Abstract
The formation of cholesterol domains in lipoplexes has been associated with enhanced serum stability and transfection rates both in cell culture and in vivo. This study utilizes the ability of saturated phosphatidylcholines to promote the formation of cholesterol domains at much lower cholesterol contents than have been utilized in previous work. The results show that lipoplexes with identical cholesterol and cationic lipid contents exhibit significantly improved transfection efficiencies when a domain is present, consistent with previous work. In addition, studies assessing transfection rates in the absence of serum demonstrate that the ability of domains to enhance transfection is not dependent on interactions with serum proteins. Consistent with this hypothesis, characterization of the adsorbed proteins composing the corona of these lipoplex formulations did not reveal a correlation between transfection and the adsorption of a specific protein. Finally, we show that the interaction with serum proteins can promote domain formation in some formulations, and thereby result in enhanced transfection only after serum exposure.
Keywords: serum protein interaction, lipoplex, cholesterol domain, transfection, gene delivery, differential scanning calorimetry, mass spectrometry, protein corona
Introduction
Nucleic acids (DNA, siRNA, miRNA, aptamers) represent a new class of pharmaceuticals that have the potential to successfully treat a wide variety of diseases [1–4]. Although some nucleic acid-based pharmaceuticals have progressed through clinical trials and been marketed, delivery has proven to be the major barrier toward realizing the full potential of this new class of pharmaceutical agents [5]. In contrast to traditional pharmaceuticals that can diffuse across the cell membrane, most nucleic acids must be delivered into the cytoplasm to elicit a therapeutic effect. Intracellular delivery has proven to be a difficult challenge, especially in vivo, and researchers have identified numerous methods of enhancing uptake and cytoplasmic delivery. Unfortunately, none of these approaches has proven sufficiently efficacious in clinical trials, and improved delivery systems are needed to enhance intracellular delivery.
Considering that nucleic acids are predominantly administered via the intravenous route, delivery systems must maintain stability in the blood in order to access the target site. Earlier work has documented the ability of cholesterol to greatly enhance the serum stability of lipid-based delivery systems, and researchers have proposed that the enhanced membrane rigidity imparted by cholesterol allows such systems to resist aggregation and dissociation upon interactions with serum proteins [6–9]. More specifically, our previous studies have clearly shown that increasing cholesterol content is correlated with the ability of lipoplexes to resist serum-induced aggregation and maintain transfection rates [8,9]. Furthermore, the enhanced stability and transfection rates observed in cell culture studies have translated to improved tumor accumulation and reporter gene expression in vivo [10,11].
Studies characterizing formulations containing high cholesterol have shown that cholesterol contents above 66 mole percent (49% by weight) result in the formation of a phase-separated cholesterol domain that appears to be coincident with significant increases in stability and delivery observed both in cell culture and in vivo [9,11,12]. In addition, studies on serum protein binding have suggested that cholesterol domains do not adsorb detectable levels of protein, and thus might offer an optimal environment for small molecule ligands that could otherwise be obscured by protein binding [9]. Experiments with ligands (i.e., folate) have demonstrated that transfection in 50% serum is only enhanced if formulations possess a cholesterol domain [12]. Further experiments with formulations possessing a cholesterol domain have established that the anchor to which the ligand is attached is critical for enhancing transfection; i.e., transfection is not enhanced when the ligand is conjugated to a lipid anchor that is excluded from the domain [12]. In contrast, ligands conjugated to anchors that are able to partition into the cholesterol domain resulted in significantly enhanced transfection. This same effect was recently documented for tumor delivery in vivo [11].
The studies summarized above clearly indicate that cholesterol domains are advantageous for both transfection and stability. However, the use of the very high cholesterol contents required for the formation of a domain raises concerns about the storage stability of such formulations and potential immunogenic effects that have been previously observed with formulations employing high cholesterol [5,13–15]. Accordingly, it would be advantageous to develop strategies that allow the formation of cholesterol domains under conditions that avoid concerns about storage stability and immunogenicity, i.e., lower cholesterol contents. More specifically, previous studies have shown that lipids possessing saturated acyl chains can promote the formation of cholesterol domains [16], and we assess this potential strategy for creating domains in lipoplexes at reduced cholesterol contents. Furthermore, transfection with these novel lipoplex formulations was characterized in the presence and absence of serum to assess the potential role of protein binding in enhancing transfection in vitro. Our results are consistent with our previous studies establishing that the formation of cholesterol domains in lipoplex formulations greatly enhances vector performance, and demonstrate that these benefits can be realized at much lower cholesterol contents that might be advantageous for in vivo applications and more amenable to commercial development.
Materials and Methods
Lipoplex Preparation
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). Lipid formulations were prepared by mixing stock lipids in chloroform and then evaporating chloroform from the mixtures under a stream of nitrogen gas for 15 minutes. The resulting lipid film was then dried overnight under vacuum to ensure all residual chloroform was removed. Films were re-suspended at 65 °C in double distilled water and sonicated for 1 minute. Lipoplexes were then prepared at a +/− charge ratio of 4 by mixing equal volumes of plasmid encoding luciferase with the suspended liposomes as previously described [9]. They were allowed to associate at room temperature for 15 minutes prior to use in experiments.
Transfection Protocol
MCF-7 cells (American Type Culture Collection # HTB-22; human breast adenocarcinoma cells) were cultured at 37 °C, 5% carbon dioxide with 100% humidity in Minimum Essential Media (MEM), 10% fetal bovine serum (FBS), 50 U/ml penicillin, 50 μg/ml streptomycin (all media from Cellgro MediaTech Inc., a Corning Acquisition, Manassas, VA). Media was filtered prior to use with either a 0.22 μm syringe filter or a 0.22 μm stericup to ensure sterility (Thermo-Fisher Rockford, IL). For transfection experiments, cells were seeded at 20,000 cells/well in 96-well plates 24 hours prior to treatment. On the day of the experiment, cells were washed twice with phosphate buffered saline (PBS) before adding 100 μl of transfection media (50% MEM, 50% FBS to mimic in vivo serum protein conditions). Formulations were then added to the center of each well (2 μl total volume containing 1 μg total DNA) and allowed to incubate for 4 hours. In some experiments, lipoplexes were pre-incubated 1:1 v/v in MEM or FBS for 30 minutes prior to dilution in 100% MEM, and then administered to cells for transfection as described above. After 4 hours, the transfection media was removed, and cells were again washed twice with PBS before being reintroduced to their normal 10% FBS growth media. After resting for 48 hours, the cells were again washed twice with PBS before being lysed with 30 μl Promega lysis buffer in the −80 °C freezer according to manufacturer’s instructions (Promega, Madison, WI). Lysate was assayed for protein content using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) on a 96-well THERMOmax plate reader (Molecular Devices, Sunnyvale, CA). Luminescence was quantified using a Monolight Luminometer according to manufacturer’s instructions (BD Biosciences, San Jose, CA).
Particle Sizing by DLS
Sizes of the lipoplexes were measured by dynamic light scattering with a Nicomp 380 ZLS (PSS Nicomp, Santa Barbara, CA). The instrument was set on vesicle mode using the volume-weight Gaussian distribution analysis. Samples were pre-incubated 1:1 v/v in MEM or FBS for 30 minutes prior to making the size measurements. Incubated samples were then diluted 1:100 in PBS prior to sizing to reduce light scattering.
Differential Scanning Calorimetry
Calorimetry measurements were made on each formulation using Microcal VP-DSC (Microcal Inc., Northampton, MA). Samples prepared in double distilled water as described above were loaded into the instrument. As described above for transfection and particle sizing, some experiments involved pre-incubation 1:1 v/v with MEM or FBS for 30 minutes prior to analysis. The concentration of lipid in all samples was 0.5 mM total lipid as previously described [11]. Samples were degassed with the MicroCal ThermoVac (Microcal Inc., Northampton, MA) for 20 minutes at a temperature of 4 °C and then loaded carefully with gastight Hamilton syringes (Hamilton Company, Reno, NV) using the technique described by Microcal. The instrument was set to scan from 10–90 °C at 90 °C/h. Melting curves were analyzed using Origin 7.0 software.
Serum Protein Pull-down Experiments
Lipoplex formulations were mixed 1:1 v/v with either MEM or FBS, and incubated at room temperature for 30 minutes. Samples were then centrifuged at 13,000 x g for 60 min. The supernatant was removed and the pellet was re-suspended in 10% w/v sodium dodecyl sulfate (SDS). Surface proteins were removed by boiling (95 °C) re-suspended pellets in SDS for 5 minutes according to the methods of Tandia et al., 2003 [17]. Samples were then dialyzed into PBS using the lowest MW (2000 Da) dialysis cassette from Pierce Thermo-Fisher (Rockford, IL) for 24 h with three buffer changes. Total protein content of each pull-down was measured with the Bio-Rad protein assay as mentioned above. The remainder of the sample was used for mass spectrometry analysis.
Protein Analysis by MALDI/MS
Dialyzed proteins from the pull-down experiments were prepared for mass spectrometry using Millipore C4 ZipTips (Billerica, MA). Trifluoroacetic acid (TFA) was added at 0.1% to each sample. The ZipTip was then prepared according to the manufacturer’s suggestion before eluting with 0.1%TFA/50%acetonitrile (ACN)/water. The ZipTipped sample was then mixed 1:1 v/v with saturated matrix solution (either α-cyano-4-hydroxycinnamic [CHCA] for lower MW protein detection or sinapic acid both at ~10 mg/ml or saturated in 0.1%TFA/50% ACN/water). The 1:1 matrix/protein solutions were spotted at a volume of 1 μl on a clean plate. The plate was loaded in the Bruker Omniflex MALDI-TOF (Bruker Corp., Fremont, CA). The laser was set at 65% power with 100 shots per run. The spectra were analyzed by Flex Analysis software. Due to the mass accuracy and possible modifications/degradation of proteins extracted from the protein corona, it is difficult to definitively identify proteins associated with each approximate mass from this procedure. However similar to other profiling studies, MALDI provided insight into differences in protein coronas based on formulation that could be investigated further [18]. Additional experiments utilized digestion for identification of individual proteins present in the corona (see below).
Digestion Procedure for Mass Spec Analysis
Chemical denaturation for mass spec analysis was performed according to the procedure described by Russell and Park [19]. Briefly, 1 M guanidine hydrochloride and 5 mM dithiothreitol were added to each pull-down after dialysis. The mixtures were incubated at 70 °C for 20 minutes on a heating block. After denaturation was completed, 25 mM iodoacetamide was added, and this mixture was incubated at room temperature for 30 minutes. Porcine trypsin was then added to the solution at approximately a 1:40 weight ratio of trypsin to protein. The solution was allowed to digest for 5 hours at 37 °C (all chemicals for this procedure came from Sigma Aldrich, St. Louis, MO).
Results
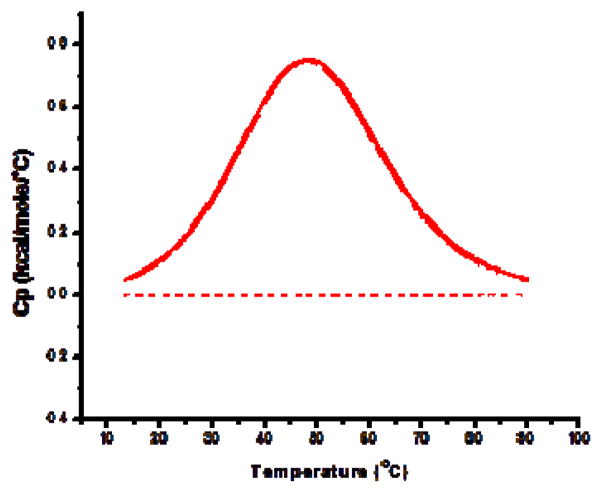
Our previous work has demonstrated that the formation of cholesterol domains in lipoplexes can be monitored with differential scanning calorimetry (DSC) [9]. More specifically, anhydrous cholesterol domains that form in our lipoplexes exhibit a heat signal that can be used to calculate the number of molecules that comprise the domain [9]. These studies have clearly established that cholesterol domains form in DOTAP/cholesterol lipoplexes at cholesterol contents of approximately 66 mol% (49% by weight). Similar cholesterol domains have been thoroughly characterized by other researchers, and it has been shown that both the saturation and the length of the lipid chains can affect domain formation [16,20,21]. Accordingly, we utilized DSC to determine if saturated phosphatidylcholines (PC) could be used to promote domain formation in our lipoplexes. Figure 1 depicts the distinct heat signal that is readily detectable by DSC when a domain is present as compared to a formulation lacking a cholesterol domain. This approach was utilized to quantify both the enthalpy and the transition temperature associated with domains that formed in lipoplexes incorporating saturated PCs of different chain lengths.
Figure 1.
Detection of domain formation by DSC. The formation of a cholesterol domain in lipoplexes is indicated by a heat signal from anhydrous cholesterol as previously described [9]. The DSC thermogram from lipoplexes incorporating 50% long chain PC (24C) demonstrates the distinct thermal signature characteristic of domain formation. In contrast, no heat signal is observed (dashed line) in formulations incorporating 50% short chain PC (14C). Each trace is the average heat signal from 8 individual samples. None of the individual lipid components exhibit transition temperatures from 20–80 °C.
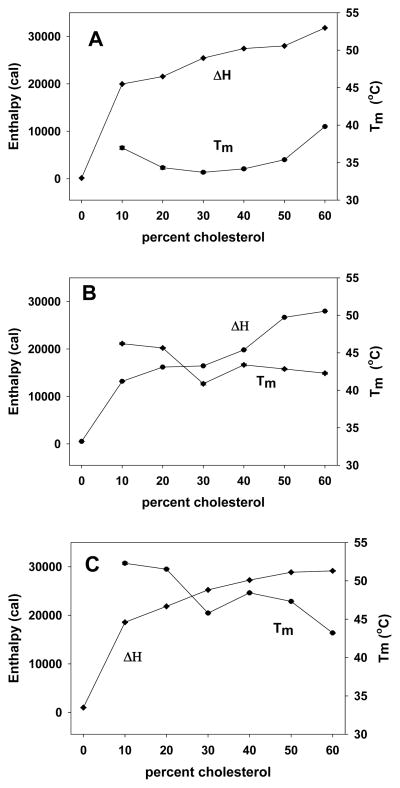
Considering our previous work showing that cholesterol domains are detected at DOTAP/cholesterol mole ratios of 30:70, experiments were performed at a constant DOTAP molar percentage of 30%. Substituting saturated PC with symmetrical chain lengths of 14, 16, 18, 20, 22, or 24 carbons for cholesterol, we progressively reduced the cholesterol content from 60 to 0 mol%, and utilized DSC to monitor domain formation. It is important to recognize that neither DOTAP nor the PCs used in these experiments exhibited detectable transition temperatures under the conditions used for determining domain formation (i.e., 0.5 mM lipid). Our results indicated that incorporation of PCs with chain lengths of 14, 16, and 18 carbons did not promote domain formation, and no heat signal was observed even in lipoplexes containing 60% cholesterol (data not shown). In contrast, a domain was clearly observed when longer PCs were incorporated into the lipoplexes, even at very low cholesterol contents (Fig. 2). The enthalpies and transition temperatures associated with domains formed in the presence of saturated PCs with chain lengths of 20, 22, and 24 carbons are shown in Figures 2A–C, respectively, and indicate that domains are observed at cholesterol contents as low as 10 mol%! Overall, Tm varied from 33–53 °C in the different lipoplex formulations, and enthalpies progressively rose at cholesterol contents above 10%, consistent with increasing amounts of cholesterol participating in the domain (Fig. 2) [9].
Figure 2.
Characterization of domain formation in lipoplexes incorporating long chain PCs. PCs composed of saturated acyl chains of different lengths were investigated for their ability to promote domain formation. Lipoplexes incorporating 20C (A), 22C (B), and 24C (C) PCs each induced the formation of domains at cholesterol contents ranging from 10–60 mol%. Symbols represent the mean of 8 replicates; the standard error is within the symbol size. Chain lengths of 14, 16 and 18 carbons did not induce the formation of cholesterol domains.
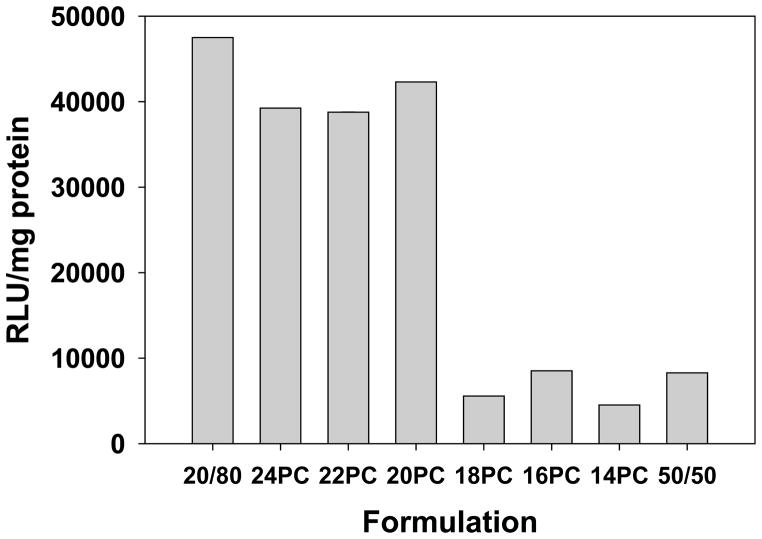
Because our previous studies have demonstrated enhanced transfection with lipoplexes possessing a cholesterol domain, transfection experiments were conducted with lipoplexes formulated with 30% DOTAP/20% cholesterol/50% PC. Formulations incorporating each of the PC chain lengths (14–24 C) were assessed for their ability to transfect MCF-7 cells in culture as compared to a standard formulation of 50% DOTAP/50% cholesterol and our best-performing formulation of 20% DOTAP/80% cholesterol [8–12]. Consistent with our previous studies, all transfection experiments were performed in 50% serum, and lipoplexes were formulated at +/− = 4. As documented in our earlier studies, lipoplexes formulated at 80% cholesterol exhibited significantly greater transfection rates as compared to a standard formulation containing 50% cholesterol (Fig. 3). Incorporation of PCs with progressively longer chain lengths resulted in a significant increase in transfection (≈ 8-fold) when the chain length was increased from 18 to 20 carbons. Furthermore, transfection rates comparable to our formulation containing 80% cholesterol were observed when 20–24C PCs were incorporated even though these formulations possessed only 20% cholesterol (Fig. 3). These findings are consistent with our previous studies showing that the formation of a cholesterol domain significantly enhances transfection rates [9,11,12].
Figure 3.
Transfection rates of lipoplexes incorporating saturated PCs in serum. The ability of lipoplexes incorporating saturated PCs with chain lengths of 14–24 carbons was assessed in MCF-7 cells. Transfections were performed in 50% serum at a +/− charge ratio of 4, and standard formulations (20% DOTAP/80% cholesterol, 50% DOTAP/50% cholesterol) are shown for comparison. Each bar represents the mean transfection of 8 individual wells, and the error bars (not visible) represent the standard errors.
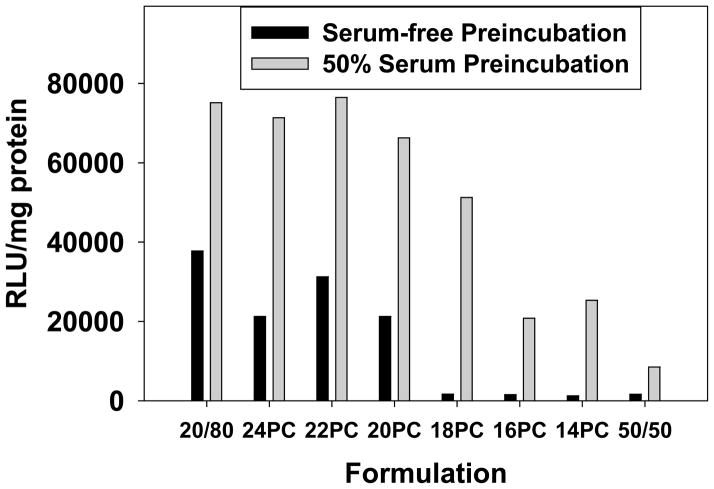
One of the initial observations that motivated us to investigate high cholesterol formulations was their ability to resist aggregation and dissociation in the presence of serum [8]. Many studies have demonstrated the adverse effects of serum protein binding on lipoplexes [22–25], and we have previously shown that high cholesterol formulations maintain elevated transfection levels in the presence of serum [8]. Considering the detrimental effects of serum, it would be expected that lipoplexes lacking a domain should exhibit higher transfection in serum-free media as compared to that observed in 50% serum. To assess the effect of serum protein binding on transfection, lipoplex formulations were pre-incubated in either serum-free media or 50% serum for 30 min before being diluted 50-fold in serum-free media and used to transfect MCF-7 cells in culture. Control experiments performed in the presence of 1% serum to mimic the low serum levels present after dilution demonstrated that transfection is not affected by these conditions (data not shown). The data in figure 4 show that pre-incubation in serum enhanced transfection rates of all lipoplexes containing saturated PCs, suggesting that serum instability is not responsible for the lower transfection rates observed with lipoplexes lacking a cholesterol domain. Furthermore, pre-incubation in serum resulted in the highest transfection rates when lipoplexes possessed a cholesterol domain (20–24C PC), whereas formulations incorporating short-chain PCs (14+16C) exhibited 3–4 fold lower transfection under these conditions. When all formulations are compared under serum-free conditions, lipoplexes possessing a domain exhibited a sharp increase in transfection rates, suggesting that the beneficial effects of a cholesterol domain are not dependent on protein binding (Fig. 4). Curiously, the lipoplex formulation incorporating 18C PC transfected at distinctly higher rates in serum than the other formulations in which a domain was not detected by DSC (Fig. 2).
Figure 4.
The effect of serum on transfection. The ability of lipoplexes incorporating saturated PCs with chain lengths of 14–24 carbons was assessed after a 30-min incubation in either 50% serum or serum-free media. Transfection of MCF-7 cells occurred in serum-free media at a +/− charge ratio of 4; standard formulations (20% DOTAP/80% cholesterol, 50% DOTAP/50% cholesterol) are shown for comparison. Each bar represents the mean transfection of 8 individual wells, and the error bars (not visible) represent the standard errors.
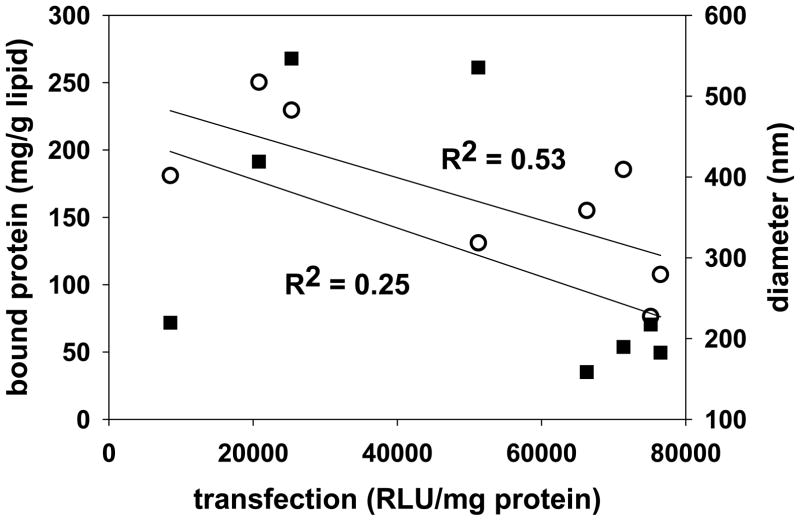
Although the beneficial effects of a cholesterol domain are not dependent on serum pre-incubation, the data in figure 4 clearly show that pre-exposure to serum proteins enhances transfection in all formulations, with the exception of the 50% DOTAP/50% cholesterol formulation. While some studies have suggested that interactions with serum proteins might be able to enhance cell uptake mechanisms or endosomal escape such that transfection is improved [26,27], exposure to serum more typically causes aggregation/dissociation that results in dramatic reductions in transfection [8,22–25,28]. It follows that serum proteins which bind to our formulations might promote cell uptake, internalization and/or advantageous intracellular trafficking. Accordingly, we endeavored to characterize proteins that bound to each of our formulations after serum exposure, i.e., “the protein corona”. Lipoplexes incubated in 50% serum for 30 min (mimicking the exposure in Fig. 4) were isolated from the media, and MALDI/MS was used to characterize proteins associated with each formulation. In addition, the total amount of protein bound to each formulation was quantified, as was the diameter of lipoplexes before and after serum exposure. The results of these experiments are displayed in Table I, and show distinct differences in the proteins that bind to these formulations. Although we do not observe any protein that binds to all formulations, we do observe striking differences in the proteins adsorbed to lipoplexes incorporating long chain PCs (20–24C) as compared to short chain PCs (14–18C). More specifically, five proteins (13,300 Da, IgG 2C, IgG 1C, alpha-2-HS-glycoprotein, collagen alpha-1 chain) bind to formulations with long chain PCs, and three proteins (3530 Da, 12,600 Da, albumin) bind to lipoplexes incorporating short-chain PCs (Table I). However, comparing these proteins with proteins adsorbed to our high transfecting 20/80 formulation and the lower transfecting 50/50 formulation reveals that none of these proteins can explain the enhanced transfection observed after serum incubation, i.e., no single protein adsorbed specifically to only the lipoplexes exhibiting cholesterol domains and high transfection. We also did not observe a good correlation between transfection and total protein bound or size after serum exposure (Fig. 5).
Table I.
Proteins Associated with Lipoplex Formulations. Proteins associated with each formulation were identified by MALDI/MS. The 50:50 formulation adsorbed many additional proteins, but only those in common with the other formulations are listed for comparison. The total quantity of adsorbed protein on each formulation is also shown, as is the diameter (nm) of each lipoplex before and after incubation in fetal calf serum (FCS).
| 24PC | 22PC | 20PC | 18PC | 16PC | 14PC | 20:80 | 50:50 | |
|---|---|---|---|---|---|---|---|---|
| Unidentified (3,530 Da) | - | - | - | x | x | x | x | x |
| Unidentified (12,600 Da) | - | - | - | x | x | x | - | - |
| Unidentified (13,300 Da) | x | x | x | - | - | - | - | x |
| Cationic trypsin (25,768 Da) | - | - | - | - | - | - | x | x |
| Immunoglobin gamma-2 C region (35,878 Da) | x | x | x | - | - | - | x | x |
| Immunoglobin gamma-1 C region (36,083 Da) | x | x | x | - | - | - | x | x |
| Alpha-2-HS-glycoprotein (38,394 Da) | x | x | x | - | - | - | - | x |
| Albumin (69,248 Da) | - | - | - | x | x | x | x | x |
| Collagen alpha-1 chain (93,594 Da) | x | x | x | - | - | - | - | - |
| mg protein/g lipid | 185.5 | 107.6 | 155.1 | 131 | 250.3 | 229.6 | 76.4 | 181.1 |
| diameter before FCS | 174.5 | 178.4 | 162.4 | 204.7 | 293.4 | 290.5 | 209.1 | 204.6 |
| diameter after FCS | 189.7 | 182.6 | 158.5 | 535.4 | 419 | 546.5 | 217.3 | 219.6 |
Figure 5.
Correlation of transfection with bound protein or particle size after serum exposure. Transfection levels after pre-incubation in serum were plotted against bound protein (open circles, R2 = 0.25) and particle diameter after serum exposure (filled squares, R2 = 0.53). Linear regression analysis was performed, and very low correlation coefficients were obtained.
Discussion
It is well-established that the delivery of nucleic acids to the interior of the target cell represents a major barrier to the therapeutic use of genes and RNA. Successful delivery systems for nucleic acids must exhibit stability in the blood, accumulation at the target site, uptake and efficient trafficking within the target cell. It is generally accepted that accumulation in the tumor is primarily governed by the Enhanced Permeation and Retention (EPR) effect whereby leaky tumor vasculature allows particles of sufficiently small size to extravasate into the tumor, at least in animal models [29]. Studies have shown that the mobility of nanoparticles is restricted in the extracellular tumor environment, and the use of ligands enhances the uptake of targeted nanoparticles by the tumor cells [30]. Our previous work has demonstrated that lipoplexes endowed with a cholesterol domain offer the potential to locate ligands within a “nanoenvironment” that facilitates gene delivery both in cell culture and in vivo [9,11,12]. Although the exploitation of cholesterol domains is a new strategy that can significantly improve intracellular delivery, the very high levels of cholesterol needed to form domains raise concerns about shelf stability and immunogenicity [5,13–15]. However, researchers working on cholesterol domains as models for membrane rafts have demonstrated that saturated lipids possessing long carbon chains can promote domain formation [16,20], and this approach was applied to lipoplexes in the current study.
The ability to detect the formation of cholesterol domains in lipoplexes by DSC allows us to assess the ability of different saturated phosphatidylcholines to promote domain formation. Our results clearly demonstrate that long chain PCs (20–24 C) greatly facilitate the formation of a cholesterol domain, and domains are detected in these formulations at cholesterol contents as low as 10% (Fig. 2). Consistent with our earlier work showing the ability of domains to enhance transfection [9,12], lipoplexes formulated with long chain PCs and 20 mol% cholesterol possessed transfection rates comparable to our best-performing formulation containing 80 mol% cholesterol (Fig. 3). Although this finding might be expected based purely on the presence of a domain, PC is known to inhibit non-bilayer phases that are thought to be critical for membrane fusion events involved in transfection [31,32]. Therefore, the ability of these formulations to efficiently transfect cells even though they possess 50 mol% PC is quite surprising, and in stark contrast to previous studies in which the incorporation of PC abolished transfection [8,31,33]. This finding suggests that the ability of PC to inhibit transfection in some studies is not straightforward.
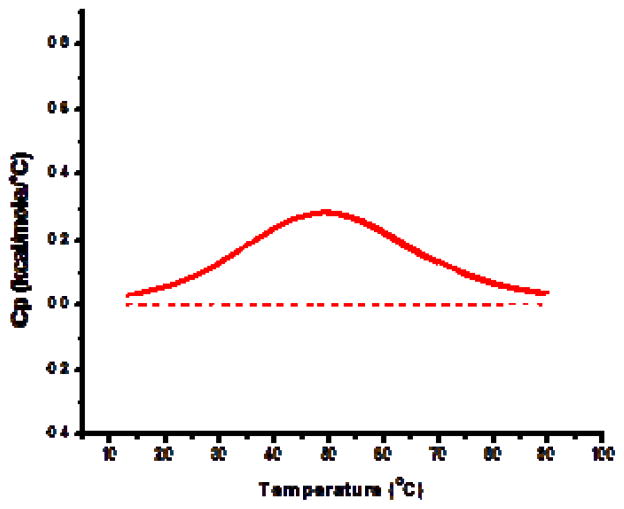
In contrast to the lipoplexes incorporating long chain PCs, short chain PCs (14, 16C) that did not promote domain formation had significantly lower transfection rates (Fig. 3). Surprisingly, lipoplexes formulated with 18C PC exhibited relatively high transfection rates after pre-incubation in serum even though a cholesterol domain was not detected in the DSC experiments (Fig. 2). More specifically, the 18C PC formulation exhibited transfection rates more consistent with domain formation after a pre-incubation in serum, but performed comparable to lipoplexes lacking a domain in the absence of serum (Fig. 4). This apparent paradox can be explained by the fact that pre-exposure to serum induces the formation of a relatively small domain in this lipoplex that is not present prior to serum exposure (Fig. 6). The ability of membrane proteins to stabilize cholesterol domains has been proposed by McConnell et al. [34], but ours is the first study to demonstrate the ability of serum proteins to promote cholesterol domains, and also to establish that this can improve delivery by a lipid-based vehicle. The formation of a domain in response to serum protein exposure was only observed with the formulation incorporating 18C PC, but this finding serves to illustrate that changes to the physical properties of the lipid vehicle upon serum protein binding have the potential to dramatically improve transfection. However, this effect cannot account for the approximately 2-fold enhancement in transfection observed for all other formulations possessing saturated PCs (Fig. 4). While some studies have suggested that interactions with serum proteins might be able to enhance cell uptake mechanisms such that transfection is improved [26], exposure to serum more typically causes aggregation/dissociation that results in dramatic reductions in transfection [8,22–25,28].
Figure 6.
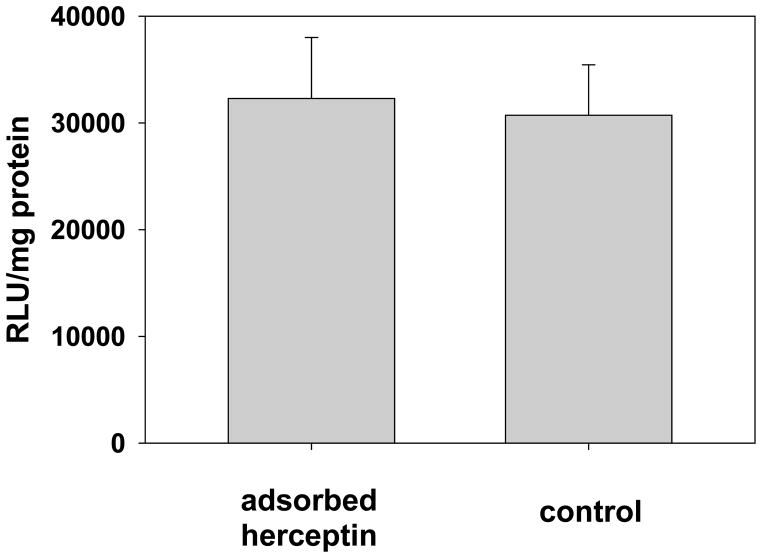
Exploiting endogenous IgG binding for targeted delivery. An antibody (Herceptin) that is known to bind the Her-2 receptor was adsorbed to lipoplexes (20% DOTAP/80% cholesterol), and used to transfect Her-18 cells that overexpress this receptor. No enhancement of transfection was observed. Each bar represents the mean transfection of 5 individual wells, and the error bars represent the standard errors.
The role of adsorbed proteins is not well understood, and studies have clearly shown that opsonization enhances uptake by liver macrophages and clearance in vivo [35]. Interestingly, previous work has suggested that adsorbed serum components might alter particle trafficking within cells [27,36,37], and such an effect could potentially enhance transfection rates. Furthermore, it has also been proposed that certain membrane proteins might bind specifically to cholesterol domains [34]. Our analysis of the proteins bound to different formulations after serum exposure identified distinct differences between proteins bound to lipoplexes incorporating long chain PCs as compared to shorter chains (Table I). However, we did not identify any specific protein in the protein corona that unambiguously correlated with transfection and/or domain formation. For example, four of the five proteins that bound to lipoplexes incorporating long chain PCs were also adsorbed onto the 50%/50% formulation which does not possess a domain and exhibited consistently lower transfection (Figs. 3+4; Table I). The last protein bound to the lipoplexes incorporating long chain PCs (collagen alpha-1-chain) was not present on our potent 20%/80% formulation which possesses a domain and exhibits high transfection [9]. In addition, we did not observe a correlation of transfection with total protein bound or particle size after serum incubation (Fig. 5). Additional plots of transfection (50% serum, preincubation in serum-free media, preincubation in 50% serum) with particle size before serum exposure or with percent change in particle size upon serum exposure yielded similarly weak correlations (data not shown).
In conclusion, our results are consistent with previous studies showing that lipids with long, saturated acyl chains promote cholesterol domain formation [16,20]. In this study, we exploited this lipid mixing behavior to generate cholesterol domains in lipoplexes at lower cholesterol levels that might be advantageous for clinical applications and commercial development. Our data clearly demonstrate that cholesterol domain formation significantly improves transfection, and also that domain formation can be triggered by serum protein binding in certain formulations. While recent studies have suggested that cholesterol can enhance transfection by promoting membrane fusion and directing lipoplexes away from the lysosome [38], we observe distinct increases in transfection if lipoplexes possess a cholesterol domain even when compared to lipoplexes formulated at equivalent cholesterol contents. Although we characterized the individual proteins adsorbed to each of our lipoplex formulations, we were unable to establish a direct relationship between transfection or domain formation and the adsorption of a specific serum protein. Previous studies have documented that adsorption of albumin can promote endosomal escape and improve transfection, but albumin was not present on formulations incorporating long chain PCs that possessed domains and exhibited enhanced transfection. Our findings suggest that the enhanced transfection observed with lipoplexes possessing a cholesterol domain after serum exposure is not due to recruitment of a specific serum protein that improves uptake and/or trafficking. This suggestion is consistent with the observation that all formulations possessing a cholesterol domain exhibited significantly higher transfection rates even under conditions where they were not exposed to serum. We conclude that the presence of a cholesterol domain, as opposed to cholesterol content, must alter cell uptake and/or trafficking by mechanisms associated with raft/membrane sorting rather than the traditional protein-receptor interactions that are utilized in antibody-targeted delivery systems. The significant role of intracellular trafficking/sorting is also evident in our previous work demonstrating that some domain-containing formulations do not enhance internalization by the target cell despite dramatically increasing transfection both in cell culture and in vivo [9,11,12].
Figure 7.
Serum proteins induce cholesterol domain formation. Lipoplexes composed of 30% DOTAP/20% cholesterol/50% 18C PC were incubated in 50% serum for 30 minutes prior to DSC analysis. A distinct heat signal is observed (solid line) indicating the formation of a cholesterol domain resulting from the adsorption of serum proteins. In contrast, no heat signal is observed when this formulation is incubated in serum-free media (dashed line). Each trace is the average heat signal from 8 individual samples.
Executive summary.
Saturated, long chain phosphatidylcholines were able to promote cholesterol domain formation in lipoplexes at low cholesterol contents, and the presence of a cholesterol domain correlated with higher transfection levels.
Exposure of lipoplexes containing saturated, long chain phosphatidylcholines to serum enhanced transfection levels.
Analysis of the protein corona did not reveal specific serum proteins that correlated with enhanced transfection or domain formation.
Serum exposure can promote cholesterol domain formation resulting in enhanced transfection.
The ability to form cholesterol domains at very low cholesterol contents greatly increases the potential for commercial development of delivery vehicles possessing cholesterol domains.
Future Perspective.
This work clearly demonstrates that cholesterol domains that form in lipid-based delivery vehicles can have significant effects on intracellular delivery. Future work will attempt to understand the mechanism by which cholesterol domains facilitate intracellular delivery, but this study documents that the adsorption of specific proteins that might promote delivery cannot account for the enhanced transfection rates observed. Therefore, it seems likely that cholesterol domains interact with components of the cellular membrane (e.g, plasma membrane lipids, specific receptors, internal membranes) to enhance delivery. The improved understanding of the role of distinct, phase-separated lipid domains on nanoparticles could allow more sophisticated delivery systems that better exploit the lipid raft structures and mosaic characteristics of biological membranes. It is hoped that harnessing lipid phase behavior may allow the field of drug delivery to shift away from protein-based ligands and focus on approaches that are more compatible with commercial development. In addition, the use of delivery vehicles that do not require PEGylation will circumvent problems associated with PEG immunogenicity and reduced circulation lifetimes.
Acknowledgments
This work utilized the University of Colorado School of Pharmacy Mass Spectrometry Core Facility and was supported by NIH/NIGMS grant #1 RO1GM093287-01A1.
References
- 1.Cavazzana-Calvo M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 2.Hyde SC, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000;7:1156–1165. doi: 10.1038/sj.gt.3301212. [DOI] [PubMed] [Google Scholar]
- 3.Nabel GJ, et al. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restifo NP, Ying H, Hwang L, Leitner WW. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89–92. doi: 10.1038/sj.gt.3301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Anchordoquy TJ. Drug Delivery Trends in Clinical Trials and Translational Medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senior J, Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 1982;30:2123–2136. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 7.Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5:137–143. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Anchordoquy TJ. The Role of Lipid Charge Density in the Serum Stability of Cationic Lipid/DNA Complexes. Biochim Biophys Acta Biomembranes. 2004;1663:143–157. doi: 10.1016/j.bbamem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9**.Xu L, Anchordoquy TJ. Cholesterol Domains in Cationic Lipid/DNA Complexes Improve Transfection. Biochim Biophys Acta Biomembranes. 2008;1778(10):2177–81. doi: 10.1016/j.bbamem.2008.04.009. This is the first paper to demonstrate that the formation of cholesterol domains in lipoplexes corresponds with enhanced transfection and serum stability. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Bradshaw-Pierce EL, DeLille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of Lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. J Pharm Sci. 2008;97:237–50. doi: 10.1002/jps.21076. [DOI] [PubMed] [Google Scholar]
- 11**.Xu L, Betker J, Yin H. Anchordoquy TJ. Ligands located within a cholesterol domain enhance gene delivery to the target tissue. J Cont Release. 2012;160:57–63. doi: 10.1016/j.jconrel.2012.03.003. This paper demonstrates the importance of locating ligands within the cholesterol domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Xu L, Anchordoquy TJ. Effect of cholesterol nanodomains on the targeting of lipid-based gene delivery in cultured cells. Molecular Pharmaceutics. 2010;7(4):1311–17. doi: 10.1021/mp100097b. This paper concludes that intracellular trafficking plays a significant role in domain-mediated transfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swartz GM, Gentry MK, Amende LM, Blanchette-Mackie EJ, Alving CR. Antibodies to cholesterol. Proc Natl Acad Sci (USA) 1988;85:1902–1906. doi: 10.1073/pnas.85.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alving CR, Swartz GM. Antibodies to cholesterol, cholesterol conjugates, and liposomes: Implications for atherosclerosis and autoimmunity. Crit Rev Immunol. 1991;10(5):441–453. [PubMed] [Google Scholar]
- 15.Szebeni J, Baranyi L, Savay S, et al. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 16.Bach D, Wachtel E. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochem Biophys Acta. 2003;1610:187–197. doi: 10.1016/s0005-2736(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 17.Tandia B-M, Vandenbranden M, Wattiez R, et al. Identification of Human Plasma Proteins that Bind to Cationic Lipid/DNA Complex and Analysis of their Effects on Transfection Efficiency: Implications for Intravenous Gene Transfer. Mol Ther. 2003;8(2):264–273. doi: 10.1016/s1525-0016(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 18.Sidransky D, Irizarry R, Califano JA, et al. Serum Protein MALDI Profiling to Distinguish Upper Aerodigestive Tract Cancer Patients from Control Subjects. J Natl Cancer Inst. 2003;95(22):1711–1717. doi: 10.1093/jnci/djg099. [DOI] [PubMed] [Google Scholar]
- 19.Park Z-Y, Russell DH. Identification of Individual Proteins in Complex Protein Mixtures by High-Resolution, High-Mass_Accuracy MALDI TOF-Mass Spectrometry Analysis of In-Solution Thermal Denaturation/Enzymatic Digestion. Anal Chemistry. 2001;73 (11):2558–2564. doi: 10.1021/ac001488p. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282:33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- 21.Mason RP, Tulenko TN, Jacob RF. Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathology. Biochim Biophys Acta Biomembranes. 2003;1610:198–207. doi: 10.1016/s0005-2736(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 22.Zelphati O, Uyechi LS, Barron LG, Szoka FC., Jr Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390:119–133. doi: 10.1016/s0005-2760(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 23.Yang JP, Huang L. Time-dependent maturation of cationic liposome-DNA complex for serum resistance. Gene Ther. 1998;5:380–387. doi: 10.1038/sj.gt.3300596. [DOI] [PubMed] [Google Scholar]
- 24.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4:950–960. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 26.Caracciolo G, Callipo L, De Sanctis SC, Cavaliere C, Pozzi D, Lagana A. Surface adsorption of protein corona controls the cell internalization mechanism of DC-Chol-DOPE/DNA lipoplexes in serum. Biochim Biophys Acta. 2009;1798:536–543. doi: 10.1016/j.bbamem.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Simões S, Slepushkin V, Pires P, et al. Human serum albumin enhances DNA transfection by lipoplexes and confers resistance to inhibition by serum. Biochim Biophys Acta. 2000;1463:459–469. doi: 10.1016/s0005-2736(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 28.Tandia B-M, Lonez C, Vandenbranden M, Ruysschaert J-M, Elouahabi A. Lipid mixing between lipoplexes and plasma lipoproteins is a major barrier for intravenous transfection mediated by cationic lipids. J Biol Chem. 2005;280:12255–12261. doi: 10.1074/jbc.M414517200. [DOI] [PubMed] [Google Scholar]
- 29.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 30.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 31.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 32.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Therapy. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 33.Hui SW, Langner M, Zhao YL, Ross P, Hurley E, Chan K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys J. 1996;71:590–599. doi: 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConnell HM, Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta Biomembranes. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 35.Moghimi SM, Patel HM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – The concept of tissue specificity. Adv Drug Del Rev. 1998;32:45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 36.Hellstrand E, Lynch I, Andersson A, et al. Complete high-density lipoproteins in nanoparticle corona. FEBS Journal. 2009;276:3372–3381. doi: 10.1111/j.1742-4658.2009.07062.x. [DOI] [PubMed] [Google Scholar]
- 37.Furumoto K, Ogawara K, Yoshida M, et al. Biliary excretion of polystyrene microspheres depends on the type of receptor-mediated uptake in rat liver. Biochim Biophys Acta. 2001;1526:221–226. doi: 10.1016/s0304-4165(01)00132-5. [DOI] [PubMed] [Google Scholar]
- 38.Pozzi D, Marchini C, Cardarelli F, et al. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim Biophys Acta. 2012;1818:2335–2343. doi: 10.1016/j.bbamem.2012.05.017. [DOI] [PubMed] [Google Scholar]