeding or evading the antiviral response is one characteristic of VBFVs, which plays an important role in viral persistence. During flavivirus infection, IFN production is induced within hours, but viral RNA replication complexes are enclosed in vesicles, which may offer protection from recognition by pathogen recognition receptors, thus delaying IFN production (Welsch et al., 2009; Gillespie et al., 2010; Overby et al., 2010; Offerdahl et al., 2012; Ye et al., 2013). In addition, some VBFVs can directly affect IFN secretion by inhibiting IFN gene transcription, suppressing IFN signaling or impairing the functions of interferon stimulated genes (Best et al., 2005; Robertson et al., 2009; Ye et al., 2013). The humoral and cell mediated immune responses are also prone to inhibition by VBFVs. Studies with WNV in C57BL/6 mice suggest that WNV specific antibodies correlate with decreased spread to the CNS (Diamond, 2003). Antibody escape mutants could also evade the T cell recognition (Diamond, 2003), but a precise role of these in viral persistence is yet to be defined.
The E protein is thought to be responsible for provoking the cytopathic effect (Isaeva et al., 1998; Prikhod'ko et al., 2001). However, the NS3 protease and NS2B NS3 protease precursor (Table 1) are also known to induce apoptosis by binding to caspase 8 (Shafee & AbuBakar, 2003; Ramanathan et al., 2006; Safronetz et al., 2013). In addition, NS2A has also been implicated in causing IFN independent cytopathic effect (Chang et al., 1999; Melian et al., 2013). Observations in our laboratory (L. Mlera, D. Offerdahl & M.E. Bloom, unpublished results) and those of others (Lancaster et al., 1998) indicate that TBFV infection in mammalian cell culture is characterized by an acute phase, which kills most infected cells; however, a small number of cells survive the initial cytolytic phase, and the culture is repopulated with cells almost all of which are infected. Clearly, VBFV induces an acutely cytopathic infection in mammalian cells, and thus, the development of a persistent infection implies that cell death factors must be evaded or modulated, although the precise mechanisms are still obscure.
The implication of specific viral proteins, domains, or sequences that combat cytolytic cell death has been rather limited, but any viral determinants that limit cell death in the face of acute infection might also enhance the initiation of persistent infection. For example, viral NS4A (Table 1) was shown to induce phosphatidylinositol 3 kinase (PI3K) dependent autophagy and thereby leading to protection from cell death (McLean et al., 2011). The manipulation of apoptosis by JEV, which activates PI3K in infected cells, was also reported (Lee et al., 2005). JEV triggers apoptosis during the late stages of infection, and the activation of PI3K is thought to provide protection from early cell death. Limited replication implies a low level of viral protein expression and may favor viral persistence.
Over the years, significant attention has been focused on defective interfering (DI) virus particles, which limit replication of the wild type virus (Schmaljohn & Blair, 1977; Debnath et al., 1991; Blitvich et al., 1999). The DI particles represent truncated genomes that can be replicated and encapsidated and compete with wild type viral particles when they infect cells. In vesicular stomatitis virus (VSV), DI particles are thought to modulate virulence (Cave et al., 1985). However, the role of VBFV DI particles in persistence is not completely certain. For TBEV, the C protein is reported to tolerate internal deletions ranging from 4 to 21 amino acids, and the deletions seem to favor attenuation and immunogenicity (Kofler et al., 2002). DI particles of VBFVs, such as Murray valley encephalitis (MVE), TBEV Sofjin strain, and WNV, are known to generate truncated NS1 proteins, following infection at high multiplicity of infection (Debnath et al., 1991; Poidinger et al., 1991; Chen et al., 1996; Bugrysheva et al., 2001). Persistent infection of Vero cells with MVE was associated with a truncated NS1, whereas this form of NS1 was not noted in the acute infection (Brinton, 1982; Lancaster et al., 1998). The truncated NS1 in MVE virus was a result of the presence of DI RNA, which contained a large internal deletion (Lancaster et al., 1998). In this case, MVE DI particles reduced the wild type MVE titer by 75–95% (Poidinger et al., 1991). However, a causal role for the MVE DI particles in maintaining persistent infection was not conclusively demonstrated. Studies of the Far Eastern Sofjin strain of the TBEV complex identified a 39 kDa truncated NS1 in both acutely and persistently infected human kidney RH cells (Bugrysheva et al., 2001). For WNV, naturally occurring DI particles interfere with transmission in mosquitoes and minimally impact pathogenesis in mice (Pesko et al., 2012). Furthermore, truncated DENV RNA species, suggesting the presence of DI particles, have been identified in acute human infections (Li et al., 2011), but have not been described in other acute VBFV infections in humans. Similarly, Banzi virus DI particles seem to have been generated in resistant C3H/RV mice (Smith, 1981). Although DI particles and the truncated NS1 may be frequently observed in persistent infections, it is not at all clear that they are independently sufficient for the establishment and maintenance of a persistent infection in vitro or in vivo. Stable expression of truncated NS1 failed to render persistent infection with JEV, suggesting that truncated NS1 is a consequence rather than a cause for viral persistence (Liao et al., 1998). The role of DI particles merits further investigation, but the role of other aspects of virus biology should also be scrutinized.
Restricted expression of the envelope (E) protein may also favor development of persistence. In KN73 cells that were persistently infected with JEV, the E protein was found to be expressed at markedly low levels compared with acutely infected cells (Feng et al., 2002). In these studies, the expression of NS3 was found to be unchanged in the acute and persistent phases of JEV infection (Feng et al., 2002). As the E protein is important for pathogenesis and immunity (CDC, 2009), low level expression could result in immune tolerance and contribute toward viral persistence.
In addition to low expression levels, mutations in the E protein of JEV, YFV, and WNV might also play a role in VBFV persistence (Ding et al., 2005; Farfan Ale et al., 2009). A 138E→K mutation in the E protein of JEV and WNV was shown to inhibit cell–cell spread of the virus and to contribute toward the development of a small plaque phenotype (Carson et al., 2006). While this mutation leads to viral attenuation (Carson et al., 2006) and is key in the attenuation of JEV SA14 14 2 vaccine (Monath et al., 2002), further elucidation of mutations that may play a role in persistence is required.
Observations that hamsters and mice infected with MBFVs obtained from urine of other MBFV infected animals do not suffer severe disease and become persistently infected indicate that the virus is attenuated (Rosen et al., 1983; Ding et al., 2005). A number of amino acid changing mutations in C, E, NS1, NS2A, NS2B, and NS5 were reportedly associated with persistence of WNV in serially passaged hamsters (Sánchez Vargas et al., 2009). These mutations are thought to have attenuated the virus (Sánchez Vargas et al., 2009). However, the same mutations have not been reported by other groups, suggesting that the mutations may not be specific to development or maintenance of viral persistence.
Mechanisms not directly involving viral proteins may also play a significant role in persistence. For instance, JEV delays the exposure of dsRNA to innate sensors and inhibits phosphorylation of IRF3 via noncoding viral short flaviviral RNA (Espada Murao & Morita, 2011; Chang et al., 2013). Similarly, WNV delays recognition by pathogen recognition receptors by activating IRF3 in a RIG I dependent manner without antagonizing the host IFN response (Fredericksen & Gale, 2006). The mechanism of evasion is currently not clear, but membrane bound vesicles that enclose the RIG I activating dsRNA of TBEV have been described (Overby et al., 2010). As the delayed recognition of viral pathogen associated molecular patterns is only temporary, these manipulations are probably just among the suite of mechanisms deployed for the establishment of viral persistence. The specific viral genes and how they manipulate apoptosis at a cellular level needs further examination.
Specific host genes that may contribute toward the development of flavivirus persistence in the vertebrate host have not been defined, but there are some suggestions. For instance, the overexpression of the proto oncogene Bcl 2 was reported to prevent apoptosis and promote persistence in JEV infected BHK and CHO cells (Liao et al., 1998), suggesting that the control of apoptosis is likely to be implicated. In addition, some inbred laboratory mice strains can carry the 2′ 5′ oligoadenylate synthetase gene, Flvr, which confers flavivirus resistance (Urosevic et al., 1997). The animals are productively infected with flaviviruses, but produce low virus titer (Brinton & Perelygin, 2003; Barkhash et al., 2010). Mice that are susceptible to flavivirus infection carry the Flvs allele. The Flvr like allele has also been characterized in wild mice and could be a partial explanation of flavivirus persistence in rodents in nature (Urosevic et al., 1997). Additional vertebrate genes, apart from the Flv, could play a role in varied susceptibilities of different mouse strains to flavivirus infection. This was suggested from recent observations that TBEV infected BALBc mice were moderately resistant, STS mice are highly resistant, whereas the BALBc/STS recombinant mice were highly sensitive to infection (Palus et al., 2013).
Immunosuppression may also be a potentiating factor for the establishment of flavivirus persistence in animal hosts. For example, WNV persistence was demonstrated by the detection of WNV RNA and immunohistochemistry in brain tissue of an immunosuppressed 57 year old man 4 months after the initial diagnosis (Penn et al., 2006). The follicular B cell lymphoma, from which he had suffered, may have facilitated, or aggravated, the persistence of WNV. In mice, transient immunosuppression with cyclophosphamide leads to WNV recrudescence (Appler et al., 2010), an observation suggesting that some aspects of the immune system operate to restrict WNV replication during persistence. However, the situation is complicated because WNV is able to persist for up to 16 months in the face of a robust humoral immune response in C57BL/6 mice (Appler et al., 2010). Furthermore, WNV persistence was reported in the brains of CD8+ T cell deficient rodents (Shrestha & Diamond, 2004), but the CD8+ T cell deficiency did not affect the antibody response in this mouse model. Knowledge about specific immune system components that could facilitate or control viral persistence remains to be characterized.
Infection by VBFVs of arthropod cells has not received the same degree of study; however, acute infection is not accompanied by the same cytopathic response observed in mammalian cells. In addition, very little is known about how or in what tissues TBFVs persist in the ticks, but the viruses likely evolved mechanisms of modulating or evading the tick immune system over the millenia (Robertson et al., 2009). Persistent infection of the I. scapularis cell line ISE 6 (Munderloh et al., 1994) was readily established and was noncytopathic (Offerdahl et al., 2012). Detailed studies of persistent TBFV infection in arthropod cell lines using contemporary techniques and methods are certain to yield useful and interesting information.
The mechanism(s) of how MBFVs persist in mosquitoes also remains a suitable topic for investigation. SLEV persists in the midgut of C. pipiens for hours before infecting the midgut epithelium (Whitfield et al., 1973; Brackney et al., 2008a), but this is a short time. Interestingly, approximately two thirds of flavivirus related sequences were reportedly detected as integrated dsDNA form in laboratory bred and wild Aedes mosquitoes (Crochu et al., 2004), although detection of a complete flavivirus genome was not reported. Furthermore, the finding of partial flavivirus like sequences in DNA form is not clear. Clearly, research into the biology of flavivirus persistence in mosquitoes and ticks has been limited and is worthy of extensive additional research.
In summary, it should be apparent that both viral and host factors play a role in the initiation and maintenance of persistent infection at both the cellular and organismal levels. In addition, successful persistence in nature almost certainly depends upon ecological and environmental forces, but these latter factors are wholly beyond the scope of this limited review.
Lessons from other viral systems
Multiple RNA and DNA viruses are known to establish persistence in culture as well as in humans and animals. Consideration of several may provide insight, if not direct parallels, useful in the study of biology of persistent VBFV infections.
The Hepacivirus genus, containing hepatitis C virus (HCV) species, belongs to the Flaviviridae family, together with the Flavivirus genus under which VBFVs are classified. Despite the virus specific cytotoxic T lymphocytes and antibodies, persistent HCV infections, which are established with high efficiency, are known to occur in humans and animals, such as chimpanzees (Main et al., 1979; Caini et al., 2012; McNally et al., 2012). HCV persistence is associated with various strategies, such as the high genetic variability that facilitates passive immune evasion. In vivo, HCV fails to activate CD4+ T cells, leading to exhaustion of CD8+ T cells. At cellular level, HCV can block interferon induction by blocking RIG I and mitochondrial antiviral signaling (MAVS) using its NS3 NS4A protease, which cleaves the IFN promoter stimulator 1 (Gould, 2001; Muñoz Jordán et al., 2005; Baril et al., 2009; Hudopisk et al., 2013; Perera Lecoin et al., 2013).
Hantavirus infections are another interesting example of viral persistence. Hantaviruses are segmented, RNA viruses that cause lifelong infections in their reservoir rodent hosts, despite high levels of neutralizing antibodies (Botten et al., 2000; Meyer & Schmaljohn, 2000; Easterbrook & Klein, 2008). Pathogen recognition receptors, such as RIG I and TLR7, are not elevated in the lungs of infected rats, suggesting that evasion of viral recognition may contribute toward the establishment of a persistent infection. Perhaps, the reason for noninduction of RIG I is the fact that hantaviruses do not produce detectable amounts of dsRNA (Wang et al., 2011). IFNs, such as IFN β, IFN λ, MxA, and pro inflammatory chemokines, cytokines, and transcription factor genes are elevated midway in the infection followed by a down regulation that favors the expression of TGF β (Schountz et al., 2012). A continued up regulation of the cytokines could be detrimental to the cell, and the virus would fail to persist. Results of a recently established animal model also show that host adapted SNV achieves prolonged and disseminated infection, with no disease in hamsters (Safronetz et al., 2013). Therefore, hantaviruses may have evolved mechanisms of manipulating target genes, which establishes persistence when induced.
One of the best studied models of persistent RNA virus infection is the rodent borne arenavirus, lymphocytic choriomeningitis virus (LCMV). In LCMV clone 13 (Cl13), a glutamine at position 1079 in the glycoprotein (lysine in the ARM 53b strain of LCMV) is important for persistence, but its precise function is unknown (Salvato et al., 1991; Moskophidis et al., 1995). The mechanisms of LCMV persistence are linked to the down regulation of MHC and costimulatory molecules, inflammatory cytokines, as well as virus induced production of immunosuppressive cytokines (Ng et al., 2011). The inability of CD134 deficient mice to control LCMV infection over time was reported to be a result of CD4 and CD8 responses (Boettler et al., 2012). In addition, OX40 also has a role in the establishment of persistent LMCV infection (Boettler et al., 2012). A recent report showed that IFN blockade of type I IFN signaling results in a CD4 T cell dependent clearance of LCMV Cl13 (Teijaro et al., 2013). This is an interesting observation considering that IFN induction is part of the antiviral response and that its induction can lead to apoptosis. However, the precise mechanism of persistence is not completely clear (Easterbrook & Klein, 2008), but it also demonstrates ‘clever’ viral manipulation of the host system to establish persistence.
Coxsackievirus infections are another system from which lessons might be drawn. Coxsackievirus B3 has been implicated toward the development of certain chronic muscle diseases, such as chronic inflammatory myopathy (Lodge et al., 1987; Tam & Messner, 1999). Coxsackievirus persistence in cell culture can take two forms: (1) an incurable steady state characterized by nonlytic virus infection and (2) an antiviral curable carrier culture system (Brinton, 2013; Pierson & Kielian, 2013). While mechanisms of coxsackievirus persistence are not completely understood, down regulation of the coxsackievirus receptor (CAR) has been suggested to play a role in viral persistence (Varatharaj, 2010). Down regulation of CAR in coxsackievirus infected HL 1 cells occurs rapidly from 60% following three passages to 90% at passage 8 (Varatharaj, 2010). The importance of CAR down regulation is emphasized by the fact that in vitro CAR knockout results in reduced viral replication as well as virus induced cell lysis (Tomori, 2004; Gulati & Maheshwari, 2007).
These selected examples simply highlight the diversity of possible mechanisms that the VBFV might harness in initiating and maintaining persistence and provide concepts that might be useful to investigate.
Avenues for future studies
In the preceding sections, we surveyed a substantial literature with relevance to various aspects of flavivirus persistence. Elucidation of how flaviviruses persist in humans could help toward the development of therapeutic interventions that could alleviate morbidity and budgetary burdens associated with neurological sequelae. Despite the current knowledge, a relative dearth of knowledge still exists, and additional research is merited on these significant human pathogens. For instance, the definition of specific viral proteins and cellular factors and their interactions in the establishment and maintenance of persistent infection is very limited. As noted, for flaviviruses to persist in infected cells in culture or in vivo, specific host defenses need to be evaded or controlled.
The role of NS5 as an interferon antagonist (especially in TBFVs) has been established (Best et al., 2005), but its IFN antagonism in the context of VBFV persistence has not been fully explored. This is also true for MBFV NS4B, which impairs IFN α/β induction via JAK/STAT signaling (Muñoz Jordán et al., 2005). Furthermore, mutations in NS2A of Kunjin virus result in increased IFN levels, suggesting an IFN antagonistic role of NS2A (Liu et al., 2004). Intriguing is the fact that VBFVs antagonize IFN even in the cells that will eventually die in the acute phase of infection. As IFN antagonism seems to be important for HCV, interrogating the role of these VBFV proteins to ascertain their role in the establishment of viral persistence will be critical.
The specific mammalian host immune responses that are evaded or controlled also need to be identified precisely. Although overexpression of Bcl 2 in BHK and CHO cells resulted in the inhibition of apoptosis and JEV persistence, a direct viral effect on Bcl 2 was not determined (Liao et al., 1998). It would be useful to understand which, and how, VBFV proteins interact with the Bcl 2 pathway. Indeed, other host pathways could be involved and need to be elucidated further.
The exact correlates of persistent flavivirus infection in the arachnid or insect vector host also need to be determined. The biology of ticks and that of mammals is completely different. For ixodid tick vectors, identifying genetic or biological targets that could play a role in viral persistence is imperative. These vector mechanisms might find application, when known, in efforts to control natural flavivirus persistence cycles in ticks. The mechanism(s) could involve viral genetic changes, such as specific mutations that may render the virus less detectable in infected cells. An important question for transmission and viral evolution dynamics is why arthropod vectors do not appear to be killed by viral infection.
The down regulation of the CAR, as a coevolutionary development that favors persistence, is intriguing (Fechner et al., 2007; Pinkert et al., 2011). For flaviviruses, various receptors have been identified as possible virus entry mechanisms (Smit et al., 2011; Perera Lecoin et al., 2013). Investigations into whether there is a down regulation of flavivirus receptors, as in Coxsackievirus persistence, could be useful in defining flavivirus persistence mechanism(s).
Finally, the establishment of relevant animal models of VBFV persistence will also be crucial for understanding the dynamics of viral persistence and host responses. Animals that serve as the natural host reservoirs will be key in developing these models. Some models have been established, but may have failed to answer ecologically important question. Thus, future work will combine studies encompassing the biology of VBFVs, molecular cell biology, animal models, and eventually virus–host ecology.
Acknowledgements
This work was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). We wish to acknowledge the able assistance of Anita Mora and Heather Murphy for graphic support. Danielle Offerdahl and Jennifer Lam provided assistance in manuscript preparation.
Footnotes
This is an excellent comprehensive review on flaviviruses.
Editor: Kelly Cole
References
- Achazi K, Ruzek D, Donoso Mantke O. et al (2011) Rodents as sentinels for the prevalence of tick borne encephalitis virus. Vector Borne Zoonotic Dis 11: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizaki H, Choi KS, Liu M, Li YJ, Lai MM. (2006) Polypyrimidine tract binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J Biomed Sci 13: 469–480. [DOI] [PubMed] [Google Scholar]
- Alvarez DE, Lodeiro MF, Luduena SJ, Pietrasanta LI, Gamarnik AV. (2005) Long range RNA–RNA interactions circularize the dengue virus genome. J Virol 79: 6631–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, Andreadis TG, Main AJ, Kline DL. (2004) Prevalence of West Nile virus in tree canopy inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg 71: 112–119. [PubMed] [Google Scholar]
- Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. (2010) Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One 5: e10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenberg R, Mastrangelo E, Walter TS, Verma A, Milani M, Owens RJ, Stuart DI, Grimes JM, Mancini EJ. (2009) Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J Virol 83: 12895–12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P, Punyadee N, Noisakran S. et al (2006) Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 193: 1078–1088. [DOI] [PubMed] [Google Scholar]
- Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, Diamond MS, Atkinson JP. (2010) Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med 207: 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril M, Racine M E, Penin F, Lamarre D. (2009) MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol 83: 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhash AV, Perelygin AA, Babenko VN, Myasnikova NG, Pilipenko PI, Romaschenko AG, Voevoda MI, Brinton MA. (2010) Variability in the 2′ 5′ oligoadenylate synthetase gene cluster is associated with human predisposition to tick borne encephalitis virus induced disease. J Infect Dis 202: 1813–1818. [DOI] [PubMed] [Google Scholar]
- Bartenschlager R, Lohmann V, Wilkinson T, Koch JO. (1995) Complex formation between the NS3 serine type proteinase of the hepatitis C virus and NS4A and its importance for polyprotein maturation. J Virol 69: 7519–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty SA, Gibney KB, Staples JE. et al (2012) Evaluation for West Nile virus (WNV) RNA in urine of patients within 5 months of WNV infection. J Infect Dis 205: 1476–1477. [DOI] [PubMed] [Google Scholar]
- Beasley DW, Barrett AD. (2002) Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol 76: 13097–13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Davis CT, Whiteman M, Granwehr B, Kinney RM, Barrett AD. (2004) Molecular determinants of virulence of West Nile virus in North America. Arch Virol Suppl 18: 35–41. [DOI] [PubMed] [Google Scholar]
- Beasley DW, Whiteman MC, Zhang S, Huang CY, Schneider BS, Smith DR, Gromowski GD, Higgs S, Kinney RM, Barrett AD. (2005) Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 79: 8339–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. (2005) Inhibition of interferon stimulated JAK STAT signaling by a tick borne flavivirus and identification of NS5 as an interferon antagonist. J Virol 79: 12828–12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski L, Hindley C, Talbot SJ. (2004) A polypyrimidine tract facilitates the expression of Kaposi’s sarcoma associated herpesvirus vFLIP through an internal ribosome entry site. J Gen Virol 85: 615–620. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Mackenzie JS, Coelen RJ, Howard MJ, Hall RA. (1995) A novel complex formed between the flavivirus E and NS1 proteins: analysis of its structure and function. Arch Virol 140: 145–156. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Scanlon D, Shiell BJ, Mackenzie JS, Hall RA. (1999) Identification and analysis of truncated and elongated species of the flavivirus NS1 protein. Virus Res 60: 67–79. [DOI] [PubMed] [Google Scholar]
- Boettler T, Moeckel F, Cheng Y, Heeg M, Salek Ardakani S, Crotty S, Croft M, von Herrath MG. (2012) OX40 facilitates control of a persistent virus infection. PLoS Pathog 8: e1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati M, Alvarez K, Assenberg R. et al (2010) Structure and functionality in flavivirus NS proteins: perspectives for drug design. Antiviral Res 87: 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordignon J, Strottmann DM, Mosimann AL, Probst CM, Stella V, Noronha L, Zanata SM, Dos Santos CN. (2007) Dengue neurovirulence in mice: identification of molecular signatures in the E and NS3 helicase domains. J Med Virol 79: 1506–1517. [DOI] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. (2000) Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). P Natl Acad Sci USA 97: 10578–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Foy BD, Olson KE. (2008a) The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J Trop Med Hyg 79: 267–274. [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD. (2008b) Stable prevalence of Powassan virus in Ixodes scapularis in a Northern Wisconsin focus. Am J Trop Med Hyg 79: 971–973. [PMC free article] [PubMed] [Google Scholar]
- Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. (2004) Structure of a flavivirus envelope glycoprotein in its low pH induced membrane fusion conformation. EMBO J 23: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton MA. (1982) Characterization of West Nile virus persistent infections in genetically resistant and susceptible mouse cells. I. Generation of defective non plaquing virus particles. Virology 116: 84–98. [DOI] [PubMed] [Google Scholar]
- Brinton M. (2013) Replication cycle and molecular biology of the West Nile Virus. Viruses 6: 13–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton MA, Perelygin AA. (2003) Genetic resistance to flaviviruses. Advances in Virus Research, pp. 43–85: Academic Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- Brooks AJ, Johansson M, John AV, Xu Y, Jans DA, Vasudevan SG. (2002) The interdomain region of dengue NS5 protein that binds to the viral helicase NS3 contains independently functional importin beta 1 and importin alpha/beta recognized nuclear localization signals. J Biol Chem 277: 36399–36407. [DOI] [PubMed] [Google Scholar]
- Bugrysheva JV, Matveeva VA, Dobrikova EY, Bykovskaya NV, Korobova SA, Bakhvalova VN, Morozova OV. (2001) Tick borne encephalitis virus NS1 glycoprotein during acute and persistent infection of cells. Virus Res 76: 161–169. [DOI] [PubMed] [Google Scholar]
- Busch MP, Kleinman SH, Tobler LH. et al (2008) Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 198: 984–993. [DOI] [PubMed] [Google Scholar]
- Cagnacci F, Bolzoni L, Rosà R. et al (2012) Effects of deer density on tick infestation of rodents and the hazard of tick borne encephalitis. I: Empirical assessment. Int J Parasitol 42: 365–372. [DOI] [PubMed] [Google Scholar]
- Caini S, Szomor K, Ferenczi E, Szekelyne Gaspar A, Csohan A, Krisztalovics K, Molnar Z, Horvath J. (2012) Tick borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011. Euro Surveill 17: 20128. [PubMed] [Google Scholar]
- Carpi G, Bertolotti L, Rosati S, Rizzoli A. (2009) Prevalence and genetic variability of tick borne encephalitis virus in host seeking Ixodes ricinus in northern Italy. J Gen Virol 90: 2877–2883. [DOI] [PubMed] [Google Scholar]
- Carson PJ, Konewko P, Wold KS, Mariani P, Goli S, Bergloff P, Crosby RD. (2006) Long term clinical and neuropsychological outcomes of West Nile virus Infection. Clin Infect Dis 43: 723–730. [DOI] [PubMed] [Google Scholar]
- Cave DR, Hendrickson FM, Huang AS. (1985) Defective interfering virus particles modulate virulence. J Virol 55: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2009) West Nile virus transmission via organ transplantation and blood transfusion – Louisiana, 2008. MMWR Morb Mortal Wkly Rep 58: 1263–1267. [PubMed] [Google Scholar]
- Cecilia D, Gould EA. (1991) Nucleotide changes responsible for loss of neuroinvasiveness in Japanese encephalitis virus neutralization resistant mutants. Virology 181: 70–77. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Hahn CS, Galler R, Rice CM. (1990) Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44: 649–688. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Grakoui A, Rice CM. (1991) Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol 65: 6042–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TJ, Halevy M, Nestorowicz A, Rice CM, Lustig S. (1998) West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J Gen Virol 79: 2375–2380. [DOI] [PubMed] [Google Scholar]
- Chang KS, Luo G. (2006) The polypyrimidine tract binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res 115: 1–8. [DOI] [PubMed] [Google Scholar]
- Chang Y S, Liao C L, Tsao C H, Chen M C, Liu C I, Chen L K, Lin Y L. (1999) Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J Virol 73: 6257–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R Y, Hsu T W, Chen Y L, Liu S F, Tsai Y J, Lin Y T, Chen Y S, Fan Y H. (2013) Japanese encephalitis virus non coding RNA inhibits activation of interferon by blocking nuclear translocation of interferon regulatory factor 3. Vet Microbiol 166: 11–21. [DOI] [PubMed] [Google Scholar]
- Charlier N, Leyssen P, Clercq ED, Neyts J. (2004) Rodent models for the study of therapy against flavivirus infections. Antiviral Res 63: 67–77. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Fagbo S, Moureau G, Alqahtani MH, Temmam S, de Lamballerie X. (2007) Alkhurma hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerg Infect Dis 13: 153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LK, Liao CL, Lin CG, Lai SC, Liu CI, Ma SH, Huang YY, Lin YL. (1996) Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology 217: 220–229. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Kuo MD, Chien LJ, Hsu SL, Wang YM, Lin JH. (1997) RNA–protein interactions: involvement of NS3, NS5, and 3′ noncoding regions of Japanese encephalitis virus genomic RNA. J Virol 71: 3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Hall J, McLean R, Dunbar M, Klenk K, Bowen R, Smeraski CA. (2006) Susceptibility of greater sage grouse to experimental infection with West Nile virus. J Wildl Dis 42: 14–22. [DOI] [PubMed] [Google Scholar]
- Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. (2012) West Nile virus: biology, transmission, and human infection. Clin Microbiol Rev 25: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, Belhouchet M, Lemasson J J, de Micco P, de Lamballerie X. (2004) Sequences of flavivirus related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol 85: 1971–1980. [DOI] [PubMed] [Google Scholar]
- Crowder DW, Dykstra EA, Brauner JM, Duffy A, Reed C, Martin E, Peterson W, Carrière Y, Dutilleul P, Owen JP. (2013) West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PLoS One 8: e55006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcy A, Chaillet M, Schiering N, Villard F, Lim SP, Lefeuvre P, Erbel P. (2006) Purification and crystallization of dengue and West Nile virus NS2B NS3 complexes. Acta Crystallogr Sect F Struct Biol Cryst Commun 62: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WG, Blackwell JL, Shi PY, Brinton MA. (2007) Interaction between the cellular protein eEF1A and the 3′ terminal stem loop of West Nile virus genomic RNA facilitates viral minus strand RNA synthesis. J Virol 81: 10172–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff ER, Nofchissey RA, Cook JA, Hope AG, Tsvetkova A, Talbot SL, Ebel GD. (2013) Powassan virus in mammals, Alaska and New Mexico, USA, and Russia, 2004–2007. Emerg Infect Dis 19: 2012–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath NC, Tiernery R, Sil BK, Wills MR, Barrett AD. (1991) In vitro homotypic and heterotypic interference by defective interfering particles of West Nile virus. J Gen Virol 72: 2705–2711. [DOI] [PubMed] [Google Scholar]
- Diamond MS. (2003) Evasion of innate and adaptive immunity by flaviviruses. Immunol Cell Biol 81: 196–206. [DOI] [PubMed] [Google Scholar]
- Ding X, Wu X, Duan T, Siirin M, Guzman H, Yang Z, Tesh RB, Xiao SY. (2005) Nucleotide and amino acid changes in West Nile virus strains exhibiting renal tropism in hamsters. Am J Trop Med Hyg 73: 803–807. [PubMed] [Google Scholar]
- Dobler G, Gniel D, Petermann R, Pfeffer M. (2012) Epidemiology and distribution of tick borne encephalitis. Wien Med Wochenschr 162: 230–238. [DOI] [PubMed] [Google Scholar]
- Docherty DE, Samuel MD, Nolden CA, Egstad KF, Griffin KM. (2006) West Nile virus antibody prevalence in wild mammals, Southern Wisconsin. Emerg Infect Dis 12: 1982–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domitrovich AM, Diebel KW, Ali N, Sarker S, Siddiqui A. (2005) Role of La autoantigen and polypyrimidine tract binding protein in HCV replication. Virology 335: 72–86. [DOI] [PubMed] [Google Scholar]
- Duarte dos Santos CN, Frenkiel MP, Courageot MP, Rocha CF, Vazeille Falcoz MC, Wien MW, Rey FA, Deubel V, Despres P. (2000) Determinants in the envelope E protein and viral RNA helicase NS3 that influence the induction of apoptosis in response to infection with dengue type 1 virus. Virology 274: 292–308. [DOI] [PubMed] [Google Scholar]
- Dupuis A, II, Peters R, Prusinski M, Falco R, Ostfeld R, Kramer L. (2013) Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook JD, Klein SL. (2008) Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog 4: e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD. (2010) Update on Powassan virus: emergence of a North American tick borne flavivirus. Annu Rev Entomol 55: 95–110. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Diamond MS, Fremont DH. (2014) Structural basis of flavivirus NS1 assembly and antibody recognition. P Natl Acad Sci USA 111: 4285–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. (2002) An RNA cap (nucleoside 2′ O ) methyltransferase in the flavivirus RNA polymerase xml: crystal structure and functional characterization. EMBO J 21: 2757–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshuber S, Mandl CW. (2005) Resuscitating mutations in a furin cleavage deficient mutant of the flavivirus tick borne encephalitis virus. J Virol 79: 11813–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvang A, Melik W, Bertrand Y, Lonn M, Johansson M. (2011) Sequencing of a tick borne encephalitis virus from Ixodes ricinus reveals a thermosensitive RNA switch significant for virus propagation in ectothermic arthropods. Vector Borne Zoonotic Dis 11: 649–658. [DOI] [PubMed] [Google Scholar]
- Engel AR, Rumyantsev AA, Maximova OA, Speicher JM, Heiss B, Murphy BR, Pletnev AG. (2010) The neurovirulence and neuroinvasiveness of chimeric tick borne encephalitis/dengue virus can be attenuated by introducing defined mutations into the envelope and NS5 protein genes and the 3′ non coding region of the genome. Virology 405: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbel P, Schiering N, D'Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U. (2006) Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol 13: 372–373. [DOI] [PubMed] [Google Scholar]
- Espada Murao LA, Morita K. (2011) Delayed cytosolic exposure of Japanese encephalitis virus double stranded RNA impedes interferon activation and enhances viral dissemination in porcine cells. J Virol 85: 6736–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Seeger C. (2007) Differential effects of mutations in NS4B on West Nile virus replication and inhibition of interferon signaling. J Virol 81: 11809–11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgout B, Bray M, Schlesinger JJ, Lai CJ. (1990) Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol 64: 4356–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgout B, Pethel M, Zhang YM, Lai CJ. (1991) Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol 65: 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfan Ale JA, Loroño Pino MA, Garcia Rejon JE. et al (2009) Detection of RNA from a novel West Nile like virus and high prevalence of an insect specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg 80: 85–95. [PMC free article] [PubMed] [Google Scholar]
- Fechner H, Pinkert S, Wang X. et al (2007) Coxsackievirus B3 and adenovirus infections of cardiac cells are efficiently inhibited by vector mediated RNA interference targeting their common receptor. Gene Ther 14: 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Takegami T, Zhao G. (2002) Characterization and E protein expression of mutant strains during persistent infection of KN73 cells with Japanese encephalitis virus. Chin Med J 115: 1324–1327. [PubMed] [Google Scholar]
- Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, Gamarnik AV. (2006) A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev 20: 2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth AE, Atkins JF. (2009) A conserved predicted pseudoknot in the NS2A encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1′ may derive from ribosomal frameshifting. Virol J 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth AE, Blitvich BJ, Wills NM, Miller CL, Atkins JF. (2010) Evidence for ribosomal frameshifting and a novel overlapping gene in the genomes of insect specific flaviviruses. Virology 399: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez PM, Sessions OM, Wagner EJ, Gromeier M, Garcia Blanco MA. (2005) The polypyrimidine tract binding protein is required for efficient picornavirus gene expression and propagation. J Virol 79: 6172–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Gale M., Jr (2006) West Nile virus evades activation of interferon regulatory factor 3 through RIG I dependent and independent pathways without antagonizing host defense signaling. J Virol 80: 2913–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe P, Harris E. (2010) Interplay of RNA elements in the dengue virus 5′ and 3′ ends required for viral RNA replication. J Virol 84: 6103–6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe P, Shi PY, Harris E. (2011) The 5′ and 3′ downstream AUG region elements are required for mosquito borne flavivirus RNA replication. J Virol 85: 1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney KB, Lanciotti R, Sejvar JJ, Nugent CT, Linnen JM, Delorey MJ, Lehman JA, Boswell EN, Staples JE, Fischer M. (2011) West Nile virus RNA not detected in urine of 40 people tested 6 years after acute West Nile virus disease. J Infect Dis 203: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. (2010) The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol 84: 10438–10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard YA, Klingler KA, Higgs S. (2004) West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis 4: 109–122. [DOI] [PubMed] [Google Scholar]
- Girard YA, Popov V, Wen J, Han V, Higgs S. (2005) Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae). J Med Entomol 42: 429–444. [DOI] [PubMed] [Google Scholar]
- Glass JD, Samuels O, Rich MM. (2002) Poliomyelitis due to West Nile virus. N Engl J Med 347: 1280–1281. [DOI] [PubMed] [Google Scholar]
- Gomez A, Kilpatrick AM, Kramer LD, Dupuis AP, II, Maffei JG, Goetz SJ, Marra PP, Daszak P, Aguirre AA. (2008) Land use and West Nile virus seroprevalence in wild mammals. Emerg Infect Dis 14: 962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA. (2001) Flavivirus infections in humans. eLS, pp. 1–17. John Wiley & Sons, Ltd, NJ. [Google Scholar]
- Grard G, Moureau G, Charrel RN, Holmes EC, Gould EA, de Lamballerie X. (2010) Genomics and evolution of Aedes borne flaviviruses. J Gen Virol 91: 87–94. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Gould EA. (2007a) Direct repeats in the flavivirus 3′ untranslated region; a strategy for survival in the environment? Virology 358: 258–265. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Gould EA. (2007b) Origin and evolution of 3′UTR of flaviviruses: long direct repeats as a basis for the formation of secondary structures and their significance for virus transmission. Adv Virus Res 69: 203–248. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Venugopal K, Zanotto PM. et al (1997) Complete sequence of two tick borne flaviviruses isolated from Siberia and the UK: analysis and significance of the 5′ and 3′ UTRs. Virus Res 49: 27–39. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Frolova TV, Zhankov AI, Armesto M, Turner SL, Frolova MP, Pogodina VV, Lashkevich VA, Gould EA. (2003) Characterization of a Siberian virus isolated from a patient with progressive chronic tick borne encephalitis. J Virol 77: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Shieh WJ, Hunter S, Paddock CD, Morken T, Campbell GL, Marfin AA, Zaki SR. (2004) Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol 35: 983–990. [DOI] [PubMed] [Google Scholar]
- Gulati S, Maheshwari A. (2007) Atypical manifestations of dengue. Trop Med Int Health 12: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Gutsche I, Coulibaly F, Voss JE. et al (2011) Secreted dengue virus nonstructural protein NS1 is an atypical barrel shaped high density lipoprotein. P Natl Acad Sci USA 108: 8003–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund M, Gunther G. (2003) Tick borne encephalitis–pathogenesis, clinical course and long term follow up. Vaccine 21: S11–S18. [DOI] [PubMed] [Google Scholar]
- Haglund M, Forsgren M, Lindh G, Lindquist L. (1996) A 10 year follow up study of tick borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand J Infect Dis 28: 217–224. [DOI] [PubMed] [Google Scholar]
- Hahn CS, Hahn YS, Rice CM, Lee E, Dalgarno L, Strauss EG, Strauss JH. (1987) Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J Mol Biol 198: 33–41. [DOI] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Rosen ME, Walker ED, Tsao JI. (2011) Diverse Borrelia burgdorferi strains in a bird tick cryptic cycle. Appl Environ Microbiol 77: 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka D, Nagata N, Hasegawa H, Sata T, Takashima I, Koike S. (2010) Early mortality following intracerebral infection with the Oshima strain of tick borne encephalitis virus in a mouse model. J Vet Med Sci 72: 391–396. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Stiasny K, Puschner Auer G, Holzmann H, Allison SL, Mandl CW, Kunz C. (1994) Structural changes and functional control of the tick borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198: 109–117. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Tadano M, Men R, Lai CJ. (1996) Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224: 437–445. [DOI] [PubMed] [Google Scholar]
- Holbrook MR, Aronson JF, Campbell GA, Jones S, Feldmann H, Barrett ADT. (2005) An animal model for the tickborne flavivirus Omsk hemorrhagic fever virus. J Infect Dis 191: 100–108. [DOI] [PubMed] [Google Scholar]
- Holzmann H, Heinz FX, Mandl CW, Guirakhoo F, Kunz C. (1990) A single amino acid substitution in envelope protein E of tick borne encephalitis virus leads to attenuation in the mouse model. J Virol 64: 5156–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudopisk N, Korva M, Janet E, Simetinger M, Grgic Vite M, Gubensek J, Natek V, Kraigher A, Strle F, Avsic Zupanc T. (2013) Tick borne encephalitis associated with consumption of raw goat milk, Slovenia, 2012. Emerg Infect Dis 19: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen RR, Liggitt HD, Kelley ST, Grant R, Anderson DM, Hall RA, Tesh RB, Travassos DaRosa AP, Bielefeldt Ohmann H. (2006) West Nile and St Louis encephalitis virus antibody seroconversion, prevalence, and persistence in naturally infected pig tailed macaques (Macaca nemestrina). Clin Vaccine Immunol 13: 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A. (2002) Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control. Japanese Encephalitis and West Nile Viruses (Mackenzie J, Barrett AT, Deubel V, eds), pp. 139–152. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Isaeva MP, Leonova GN, Kozhemiako VB, Borisevich VG, Maistrovskaia OS, Rasskazov VA. (1998) Apoptosis as a mechanism for the cytopathic action of tick borne encephalitis virus. Vopr Virusol 43: 182–186. [PubMed] [Google Scholar]
- Jacobson ER, Ginn PE, Troutman JM, Farina L, Stark L, Klenk K, Burkhalter KL, Komar N. (2005) West Nile virus infection in farmed American alligators (Alligator mississippiensis) in Florida. J Wildl Dis 41: 96–106. [DOI] [PubMed] [Google Scholar]
- Jaenson T, Hjertqvist M, Bergstrom T, Lundkvist A. (2012) Why is tick borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors 5: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yao H, Duan X, Lu X, Liu Y. (2009) Polypyrimidine tract binding protein influences negative strand RNA synthesis of dengue virus. Biochem Biophys Res Commun 385: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Brooks AJ, Jans DA, Vasudevan SG. (2001) A small region of the dengue virus encoded RNA dependent RNA polymerase, NS5, confers interaction with both the nuclear transport receptor importin beta and the viral helicase, NS3. J Gen Virol 82: 735–745. [DOI] [PubMed] [Google Scholar]
- Jones CT, Ma L, Burgner JW, Groesch TD, Post CB, Kuhn RJ. (2003) Flavivirus capsid is a dimeric alpha helical protein. J Virol 77: 7143–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Kar K, Anthony K, Gould LH, Ledizet M, Fikrig E, Marasco WA, Koski RA, Modis Y. (2006) Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J Virol 80: 11000–11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoora H, Signsb K, Somsela P, Downesa FP, Clarka PA, Masseya JP. (2004) Persistence of West Nile Virus (WNV) IgM antibodies in cerebrospinal fluid from patients with CNS disease. J Clin Virol 31: 289–291. [DOI] [PubMed] [Google Scholar]
- Khromykh AA, Kondratieva N, Sgro JY, Palmenberg A, Westaway EG. (2003) Significance in replication of the terminal nucleotides of the flavivirus genome. J Virol 77: 10623–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. (2006) Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond B Biol Sci 273: 2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Yun SI, Song BH, Hahn YS, Lee CH, Oh HW, Lee YM. (2008) A single N linked glycosylation site in the Japanese encephalitis virus prM protein is critical for cell type specific prM protein biogenesis, virus particle release, and pathogenicity in mice. J Virol 82: 7846–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Sasaki M, Okumur M, Kim E, Sawa H. (2010) Flavivirus encephalitis: pathological aspects of mouse and other animal models. Vet Pathol 47: 806–818. [DOI] [PubMed] [Google Scholar]
- Klaus C, Beer M, Saier R, Schau U, Moog U, Hoffmann B, Diller R, Suss J. (2012) Goats and sheep as sentinels for tick borne encephalitis (TBE) virus epidemiological studies in areas endemic and non endemic for TBE virus in Germany. Ticks Tick Borne Dis 3: 27–37. [DOI] [PubMed] [Google Scholar]
- Knap N, Korva M, Dolinsek V, Sekirnik M, Trilar T, Avsic Zupanc T. (2012) Patterns of tick borne encephalitis virus infection in rodents in Slovenia. Vector Borne Zoonotic Dis 12: 236–242. [DOI] [PubMed] [Google Scholar]
- Kofler RM, Heinz FX, Mandl CW. (2002) Capsid protein C of tick borne encephalitis virus tolerates large internal deletions and is a favorable target for attenuation of virulence. J Virol 76: 3534–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler RM, Hoenninger VM, Thurner C, Mandl CW. (2006) Functional analysis of the tick borne encephalitis virus cyclization elements indicates major differences between mosquito borne and tick borne flaviviruses. J Virol 80: 4099–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Ebel GD. (2003) Dynamics of flavivirus infection in mosquitoes. Advances in Virus Research, Vol 60 (Chambers TJMonath TP, eds), pp. 187–232. Elsevier Academic Press, UK. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. (1997) Antibodies protect mice against challenge with tick borne encephalitis virus (TBEV) infected macrophages. Clin Exp Immunol 110: 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn RJ, Zhang W, Rossmann MG. et al (2002) Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda M, Danielova V, Jones LD, Nuttall PA. (1993a) Amplification of tick borne encephalitis virus infection during co feeding of ticks. Med Vet Entomol 7: 339–342. [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Danielova V, Nuttall PA. (1993b) Efficient transmission of tick borne encephalitis virus between cofeeding ticks. J Med Entomol 30: 295–299. [DOI] [PubMed] [Google Scholar]
- Labuda M, Austyn JM, Zuffova E, Kozuch O, Fuchsberger N, Lysy J, Nuttall PA. (1996) Importance of localized skin infection in tick borne encephalitis virus transmission. Virology 219: 357–366. [DOI] [PubMed] [Google Scholar]
- Lancaster MU, Hodgetts SI, Mackenzie JS, Urosevic N. (1998) Characterization of defective viral RNA produced during persistent infection of Vero cells with Murray Valley encephalitis virus. J Virol 72: 2474–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Hall RA, Lobigs M. (2004) Common E protein determinants for attenuation of glycosaminoglycan binding variants of Japanese encephalitis and West Nile viruses. J Virol 78: 8271–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Liao CL, Lin YL. (2005) Flavivirus activates phosphatidylinositol 3 kinase signaling to block caspase dependent apoptotic cell death at the early stage of virus infection. J Virol 79: 8388–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Wright PJ, Davidson A, Lobigs M. (2006) Virulence attenuation of Dengue virus due to augmented glycosaminoglycan binding affinity and restriction in extraneural dissemination. J Gen Virol 87: 2791–2801. [DOI] [PubMed] [Google Scholar]
- Lescar J, Luo D, Xu T, Sampath A, Lim SP, Canard B, Vasudevan SG. (2008) Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from Dengue virus as a target. Antiviral Res 80: 94–101. [DOI] [PubMed] [Google Scholar]
- Li D, Lott WB, Lowry K, Jones A, Thu HM, Aaskov J. (2011) Defective interfering viral particles in acute Dengue infections. PLoS One 6: e19447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CL, Lin YL, Shen SC, Shen JY, Su HL, Huang YL, Ma SH, Sun YC, Chen KP, Chen LK. (1998) Antiapoptotic but not antiviral function of human bcl 2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J Virol 72: 9844–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Amberg SM, Chambers TJ, Rice CM. (1993) Cleavage at a novel site in the NS4A region by the yellow fever virus NS2B 3 proteinase is a prerequisite for processing at the downstream 4A/4B signalase site. J Virol 67: 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Liao CL, Lin E, Lin YL. (2004) Blocking of the alpha interferon induced Jak Stat signaling pathway by Japanese encephalitis virus infection. J Virol 78: 9285–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Lin KH, Lyu PC, Chen WJ. (2006) Japanese encephalitis virus NS2B NS3 protease binding to phage displayed human brain proteins with the domain of trypsin inhibitor and basic region leucine zipper. Virus Res 116: 106–113. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, Thiel H J, Rice CM. (2007). Fields Virology, Vol 1 (Knipe DM, Howley PM, eds), pp. 1153–1252. Lippincott Raven, Philadelphia. [Google Scholar]
- Liu WJ, Chen HB, Wang XJ, Huang H, Khromykh AA. (2004) Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus non structural protein NS2A in inhibition of beta interferon promoter driven transcription. J Virol 78: 12225–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Wang XJ, Mokhonov VV, Shi PY, Randall R, Khromykh AA. (2005) Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J Virol 79: 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. (2006) A single amino acid substitution in the West Nile virus non structural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol 80: 2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wimmer E, Paul AV. (2009) Cis acting RNA elements in human and animal plus strand RNA viruses. Biochim Biophys Acta 1789: 495–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodeiro MF, Filomatori CV, Gamarnik AV. (2009) Structural and functional studies of the promoter element for dengue virus RNA replication. J Virol 83: 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge PA, Herzum M, Olszewski J, Huber SA. (1987) Coxsackievirus B 3 myocarditis Acute and chronic forms of the disease caused by different immunopathogenic mechanisms. Am J Pathol 128: 455–463. [PMC free article] [PubMed] [Google Scholar]
- Lorenz IC, Allison SL, Heinz FX, Helenius A. (2002) Folding and dimerization of tick borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol 76: 5480–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. (1998) Subcellular localization and some biochemical properties of the flavivirus Kunjin non structural proteins NS2A and NS4A. Virology 245: 203–215. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Barrett ADT, Deubel V. (2002) The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. Japanese Encephalitis and West Nile Viruses (Mackenzie J, Barrett AT, Deubel V, eds), pp. 1–10. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10: S98–S109. [DOI] [PubMed] [Google Scholar]
- Madden K. (2003) West Nile virus infection and its neurological manifestations. Clin Med Res 1: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AJ, Carey AB, Downs WG. (1979) Powassan virus in Ixodes cookei and Mustelidae in New England. J Wildl Dis 15: 585–591. [DOI] [PubMed] [Google Scholar]
- Malet H, Egloff MP, Selisko B. et al (2007) Crystal structure of the RNA polymerase domain of the West Nile virus non structural protein 5. J Biol Chem 282: 10678–10689. [DOI] [PubMed] [Google Scholar]
- Mandl CW. (2005) Steps of the tick borne encephalitis virus replication cycle that affect neuropathogenesis. Virus Res 111: 161–174. [DOI] [PubMed] [Google Scholar]
- Mandl CW, Holzmann H, Meixner T, Rauscher S, Stadler PF, Allison SL, Heinz FX. (1998) Spontaneous and engineered deletions in the 3′ noncoding region of tick borne encephalitis virus: construction of highly attenuated mutants of a flavivirus. J Virol 72: 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. (2009) Tick borne encephalitis virus – a review of an emerging zoonosis. J Gen Virol 90: 1781–1794. [DOI] [PubMed] [Google Scholar]
- Markoff L. (2003) 5′ and 3′ noncoding regions in flavivirus RNA. Adv Virus Res 59: 177–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschang RE. (2011) Viruses infecting reptiles. Viruses 3: 2087–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina BEE, Koraka P, Osterhaus ADME. (2009) Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 22: 564–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo E, Pezzullo M, De Burghgraeve T. et al (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother 67: 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Arora KL, Rawat S, Chaturvedi UC. (1986a) Japanese encephalitis virus latency following congenital infection in mice. J Gen Virol 67: 945–947. [DOI] [PubMed] [Google Scholar]
- Mathur A, Arora KL, Rawat S, Chaturvedi UC. (1986b) Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol 67: 381–385. [DOI] [PubMed] [Google Scholar]
- Mazzon M, Jones M, Davidson A, Chain B, Jacobs M. (2009) Dengue virus NS5 inhibits interferon alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J Infect Dis 200: 1261–1270. [DOI] [PubMed] [Google Scholar]
- McGee CE, Shustov AV, Tsetsarkin K, Frolov IV, Mason PW, Vanlandingham DL, Higgs S. (2010) Infection, dissemination, and transmission of a West Nile virus green fluorescent protein infectious clone by Culex pipiens quinquefasciatus mosquitoes. Vector Borne Zoonotic Dis 10: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie V, Goulet N. (2010) Bird community composition linked to human West Nile virus cases along the Colorado Front Range. EcoHealth 7: 439–447. [DOI] [PubMed] [Google Scholar]
- McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. (2011) Flavivirus NS4A induced autophagy protects cells against death and enhances virus replication. J Biol Chem 286: 22147–22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn PC. (1997) The molecular basis of virulence of the encephalitogenic flaviviruses. J Gen Virol 78: 2711–2722. [DOI] [PubMed] [Google Scholar]
- McNally KL, Mitzel DN, Anderson JM, Ribeiro JMC, Valenzuela JG, Myers TG, Godinez A, Wolfinbarger JB, Best SM, Bloom ME. (2012) Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick Borne Dis 3: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melian EB, Hinzman E, Nagasaki T. et al (2010) NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frame shifting and plays a role in viral neuroinvasiveness. J Virol 84: 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melian EB, Edmonds JH, Nagasaki TK, Hinzman E, Floden N, Khromykh AA. (2013) West Nile virus NS2A protein facilitates virus induced apoptosis independently of interferon response. J Gen Virol 94: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ, Schmaljohn CS. (2000) Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol 8: 61–67. [DOI] [PubMed] [Google Scholar]
- Miller S, Sparacio S, Bartenschlager R. (2006) Subcellular localization and membrane topology of the Dengue virus type 2 Non structural protein 4B. J Biol Chem 281: 8854–8863. [DOI] [PubMed] [Google Scholar]
- Miller S, Kastner S, Krijnse Locker J, Buhler S, Bartenschlager R. (2007) The non structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K regulated manner. J Biol Chem 282: 8873–8882. [DOI] [PubMed] [Google Scholar]
- Miorin L, Maiuri P, Hoenninger VM, Mandl CW, Marcello A. (2008) Spatial and temporal organization of tick borne encephalitis flavivirus replicated RNA in living cells. Virology 379: 64–77. [DOI] [PubMed] [Google Scholar]
- Mitzel DN, Wolfinbarger JB, Long RD, Masnick M, Best SM, Bloom ME. (2007) Tick borne flavivirus infection in Ixodes scapularis larvae: development of a novel method for synchronous viral infection of ticks. Virology 365: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. (2003) A ligand binding pocket in the dengue virus envelope glycoprotein. P Natl Acad Sci USA 100: 6986–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. (2005) Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 79: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar E, Gulyas MS, Kubinyi L, Nosek J, Kozuch O, Ernek E, Labuda M, Grulich I. (1976) Studies on the occurrence of tick borne encephalitis in Hungary. Acta Vet Acad Sci Hung 26: 419–437. [PubMed] [Google Scholar]
- Monath TP, Arroyo J, Levenbook I, Zhang Z X, Catalan J, Draper K, Guirakhoo F. (2002) Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J Virol 76: 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SP, Brown JA, Kuehnert M, Smith TL, Crall N, Lanciotti RS, Macedo de Oliveira A, Boo T, Marfin AA. (2006) Transfusion associated transmission of West Nile virus, United States 2003 through 2005. Transfusion 46: 2038–2046. [DOI] [PubMed] [Google Scholar]
- Morchang A, Yasamut U, Netsawang J. et al (2011) Cell death gene expression profile: role of RIPK2 in dengue virus mediated apoptosis. Virus Res 156: 25–34. [DOI] [PubMed] [Google Scholar]
- Mori Y, Okabayashi T, Yamashita T. et al (2005) Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J Virol 79: 3448–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, Cheney CD, Blatt LM. (2004) Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res 63: 41–50. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Battegay M, van den Broek M, Laine E, Hoffmann Rohrer U, Zinkernagel RM. (1995) Role of virus and host variables in virus persistence or immunopathological disease caused by a non cytolytic virus. J Gen Virol 76: 381–391. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Liu Y, Wang M, Chen C, Kurtti TJ. (1994) Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol 80: 533–543. [PubMed] [Google Scholar]
- Muñoz Jordán JL, Sanchez Burgos GG, Laurent Rolle M, Garcia Sastre A. (2003) Inhibition of interferon signaling by dengue virus. P Natl Acad Sci USA 100: 14333–14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz Jordán JL, Laurent Rolle M, Ashour J, Martinez Sobrido L, Ashok M, Lipkin WI, Garcia Sastre A. (2005) Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol 79: 8004–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz Jordán JL, Collins CS, Vergne E, Santiago GA, Petersen L, Sun W, Linnen JM. (2009) Highly sensitive detection of dengue virus nucleic acid in samples from clinically ill patients. J Clin Microbiol 47: 927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fisher Hoch S. (2010) Persistent infection with West Nile virus years after initial infection. J Infect Dis 201: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth N, Young G, Ndaluka C, Bielefeldt Ohmann H, Komar N, Bowen R. (2009) Persistent West Nile virus infection in the house sparrow. (Passer domesticus). Arch Virol 154: 783–789. [DOI] [PubMed] [Google Scholar]
- Netsawang J, Noisakran S, Puttikhunt C, Kasinrerk W, Wongwiwat W, Malasit P, Yenchitsomanus PT, Limjindaporn T. (2010) Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res 147: 275–283. [DOI] [PubMed] [Google Scholar]
- Ng CT, Sullivan BM, Oldstone MB. (2011) The role of dendritic cells in viral persistence. Curr Opin Virol 1: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Barrett AD. (1996) Molecular differences between wild type Japanese encephalitis virus strains of high and low mouse neuroinvasiveness. J Gen Virol 77: 1449–1455. [DOI] [PubMed] [Google Scholar]
- Nosek J, Korolev MB, Chunikhin SP, Kozuch O, Ciampor F. (1984) The replication and eclipse phase of the tick borne encephalitis virus in Dermacentor reticulatus. Folia Parasitol 31: 187–189. [PubMed] [Google Scholar]
- Nuttall PA, Labuda M. (2003) Dynamics of infection in tick vectors and at the tick–host interface. Adv Virus Res, 60: 233–272. Elsevier Academic Press. [DOI] [PubMed] [Google Scholar]
- Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. (2006) Crystal structure of the West Nile virus envelope glycoprotein. J Virol 80: 11467–11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. (2012) A three dimensional comparison of tick borne flavivirus infection in mammalian and tick cell lines. PLoS One 7: e47912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W, Yang MR, Lee EW, Park KM, Pyo S, Yang JS, Lee HW, Song J. (2006) Jab1 mediates cytoplasmic localization and degradation of West Nile virus capsid protein. J Biol Chem 281: 30166–30174. [DOI] [PubMed] [Google Scholar]
- Overby AK, Popov VL, Niedrig M, Weber F. (2010) Tick borne encephalitis virus delays interferon induction and hides its double stranded RNA in intracellular membrane vesicles. J Virol 84: 8470–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palus M, Vojtiskova J, Salat J, Kopecky J, Grubhoffer L, Lipoldova M, Demant P, Ruzek D. (2013) Mice with different susceptibility to tick borne encephalitis virus infection show selective neutralizing antibody response and inflammatory reaction in the central nervous system. J Neuroinflammation 10: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GS, Morris KL, Hallett RG, Bloom ME, Best SM. (2007) Identification of residues critical for the interferon antagonist function of Langat virus NS5 reveals a role for the RNA dependent RNA polymerase domain. J Virol 81: 6936–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Landt O, Kaiser M, Faye O, Koppe T, Lass U, Sall AA, Niedrig M. (2013) Development of one step quantitative reverse transcription PCR for the rapid detection of flaviviruses. Virol J 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pealer LN, Marfin AA, Petersen LR. et al (2003) Transmission of West Nile Virus through blood transfusion in the United States in 2002. N Engl J Med 349: 1236–1245. [DOI] [PubMed] [Google Scholar]
- Penn RG, Guarner J, Sejvar JJ, Hartman H, McComb RD, Nevins DL, Bhatnagar J, Zaki SR. (2006) Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis 42: 680–683. [DOI] [PubMed] [Google Scholar]
- Perera Lecoin M, Meertens L, Carnec X, Amara A. (2013) Flavivirus entry receptors: an update. Viruses 6: 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko KN, Fitzpatrick KA, Ryan EM, Shi PY, Zhang B, Lennon NJ, Newman RM, Henn MR, Ebel GD. (2012) Internally deleted WNV genomes isolated from exotic birds in New Mexico: function in cells, mosquitoes, and mice. Virology 427: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC. (2010) Modeling antibody enhanced Dengue virus infection and disease in mice: protection or pathogenesis? Cell Host Microbe 7: 85–86. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Diamond MS. (2012) Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Kielian M. (2013) Flaviviruses: braking the entering. Curr Opin Virol 3: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert S, Klingel K, Lindig V, Dörner A, Zeichhardt H, Spiller OB, Fechner H. (2011) Virus host co evolution in a persistent Coxsackievirus B3 infected cardiomyocyte cell line. J Virol 85: 13409–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnev AG. (2001) Infectious cDNA clone of attenuated Langat tick borne flavivirus (strain E5) and a 3′ deletion mutant constructed from it exhibit decreased neuroinvasiveness in immunodeficient mice. Virology 282: 288–300. [DOI] [PubMed] [Google Scholar]
- Pogodina VV. (1983) Persistence of the tick borne encephalitis virus and its consequences. Vest Akad Med Nauk SSSR 5: 67–73. [PubMed] [Google Scholar]
- Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Levina LS, Mamonenko LL, Koreshkova GV, Ralf NM. (1981) Persistence of tick borne encephalitis virus in monkeys I Features of experimental infection. Acta Virol 25: 337–343. [PubMed] [Google Scholar]
- Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM. (1983) Study on West Nile virus persistence in monkeys. Arch Virol 75: 71–86. [DOI] [PubMed] [Google Scholar]
- Pogodina VV, Bochkova NG, Levina LS. (1984) Persistence of tick borne encephalitis virus in monkeys VII Some features of the immune response. Acta Virol 28: 407–415. [PubMed] [Google Scholar]
- Poidinger M, Coelen RJ, Mackenzie JS. (1991) Persistent infection of Vero cells by the flavivirus Murray Valley encephalitis virus. J Gen Virol 72: 573–578. [DOI] [PubMed] [Google Scholar]
- Prikhod'ko GG, Prikhod'ko EA, Cohen JI, Pletnev AG. (2001) Infection with Langat flavivirus or expression of the envelope protein induces apoptotic cell death. Virology 286: 328–335. [DOI] [PubMed] [Google Scholar]
- Prikhod'ko GG, Prikhod'ko EA, Pletnev AG, Cohen JI. (2002) Langat flavivirus protease NS3 binds caspase 8 and induces apoptosis. J Virol 76: 5701–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince HE, Lape Nixon M, Yeh C, Tobler LH, Busch MP. (2008) Persistence of antibodies to West Nile virus nonstructural protein 5. J Clin Virol 43: 102–106. [DOI] [PubMed] [Google Scholar]
- Pryor MJ, Rawlinson SM, Butcher RE, Barton CL, Waterhouse TA, Vasudevan SG, Bardin PG, Wright PJ, Jans DA, Davidson AD. (2007) Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta recognized nuclear localization sequences is integral to viral infection. Traffic 8: 795–807. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Beltramo T, Torre D. (2007) Seroprevalence study of tick borne encephalitis, Borrelia burgdorferi, Dengue and Toscana virus in Turin Province. Cell Biochem Funct 25: 185–188. [DOI] [PubMed] [Google Scholar]
- Rajagopalan PK, Paul SD, Sreenivasan MA. (1969) Isolation of Kyasanur forest disease virus from the insectivorous bat, Rhinolophus rouxi and from Ornithodoros ticks. Indian J Med Res 57: 805–808. [PubMed] [Google Scholar]
- Ramanathan MP, Chambers JA, Pankhong P, Chattergoon M, Attatippaholkun W, Dang K, Shah N, Weiner DB. (2006) Host cell killing by the West Nile Virus NS2B NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase 8 based apoptotic pathway. Virology 345: 56–72. [DOI] [PubMed] [Google Scholar]
- Ravi V, Desai AS, Shenoy PK, Satishchandra P, Chandramuki A, Gourie Devi M. (1993) Persistence of Japanese encephalitis virus in the human nervous system. J Med Virol 40: 326–329. [DOI] [PubMed] [Google Scholar]
- Rawlinson SM, Pryor MJ, Wright PJ, Jans DA. (2009) CRM1 mediated nuclear export of dengue virus RNA polymerase NS5 modulates interleukin 8 induction and virus production. J Biol Chem 284: 15589–15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi Wagner P, Kund M, Zent O, Dvorak G, Jaehnig P, Holzmann H, Mikolasek A, Kollaritsch H. (2004) Persistence of protective immunity following vaccination against tick borne encephalitis – longer than expected? Vaccine 22: 2743–2749. [DOI] [PubMed] [Google Scholar]
- Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. (1995) The envelope glycoprotein from tick borne encephalitis virus at 2 A resolution. Nature 375: 291–298. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Mitzel DN, Taylor RT, Best SM, Bloom ME. (2009) Tick borne flaviviruses: dissecting host immune responses and virus countermeasures. Immunol Res 43: 172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal J, Westaway EG, Khromykh A, Mackenzie JM. (2006) Regulated cleavages at the West Nile virus NS4A 2K NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein. J Virol 80: 4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JJ, Hall JS, McLean RG, Marlenee NL, Beaty BJ, Gansowski J, Clark L. (2005) Serologic evidence of exposure of wild animals to flaviviruses in the central and eastern United States. Am J Trop Med Hyg 72: 622–630. [PubMed] [Google Scholar]
- Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. (1983) Transovarial transmission of Dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. Am J Trop Med Hyg 32: 1108–1119. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Chaves L, Hamer G, Su T, Brown W, Walker E, Haramis L, Goldberg T, Kitron U. (2010) Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžek D, Vancová M, Tesařová M, Ahantarig A, Kopecký J, Grubhoffer L. (2009) Morphological changes in human neural cells following tick borne encephalitis virus infection. J Gen Virol 90: 1649–1658. [DOI] [PubMed] [Google Scholar]
- Ryan MD, Monaghan S, Flint M. (1998) Virus encoded proteinases of the Flaviviridae. J Gen Virol 79: 947–959. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Pergam SA, Harrington JA. et al (2010) Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol 32: 81–87. [DOI] [PubMed] [Google Scholar]
- Safronetz D, Prescott J, Haddock E, Scott DP, Feldmann H, Ebihara H. (2013) Hamster adapted Sin Nombre virus causes disseminated infection and efficiently replicates in pulmonary endothelial cells without signs of disease. J Virol 87: 4778–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M, Borrow P, Shimomaye E, Oldstone MB. (1991) Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T lymphocyte response and establishment of persistence. J Virol 65: 1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez IJ, Ruiz BH. (1996) A single nucleotide change in the E protein gene of dengue virus 2 Mexican strain affects neurovirulence in mice. J Gen Virol 77: 2541–2545. [DOI] [PubMed] [Google Scholar]
- Sánchez Seco MP, Rosario D, Domingo C, Hernández L, Valdés K, Guzmán MG, Tenorio A. (2005) Generic RT nested PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods 126: 101–109. [DOI] [PubMed] [Google Scholar]
- Sánchez Vargas I, Scott JC, Poole Smith BK, Franz AWE, Barbosa Solomieu V, Wilusz J, Olson KE, Blair CD. (2009) Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathog 5: e1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Xie G, Li B. et al (2013) A hamster derived West Nile virus isolate induces persistent renal infection in mice. PLoS Negl Trop Dis 7: e2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C, Blair CD. (1977) Persistent infection of cultured mammalian cells by Japanese encephalitis virus. J Virol 24: 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schountz T, Acuna Retamar M, Feinstein S, Prescott J, Torres Perez F, Podell B, Peters S, Ye C, Black WC, Hjelle B. (2012) Kinetics of immune responses in deer mice experimentally infected with Sin Nombre virus. J Virol 86: 10015–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee N, AbuBakar S. (2003) Dengue virus type 2 NS3 protease and NS2B NS3 protease precursor induce apoptosis. J Gen Virol 84: 2191–2195. [DOI] [PubMed] [Google Scholar]
- Sharma S, Mathur A, Prakash V, Kulshreshtha R, Kumar R, Chaturvedi UC. (1991) Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol 85: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ST, Lai MM. (2005) Viral and cellular proteins involved in coronavirus replication. Curr Top Microbiol Immunol 287: 95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. (2004) Role of CD8+ T cells in control of West Nile virus infection. J Virol 78: 8312–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siirin MT, Duan T, Lei H, Guzman H, da Rosa AP, Watts DM, Xiao SY, Tesh RB. (2007) Chronic St Louis encephalitis virus infection in the golden hamster (Mesocricetus auratus). Am J Trop Med Hyg 76: 299–306. [PubMed] [Google Scholar]
- Smit J, Moesker B, Rodenhuis Zybert I, Wilschut J. (2011) Flavivirus cell entry and membrane fusion. Viruses 3: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL. (1981) Genetic resistance to lethal flavivirus encephalitis: effect of host age and immune status and route of inoculation on production of interfering Banzi virus in vivo. Am J Trop Med Hyg 30: 1319–1323. [DOI] [PubMed] [Google Scholar]
- Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TTN, Ha DQ, Day NPJ, Nisalak A, Vaughn DW, White NJ. (1998) Poliomyelitis like illness due to Japanese encephalitis virus. Lancet 351: 1094–1097. [DOI] [PubMed] [Google Scholar]
- Steele KE, Linn MJ, Schoepp RJ. et al (2000) Pathology of Fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol 37: 208–224. [DOI] [PubMed] [Google Scholar]
- Steinman A, Banet Noach C, Tal S, Levi O, Simanov L, Perk S, Malkinson M, Shpigel N. (2003) West Nile virus infection in crocodiles. Emerg Infect Dis 9: 887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA. (2011) Persistence of virus specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol 12: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny K, Aberle JH, Chmelik V, Karrer U, Holzmann H, Heinz FX. (2012) Quantitative determination of IgM antibodies reduces the pitfalls in the serodiagnosis of tick borne encephalitis. J Clin Virol 54: 115–120. [DOI] [PubMed] [Google Scholar]
- Strausbaugh LJ, Marfin AA, Gubler DJ. (2001) West Nile encephalitis: an emerging disease in the United States. Clin Infect Dis 33: 1713–1719. [DOI] [PubMed] [Google Scholar]
- Sukupolvi Petty S, Austin SK, Purtha WE. et al (2007) Type and subcomplex specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 81: 12816–12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. (2007) Antiplatelet autoantibodies elicited by dengue virus non structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost 5: 2291–2299. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Takada K, Kurane I. (2001) Electron microscopic study of persistent Dengue virus infection: analysis using a cell line persistently infected with Dengue 2 virus. Intervirology 44: 48–54. [DOI] [PubMed] [Google Scholar]
- Tam PE, Messner RP. (1999) Molecular mechanisms of Coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double stranded complex without associated genomic mutations or evolution. J Virol 73: 10113–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, Best SM. (2011) TRIM79alpha, an interferon stimulated gene product, restricts tick borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe 10: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. (2013) Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A. (1997) A new tick borne encephalitis like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY. (2005) Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis 192: 287–295. [DOI] [PubMed] [Google Scholar]
- Tischendorf JJ, Beger C, Korf M, Manns MP, Kruger M. (2004) Polypyrimidine tract binding protein (PTB) inhibits hepatitis C virus internal ribosome entry site (HCV IRES) mediated translation, but does not affect HCV replication. Arch Virol 149: 1955–1970. [DOI] [PubMed] [Google Scholar]
- Tomori O. (2004) Yellow fever: the recurring plague. Crit Rev Clin Lab Sci 41: 391–427. [DOI] [PubMed] [Google Scholar]
- Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. (2005) Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg 72: 320–324. [PubMed] [Google Scholar]
- Tuplin A, Evans DJ, Buckley A, Jones IM, Gould EA, Gritsun TS. (2011) Replication enhancer elements within the open reading frame of tick borne encephalitis virus and their evolution within the Flavivirus genus. Nucleic Acids Res 39: 7034–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic N, van Maanen M, Mansfield JP, Mackenzie JS, Shellam GR. (1997) Molecular characterization of virus specific RNA produced in the brains of flavivirus susceptible and resistant mice after challenge with Murray Valley encephalitis virus. J Gen Virol 78: 23–29. [DOI] [PubMed] [Google Scholar]
- Ustinova O, Volechova G, Deviatkov M, Gusmanova A. (1997) Clinical and epidemiological features of tick borne encephalitis in Perm region. Zh Mikrobiol Epidemiol Immunobiol 3: 33–36. [PubMed] [Google Scholar]
- Valle RP, Falgout B. (1998) Mutagenesis of the NS3 protease of dengue virus type 2. J Virol 72: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk AF, Ritchie SA, Mackenzie JS. (2009) Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol 54: 17–35. [DOI] [PubMed] [Google Scholar]
- Van Gerpen JA. (2003) Neurologic sequelae of West Nile virus infection. Ochsner J 5: 18–20. [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A. (2010) Encephalitis in the clinical spectrum of dengue infection. Neruol India 58: 585–591. [DOI] [PubMed] [Google Scholar]
- Vereta LA, Skorobrekha VZ, Nikolaeva SP, Aleksandrov VI, Tolstonogova VI, Zakharycheva TA, Red'ko AP, Lev MI, Savel'eva NA. (1991) The transmission of the tick borne encephalitis virus via cow’s milk. Med Parazitol (Mosk) 3: 54–56. [PubMed] [Google Scholar]
- Villordo SM, Gamarnik AV. (2009) Genome cyclization as strategy for flavivirus RNA replication. Virus Res 139: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Vaheri A, Weber F, Plyusnin A. (2011) Old World hantaviruses do not produce detectable amounts of dsRNA in infected cells and the 5′ termini of their genomic RNAs are monophosphorylated. J Gen Virol 92: 1199–1204. [DOI] [PubMed] [Google Scholar]
- Weidmann M, Ruzek D, Krivanec K, Zoller G, Essbauer S, Pfeffer M, Zanotto PM, Hufert FT, Dobler G. (2011) Relation of genetic phylogeny and geographical distance of tick borne encephalitis virus in central Europe. J Gen Virol 92: 1906–1916. [DOI] [PubMed] [Google Scholar]
- Welsch S, Miller S, Romero Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse Locker J, Bartenschlager R. (2009) Composition and three dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme K, Wigerius M, Johansson M. (2008) Tick borne encephalitis virus NS5 associates with membrane protein scribble and impairs interferon stimulated JAK STAT signalling. Cell Microbiol 10: 696–712. [DOI] [PubMed] [Google Scholar]
- Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. (1997) Ultrastructure of Kunjin virus infected cells: colocalization of NS1 and NS3 with double stranded RNA, and of NS2B with NS3, in virus induced membrane structures. J Virol 71: 6650–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway EG, Mackenzie JM, Khromykh AA. (2003) Kunjin RNA replication and applications of Kunjin replicons. Adv Virus Res 59: 99–140. [DOI] [PubMed] [Google Scholar]
- Wheeler SS, Langevin SA, Brault AC, Woods L, Carroll BD, Reisen WK. (2012) Detection of persistent West Nile virus RNA in experimentally and naturally Infected avian hosts. Am J Trop Med Hyg 87: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield SG, Murphy FA, Sudia WD. (1973) St Louis encephalitis virus: an ultrastructural study of infection in a mosquito vector. Virology 56: 70–87. [DOI] [PubMed] [Google Scholar]
- Xiao S Y, Guzman H, Zhang H, Travassos da Rosa APA, Tesh RB. (2001) West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis 7: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Ramanathan MP, Muthuman K. et al (2002) Induction of inflammation by West Nile virus capsid through the caspase 9 apoptotic pathway. Emerg Infect Dis 8: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MR, Lee SR, Oh W. et al (2008) West Nile virus capsid protein induces p53 mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cell Microbiol 10: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Shiu SL, Chuang PH, Lin YJ, Wan L, Lan YC, Lin CW. (2009) Japanese encephalitis virus NS2B NS3 protease induces caspase 3 activation and mitochondria mediated apoptosis in human medulloblastoma cells. Virus Res 143: 77–85. [DOI] [PubMed] [Google Scholar]
- Yap TL, Xu T, Chen YL, Malet H, Egloff MP, Canard B, Vasudevan SG, Lescar J. (2007) Crystal structure of the dengue virus RNA dependent RNA polymerase catalytic domain at 185 angstrom resolution. J Virol 81: 4753–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhu B, Fu ZF, Chen H, Cao S. (2013) Immune evasion strategies of flaviviruses. Vaccine 31: 461–471. [DOI] [PubMed] [Google Scholar]
- Yiang GT, Chen YH, Chou PL, Chang WJ, Wei CW, Yu YL. (2013) The NS3 protease and helicase domains of Japanese encephalitis virus trigger cell death via caspase dependent and independent pathways. Mol Med Rep 7: 826–830. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. (2003) Structures of immature flavivirus particles. EMBO J 22: 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. (2004) Conformational changes of the flavivirus E glycoprotein. Structure 12: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Dong H, Stein DA, Iversen PL, Shi PY. (2008) West Nile virus genome cyclization and RNA replication require two pairs of long distance RNA interactions. Virology 373: 1–13. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wang Y G, Deng Y Q, Song K Y, Li X F, Wang H J, Zhu C M, Qin E D, Qin C F. (2013) Japanese encephalitis virus RNA not detected in urine. Clin Infect Dis 57: 157–158. [DOI] [PubMed] [Google Scholar]
- Zlotnik I, Carter GB, Grant DP. (1971) The persistence of louping ill virus in immunosuppressed guinea pigs. Br J Exp Pathol 52: 395–407. [PMC free article] [PubMed] [Google Scholar]
- Zou G, Puig Basagoiti F, Zhang B, Qing M, Chen L, Pankiewicz KW, Felczak K, Yuan Z, Shi PY. (2009) A single amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 384: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]




