Abstract
Ecosystems are interactive systems involving communities of species and their abiotic environment. Tumors are ecosystems in which cancer cells act as invasive species interacting with native host cell species in an established microenvironment within the larger host biosphere. At its heart, to study ecology is to study interconnectedness. In ecologic science, an ecologic network is a representation of the biotic interactions in an ecosystem in which species (nodes) are connected by pairwise interactions (links). Ecologic networks and signaling network models have been used to describe and compare the structures of ecosystems. It has been shown that disruption of ecologic networks through the loss of species or disruption of interactions between them can lead to the destruction of the ecosystem. Often, the destruction of a single node or link is not enough to disrupt the entire ecosystem. The more complex the network and its interactions, the more difficult it is to cause the extinction of a species, especially without leveraging other aspects of the ecosystem. Similarly, successful treatment of cancer with a single agent is rarely enough to cure a patient without strategically modifying the support systems conducive to survival of cancer. Cancer cells and the ecologic systems they reside in can be viewed as a series of nested networks. The most effective new paradigms for treatment will be developed through application of scaled network disruption.
Introduction
“Cancer is no more of a disease of cells than a traffic jam is a disease of cars. A lifetime of study of the internal combustion engine would not help anyone to understand our traffic problems. The causes of congestion can be many. A traffic jam is due to failure of the normal relationship between driven cars and their environment and can occur whether they themselves are running normally or not.”
D.W. Smithers, Lancet, March 1962 (1)
An ecologic approach to understanding cancer
Cancer remains the second leading cause of death in the United States, with a projected 1.6 million new cases and 577,000 deaths in 2012 (2). The global burden of cancer continues to grow, from more than 12.7 million new cases in 2008 to an estimated 15 million by 2020 (3). The last decade has seen an explosion in the understanding of cancer as a systemic disease. Hanahan and Weinberg, in their landmark 2000 and 2011 papers, described 8 hallmarks of cancer as an organizing principle to help explain the complexity of tumorigenesis (4, 5). These hallmarks include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, avoiding immune destruction, and deregulating cellular energetics. These cancer cell properties are facilitated, at least in part, by genetic instability that results in tumor cell heterogeneity and are enabled by the proinflammatory components of the tumor microenvironment (1, 5–8). As Smithers so eloquently pointed out 50 years ago, cancer is about more than just the cancer cells.
Ecology provides a framework for understanding cancer cells and their surroundings in a human host. An ecosystem encompasses all of the organisms in a given area and their interactions with the physical environment (Table 1; ref. 9). Cancer cells exist within a complex ecosystem populated by host cells including fibroblasts, endothelial cells, and leukocytes, supported by a scaffold of extracellular matrix (10– 13). Designing new therapies for cancer based on an ecologic framework (ecotherapy) allows for the application of precision medicine principles (e.g., the use of integrative sequencing results to identify actionable targets from the mutational landscape of cancer cells) while adding in therapies that attack host cell support systems within the tumor ecosystem (10–14).
Table 1.
Cancer cells can be viewed as a species as part of an ecosystem
| Man | Cancer | |
|---|---|---|
| Species | Human and others |
Cancer cells, host cells |
| Biotope | Land | Extracellular matrix |
| Local environment | Ecosystem (City) | Microenvironment, organ |
| Metacommunity | Cities | Metastases |
| Biosphere | Earth | Patient |
Ecotherapy can be applied to the primary tumor as a single population or to metastatic cancer. Ecologic science defines a metapopulation as a group of spatially separated populations of the same species that interact at some level. Metapopulations are often part of metacommunities, sets of local communities linked by multiple, interacting species (15, 16). In cancer, this framework can be applied to metastases. Eventually, many cancers, if left untreated, take on the classic characteristics of an invasive species by metastasizing to new environments (Fig. 1; refs. 17, 18). Cancer cells extravasate from the primary organ, survive circulation by hitching a ride with normal cells, and then intravasate and set up residence in a new organ. After a period of lying dormant, these cells eventually grow, destroying the local ecosystem through proliferation and spread (ecologic impact). Prevailing thought has been that metastases act as independent populations of cancer cells once they are established in a new site. Recent evidence, however, suggests that this is likely not true. Norton and Massague have shown that metastasis is a multidirectional process whereby cancer cells can seed distant sites as well as the primary tumor itself in a process of “self-seeding” (19, 20). Similarly, it has been shown that many host cells move between tumor sites (21–25). Metastatic regions, therefore, act as metacommunities in exchanging species (tumor and host cells) and information.
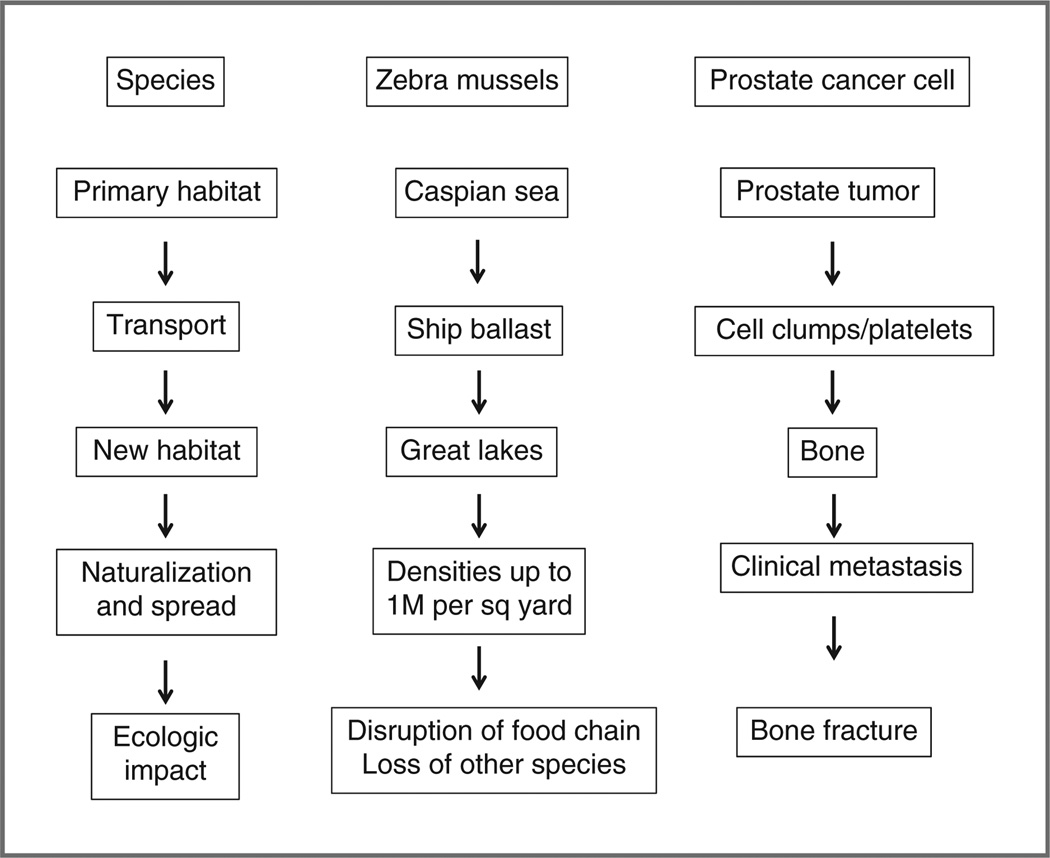
Figure 1.
Cancer cells act as an invasive species. Many cancers, if left untreated, take on the classic characteristics of an invasive species by spreading to new environments. A classic example of an invasive species is the zebra mussel. Originally only found in the Caspian Sea, it hitched a ride in the ballast tanks of ships and was dumped in the Great Lakes where it has wreaked havoc on the local ecosystem, continuing now to spread all the way to Louisiana. Similarly, cancer cells extravasate from the primary organ, survive circulation by hitching a ride with normal cells, then intravasate and set up residence in a new organ. After a period of lying dormant, these cells eventually grow, destroying the local ecosystem through proliferation and spread (ecologic impact). The migration of prostate cancer cells to bone is a classic example of metastatic spread, causing pain and fracture.
Ecologic networks model interactions within an ecosystem as links between communities or species, which serve as nodes. Disruption of the ecosystem, whether it is at the scale of a local tumor microenvironment or at the level of metacommunities present in the host biosphere, can be accomplished through extinction of a node directly or by link (communication) disruption. This paradigm allows new ways of thinking about when and how to treat cancers. For example, the eventual proliferation of disseminated tumor cells could be slowed or halted by arresting the supportive interconnections forming during their period of dormancy (26). Consideration of both node and link targeting in reference to the scaled networks of cancer will offer insights to the cancer community for the design of cancer treatment strategies (27).
The scaled networks of cancer
Cancer is best understood as a complex system of interacting scaled networks influenced by local and distant factors (Table 2; refs. 25, 27). At each network level, the interacting components can be defined in terms of ecologic network nodes and links. In the nucleus, the nodes are the DNA, RNA, and protein molecules linked by the machinery and structural and biochemical pathways that allow assembly from DNA to protein. At the cellular level, nodes consist of individual molecules interacting through the linkage of signal transduction pathways and the cytoskeleton. Within the tumor microenvironment, the cancer cells and different host cell types act as nodes that are influenced by linkages to the extracellular matrix and the exchange of soluble factors such as chemokines and cytokines. At the patient level, the primary tumor and its metastases act as metacommunities (nodes) interacting through cell trafficking and the exchange of humoral factors (links).
Table 2.
The tumor ecosystem is a complex system of intracellular and extracellular networks
| Network | Network level | Target examples |
|---|---|---|
| Cancer cell (species) |
Nucleus | Replication Epigenetic modification |
| Transcription | ||
| Translation | ||
| Protein–protein interactions |
||
| Cell | Cell structure | |
| Cell metabolism | ||
| Cell signaling | ||
| Cell invasion/metastasis machinery |
||
| Ecosystem | Tumor microenvironment |
Biotope (extracellular matrix) |
| Angiogenesis | ||
| Host cells | ||
| Immune system | ||
| Cytokines/chemokines | ||
| Metacommunity | Metastases | Individual tumors |
| Cell trafficking | ||
| Cytokines/chemokines | ||
| Biosphere | Patient | Cytokines/chemokines |
| Hormones | ||
| Treatment toxicities |
Primary and acquired resistances seem to result from the concomitant activation of multiple, often overlapping, growth and signaling hallmark pathways (28). The next generation of cancer therapy should combine personalized data derived from cancer cells through integrative sequencing technologies with agents that target all levels of the cancer ecosystem using the concept of scaled network disruption (14, 29). Such a therapeutic scheme would concurrently or successively employ agents targeting not only nuclear and cellular networks of interest, but also cell– cell interactions within a tumor microenvironment, longer range interactions among cancer metacommunities, and interplay between the host biosphere and tumor metacommunities.
The Nuclear Network
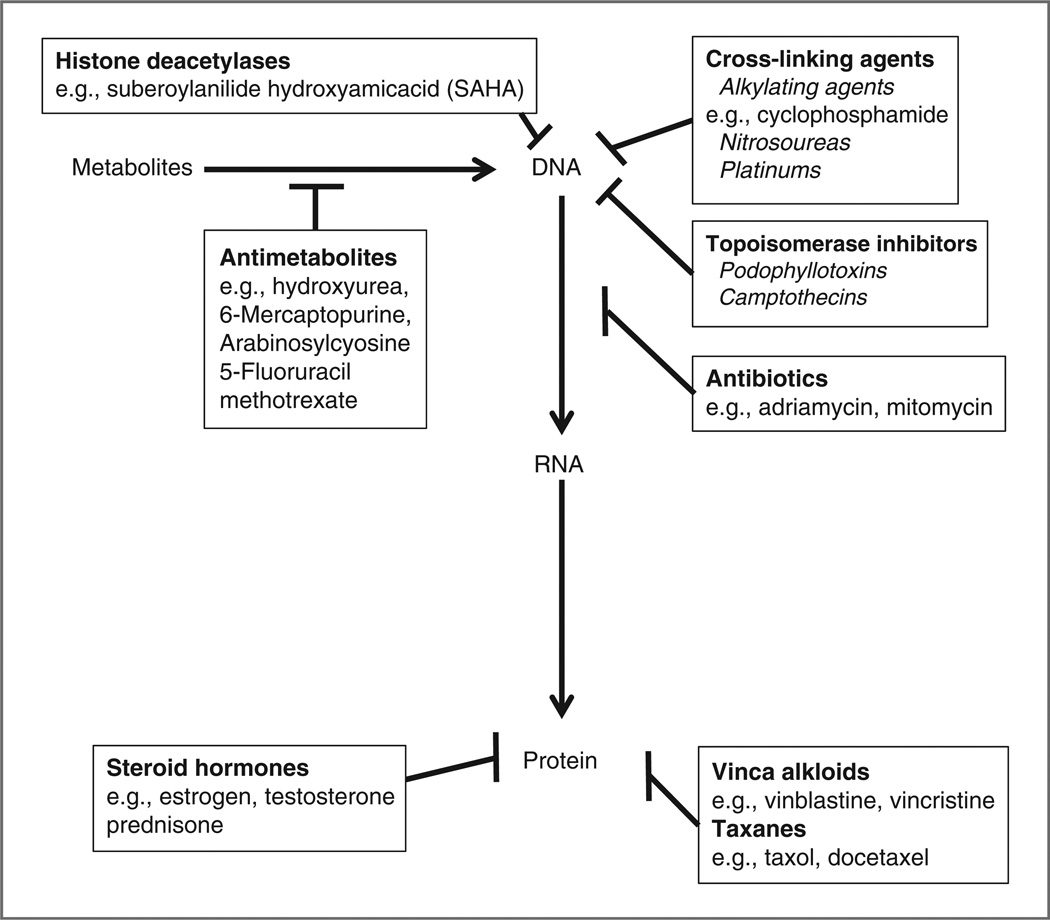
It is possible for a species within an ecosystem to be destroyed directly, for example, using pesticides to kill insects. This approach is analogous to using chemotherapy and targeted agents (30, 31). This has proven to be an inefficient approach, and, except for a few notable exceptions, for example, imantinib for chronic myelogenous leukemia, targeting just one component within the nuclear network has rarely resulted in curative cancer treatment (29, 32). Generally, success in this arena has been the result of combining multiple agents that target different levels of the nuclear network (Fig. 2). For example, CHOP chemotherapy combines agents that interrupt DNA, RNA, and protein machineries in the nucleus and cytoplasm (33, 34). Combination strategies that add modulation of histone and nonhistone protein acetylation with histone deacetylase inhibitors have improved therapeutic outcomes of multiple cancers (35, 36). Cancer cell disruption at the nuclear level is a core component of scaled network disruption, but it must be understood that the adaptive invasive species rarely succumb to one simple blow.
Figure 2.
Inhibition of DNA–RNA–protein synthesis. Traditional chemotherapeutic strategies have relied on combining multiple agents that target different levels of the nuclear network. For example, CHOP chemotherapy for lymphoma combines agents that interrupt DNA (C = cyclophosphamide), RNA (H = hydroxydaunorubicin), and protein machineries (O = oncovin or vincristine and P = prednisone) in the nucleus and cytoplasm.
The Cell Network
The networks present in the cell have classically been targeted by cell structure-disrupting agents such as micro-tubule inhibiting vinca alkyloids and taxanes (Fig. 2). The last decade has seen an explosion in “targeted therapy” to disrupt cell signaling with antibodies and small molecules directed at inhibiting receptors that activate downstream pathways and/or kinases (Fig. 3; refs. 37–40). Deregulation of receptor tyrosine kinases (RTK) has been shown in a wide variety of cancers. Multiple RTKs have been targeted through inhibition of their upstream receptors through blocking antibodies or small molecules (37–40). A second effective strategy has been to block signaling of RTK catalytic activity with small molecule inhibitors. Examples of low molecular weight TK inhibitors include imatinib, targeting tumors with mutant c-Kit, and erlotinib, targeting cancers with mutant epidermal growth factor receptor. RTK inhibition is now being combined with multiple traditional chemotherapeutics as well as newer targeted agents for network disruption at the nuclear and cellular levels.
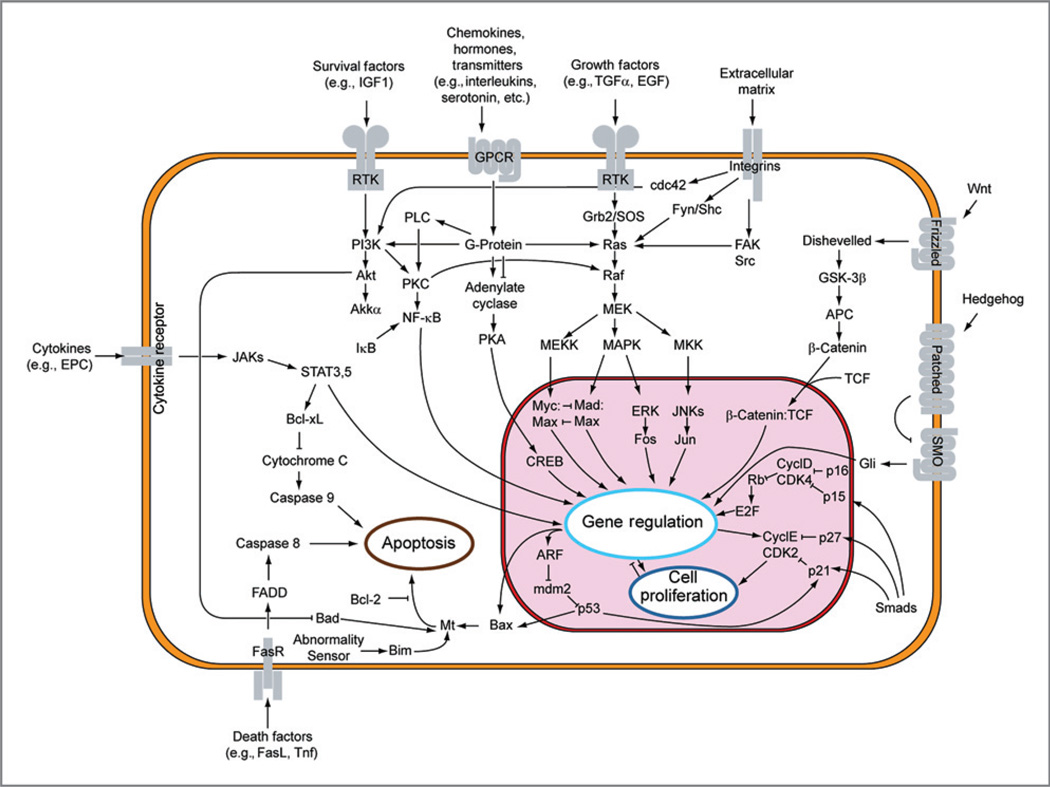
Figure 3.
Major signal transduction pathways disrupted in cancer. Targeted therapies in the form of antibodies and small molecules have been designed to inhibit receptors and downstream pathways and/or kinases that have been shown to be disrupted in cancer. Obtained from Wikipedia Commons. Originally derived from the Hallmarks of Cancer (4).
In addition, therapies are being developed that disrupt cell metabolism such as the proteasome as well as chaper-one molecules (41, 42). The ubiquitin–proteasome pathway is the major intracellular mechanism for controlled protein degradation and is an attractive target for disrupting intracellular cancer networks because the majority of cell-cycle regulators and modulators of apoptosis are degraded via this pathway. The first agent to be approved to target the proteasome is bortezomib in the treatment of multiple myeloma (41, 42). This agent is now being combined with other therapeutics to improve therapeutic outcomes (43).
The Tumor Microenvironment Ecosystem Network
Within the context of ecology and Darwinian evolution, the most efficient way to kill a species is to alter its ecosystem. For instance, it is easier to drain a swamp than to try to kill all of the mosquitoes with pesticide. This approach, however, also kills all of the other species living in the swamp. The challenge in patients with cancer is identifying and eliminating nonessential elements of the tumor environment that are promoting the growth of cancer cells. These nonessential elements may be host cells elements of the biotope that are promoting cancer growth (10). The extracellular matrix, acting as a node in the microenvironment network, has been shown to regulate cell functions, cell metabolism, and cell–cell interactions (44–46). For example, matrix-associated resistance to therapy via upregulation of cellular survival programs in response to PI3K/ mTOR inhibition was recently shown, further magnifying the apparent need for therapeutics targeting the extracellular matrix (47).
The targeting of nonessential tumor microenvironment components has already been applied to cancer treatment with success. The use of bisphosphonates (targeting the biotope of bone by binding to the bone matrix) has been shown to reduce skeletal-related events in multiple cancers including breast, prostate, and myeloma (48). Thus, the host cells act as nodes that can be targeted through direct inhibitors or strategies that interrupt the communications between them and the supporting ecosystem. At any given time, a cancer cell is interacting with more than 20 different species of host cells, providing a multitude of potential nodal and link targets (10). Examples of targeting nodes directly include inhibiting host endothelial cells through RTK antiangiogenic therapy or modulating T-cell responses to the presence of cancer (49, 50). Strategies to inhibit network links are also being used, but further development is needed. Bevacizumab is used to bind the cancer cell produced soluble signal VEGF, inhibiting the signal and subsequent growth of the host endothelial cells (49, 51). Strategies that inhibit other nonessential host cells such as tumor-associated macrophages and cancer-associated fibroblasts are being studied (52, 53).
The Metastases Metacommunity Network
It has now been established in multiple experimental systems that cancer cells traffic between tumor sites within the host (19–24). In addition, multiple host cells, including hematopoietic stem cells, endothelial progenitors, cancer-associated fibroblasts, and inflammatory mononuclear cells (T-, B-, and monocytes) seem to traffic freely between tumor sites (19–24). This is analogous to cities connected by highways with traffic moving between them. The most direct application of disrupting the metastasis metacommunity is the treatment of solitary metastases or minimal metastatic disease, resulting in decreased tumor burden and increased life for the patient. Although rarely curative, resection of metastases in colon, renal, GIST, breast, and lung cancers seem to have palliative benefits in many patients (54, 55).
The Host Biosphere Network
Cancer is an endocrine organ that affects the host at a systemic level. The traits that a cancer acquires to successfully grow and metastasize to distant sites produce multiple factors that result in different clinical syndromes that are lethal for the patient (56). The reasons patients succumb to cancer can be roughly divided into 2 categories: death due to specific organ involvement with subsequent organ function failure (e.g., metastases to the brain), or death due to a variety of clinical syndromes that have a common theme of cytokine overproduction. We have previously shown that multiple cytokines, including interleukin (IL)-6, IL-1, IL-11, CCL2, CXCL12, TGF-β, and TNF-α, are commonly produced by the majority of metastatic cancers and are important mediators of the lethal phenotype. These cytokines represent an ecologic network link between the metacommunities of cancer cells and lead to morbidity and mortality by causing cancer-related syndromes, including cachexia, thrombosis, and dyspnea. Interventions that disrupt these links have the potential not only to decrease the morbidity of cancer, but also to increase the lifespan of patients suffering from cancer (Table 3).
Table 3.
Examples of chemokines, cytokines, and other factors that play a role in the scaled networks of cancer
| Factors | Role in cancer networks |
|---|---|
| Chemokines | |
| CCL2/CCR2 | Facilitates invasion and metastasis, promotes cancer cell growth by autocrine regulation, contributes to regulation of angiogenesis. |
| CXCL12/CXCR4 | Regulates stem cell homing and plays a crucial role in facilitating those tumors which metastasize to bone. |
| CXCL16/CXCR6 | Induces chemotaxis of Th1T cells and promotes angiogenesis. |
| Cytokines | |
| IL-1 | Contributes to ability to metastasize, implicated as a tumor cell growth factor, stimulates angiogenic factors, implicated in thrombosis, cachexia, and bone metastases. |
| IL-2 | Activates Treg cells, modulates the inflammatory environment. |
| IL-6 | Promotes cancer growth, implicated as a tumor cell growth factor, stimulates angiogenic factors, implicated in thrombosis, cachexia, and bone metastases. |
| NF-κB | Key mediator and regulator of the inflammatory process, participates in feedback loop of proinflammatory cytokines, suppresses apoptosis, promotes tumor invasion and metastasis, contributes to tumor proliferation by activating the expression of growth factor genes, contributes to genomic instability of the cancer cells. |
| TNF-α | Induces DNA damage and inhibits DNA repair, promotes tumor growth, induces angiogenic factors, key in initiation of inflammatory cascade, regulates chemokines, contributes to ability for invasion, contributes to cachexia syndrome, implicated in thrombosis, contributes to bone metastases. |
| TGF-β | Contributes to angiogenesis, implicated in thrombosis, contributes to bone metastases |
| VEGF | Induces tumor angiogenesis in solid tumors and promotes tumor growth and metastasis. |
| Adipokines | Mediate inflammation, immunity, metabolism, and lipogenesis, contributing to tumor growth, metastasis, and cachexia. |
| Proteases | |
| MMPs | Enzyme involved in degradation of extracellular matrix and is upregulated in most cancers, allowing tumor cell invasion and metastasis. |
| uPA | uPA levels in both resected tumor tissue and plasma are of independent prognostic significance for patient survival in several types of human cancer. |
| Cathepsins | Cysteine proteases degrade collagen in bone and extracellular matrices in other organs. Contribute to angiogenesis and metastasis. |
| Coagulation cascade | |
| Thrombin | Thrombin generation is crucial for metastasis through fibrin and platelet deposition. Thrombin receptor upregulation has been reported in a variety of malignant tissues. |
| Tissue factor | Advanced cancer is associated with a hypercoagulable state that is triggered by tissue factor (TF). TF significantly participates in tumor-associated angiogenesis, and its expression levels have been correlated with the metastatic potential. |
| Hormones | |
| Sex hormones | Contribute to bone mass, muscle mass, energy, and metabolism across multiple cell types. |
NOTE: Adapted from Ref. 56.
Abbreviations: CCL2/CCR2, monocyte chemoattractant protein-1 and its receptor; CXCL12/CXCR4, stromal derived factor-1 and its receptor; MMPs, matrix metalloproteinases; uPA, uroplasminogen activator.
Conclusions
Despite many advances, cure rates for advanced cancer remain low. There is an imperative need to identify new targets to treat metastatic cancer using novel and existing agents. The current state of treatment based on “personalized oncology” faces the challenge that it defines cancer cell aberrations that occur only in an individual’s tumor without taking into account how these aberrations are related to the tumor microenvironment and how they relate to the host patient in which the malignancy resides. The targets identified through these advances will need to be used in context of the different biologic networks in which those mutations reside. This requires an expansion in thinking from targeting cancer cells solely at the nuclear and signal transduction levels to targeting multiple network levels by disrupting the nodes and links at every level of the cancer ecosystem, that is, scaled network disruption.
Moving theory to practice begs the question of how to take the concepts of ecotherapy and scaled network disruption to make them actionable. Virtually all therapeutic advances require the incremental addition of a single agent to a current regimen, within the jurisdiction of few scaled networks. This stepwise progressionintreatment affects one additional link or node on whose dependence the cancer cell is more easily attenuated than if a larger-scaled therapeutic approach were employed. Thus, incremental therapeutic evolution has proven to be a slow process and may never identify additive or synergistic effects of combining multiple agents across the network scales.
Data mining through bioinformatics and computational medicine applications applied to freely accessible cohorts of patients could be of major help to the community. For example, multiple studies have suggested, but never definitively, that patients on anticoagulation therapy may survive longer (57, 58). One can speculate that this may be due to direct cytotoxic effects (cell network disruption), effects on cell trafficking (metacommunity network disruption), or due to decreased thrombogenic events (host biosphere network disruption). Recent reviews of large databases by Akl and colleagues suggest that patients on parental anticoagulation therapy, but not oral therapy, have a survival benefit at 24 months (57, 58). The creation of a queriable national database of the anonymized results of the trials registered on clinicaltrials.gov would allow the virtual collection of how numerous agents may be interacting—essentially enabling electronic trials. For example, one can envision extracting the data on patients of many different cancer types who had been treated with antimetabolite chemotherapy (nuclear network) plus an antimitotic (cytoplasmic network) plus a bisphosphonate (ecosystem network) plus heparin (biosphere network). This would provide a framework to design a trial with real patients and novel combinations in nonincremental fashion.
The one thing a cancer cell cannot run from is its ecosystem. Although cancer cells may mutate and develop resistance, the host cells of the local microenvironment and greater patient biosphere do not, providing stable targets (nodes and links) for multitargeted cancer therapy.
Translational Relevance.
Although the last decade has seen an explosion in the understanding of cancer as a systemic disease, cancer remains a major cause of morbidity and mortality. Tumor growth and metastasis are consequences of genetic instability resulting in tumor cell heterogeneity and are further enabled by the proinflammatory components of the tumor microenvironment. An ecosystem is defined as the organisms in a given area interacting with their environment. Cancer cells exist within a complex ecosystem populated by host cells including fibroblasts, endothelial cells, and leukocytes within a scaffold of extracellular matrix. Designing new therapies for cancer based on an ecologic framework (ecotherapy) allows for the application of precision medicine principles (e.g., the use of integrative sequencing results to identify actionable targets from the mutational landscape of cancer cells) while implementing more comprehensive therapeutic regimens that attack host cell support systems within the tumor ecosystem.
Acknowledgments
The authors thank Gail Ash for administrative support and Robert Getzenberg and Russ Taichman for suggestions.
Grant Support
This work was supported by NIH U54 CA163124, NIH 1 U01CA143055-01A1, 2 P50 CA69568, and NIH 1 PO1 CA093900. K.J. Pienta receives support from the Prostate Cancer Foundation, the Taubman Research Institute as a Taubman Scholar, and the American Cancer Society as a Clinical Research Professor.
Footnotes
Disclosure of Potential Conflicts of Interest
K.J. Pienta is a consultant and is on the advisory board of Curis, Inc. D.F. Camacho disclosed no potential conflicts of interest.
Authors’ Contributions
Conception and design: K.J. Pienta.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): K.J. Pienta.
Writing, review, and/or revision of the manuscript: K.J. Pienta, D.F. Camacho.
References
- 1.Smithers DW. An attack on cytologism. Lancet. 1962;1:493–499. doi: 10.1016/s0140-6736(62)91475-7. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 8.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Tansley AG. The use and abuse of vegetational concepts and terms. Ecology. 1935;16:284–307. [Google Scholar]
- 10.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beltrao P, Cagney G, Krogan NJ. Quantitative genetic interactions reveal biological modularity. Cell. 2010;141:739–745. doi: 10.1016/j.cell.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mareel M, Constantino S. Ecosystems of invasion and metastasis in mammary morphogenesis and cancer. Int J Dev Biol. 2011;55:671–684. doi: 10.1387/ijdb.113386mm. [DOI] [PubMed] [Google Scholar]
- 13.Ziogas DE, Katsios C, Roukos DH. From traditional molecular biology to network oncology. Future Oncol. 2011;7:155–159. doi: 10.2217/fon.10.190. [DOI] [PubMed] [Google Scholar]
- 14.Roychowdhury S, Iyer MK, Robinson DR, Lonigro RJ, Wu YM, Cao X, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111ra21. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson DS. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology. 1992;73:1984–2000. [Google Scholar]
- 16.Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett. 2004;7:601–613. [Google Scholar]
- 17.Chen KW, Pienta KJ. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theor Biol Med Model. 2011;8:36. doi: 10.1186/1742-4682-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pienta KJ, Loberg R. The “emigration, migration, and immigration” of prostate cancer. Clin Prostate Cancer. 2005;4:24–30. doi: 10.3816/cgc.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 19.Comen E, Norton L, Massague J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 20.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2011 Nov 19; doi: 10.1007/s10555-011-9340-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Mareel M, Oliveira MJ, Madani I. Cancer invasion and metastasis: interacting ecosystems. Virchows Arch. 2009;454:599–622. doi: 10.1007/s00428-009-0784-0. [DOI] [PubMed] [Google Scholar]
- 23.Grisendi G, Bussolari R, Veronesi E, Piccinno S, Burns JS, De Santis G, et al. Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers. Am J Cancer Res. 2011;1:787–805. [PMC free article] [PubMed] [Google Scholar]
- 24.Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvi-ronment on the progression of cancer. J Pathol. 2011;223:162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 25.Tyler AL, Asselbergs FW, Williams SM, Moore JH. Shadows of complexity: what biological networks reveal about epistasis and pleiotropy. Bioessays. 2009;31:220–227. doi: 10.1002/bies.200800022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res. 2011;17:5553–5558. doi: 10.1158/1078-0432.CCR-10-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–C52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 28.Tortora G, Bianco R, Daniele G, Ciardiello F, McCubrey JA, Ricciardi MR, et al. Overcoming resistance to molecularly targeted anticancer therapies: Rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist Update. 2007;10:81–100. doi: 10.1016/j.drup.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak EL, Clark JW, Chabner B. Targeted agents: the rules of combination. Clin Cancer Res. 2007;13:5232–7. doi: 10.1158/1078-0432.CCR-07-1385. [DOI] [PubMed] [Google Scholar]
- 30.Allgayer H, Fulda S. An introduction to molecular targeted therapy of cancer. Adv Med Sci. 2008;53:130–138. doi: 10.2478/v10039-008-0025-9. [DOI] [PubMed] [Google Scholar]
- 31.Yasui H, Imai K. Novel molecular-targeted therapeutics for the treatment of cancer. Anticancer Agents Med Chem. 2008;8:470–480. doi: 10.2174/187152008784533099. [DOI] [PubMed] [Google Scholar]
- 32.Coffey DS, Getzenberg RH, DeWeese TL. Hyperthermic biology and cancer therapies: a hypothesis for the “Lance Armstrong effect”. JAMA. 2006;296:445–448. doi: 10.1001/jama.296.4.445. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematop. 2011;51:1–5. doi: 10.3960/jslrt.51.1. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RI, Miller TP, O’Connor OA. Diffuse aggressive lymphoma. Hematology Am Soc Hematol Educ Program. 2004:221–236. doi: 10.1182/asheducation-2004.1.221. [DOI] [PubMed] [Google Scholar]
- 35.Song SH, Han SW, Bang YJ. Epigenetic-based therapies in cancer: progress to date. Drugs. 2011;71:2391–403. doi: 10.2165/11596690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Miller CP, Singh MM, Rivera-Del Valle N, Manton CA, Chandra J. Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors. J Biomed Biotechnol. 2011;2011:514261. doi: 10.1155/2011/514261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenzweig SA. Acquired resistance to drugs targeting receptor tyrosine kinases. Biochem Pharmacol. 2012;83:1041–1048. doi: 10.1016/j.bcp.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: When a specificity becomes an advantage. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34:1774–1780. doi: 10.1248/bpb.34.1774. [DOI] [PubMed] [Google Scholar]
- 40.Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I. Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther [Review] 2010;10:839–853. doi: 10.4161/cbt.10.9.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman AM, Shabek N, Ciechanover A. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat Rev Mol Cell Biol. 2011;12:605–620. doi: 10.1038/nrm3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cvek B, Dvorak Z. The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib. Curr Pharm Des. 2011;17:1483–1499. doi: 10.2174/138161211796197124. [DOI] [PubMed] [Google Scholar]
- 43.de Queiroz Crusoe E, Maiso P, Fernandez-Lazaro D, San-Segundo L, Garayoa M, Garcia-Gomez A, et al. Transcriptomic rationale for the synergy observed with dasatinib + bortezomib + dexamethasone in multiple myeloma. Ann Hematol. 2012;91:257–269. doi: 10.1007/s00277-011-1287-z. [DOI] [PubMed] [Google Scholar]
- 44.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassian AR, Coloff JL, Brugge JS. Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb Symp Quant Biol. 2011 Nov 21; doi: 10.1101/sqb.2011.76.010967. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleber M, Udi J, Metzke B, Terpos E, Roodmann GD, Morgan G, et al. Challenging the current approaches to multiple myeloma- and other cancer-related bone diseases: from bisphosphonates to targeted therapy. Leuk Lymphoma. 2012 Jan 13; doi: 10.3109/10428194.2011.644548. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Gaitskell K, Martinek I, Bryant A, Kehoe S, Nicum S, Morrison J. Angiogenesis inhibitors for the treatment of ovarian cancer. Cochrane Database Syst Rev. 2011;9:CD007930. doi: 10.1002/14651858.CD007930.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwala SS, Ribas A. Current experience with CTLA4-blocking monoclonal antibodies for the treatment of solid tumors. J Immunother. 2010;33:557–569. doi: 10.1097/CJI.0b013e3181dcd260. [DOI] [PubMed] [Google Scholar]
- 51.Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci U S A. 2006;103:13474–13479. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kycler W, Laski P. Surgical approach to pulmonary metastases from breast cancer. Breast J. 2012;18:52–57. doi: 10.1111/j.1524-4741.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42:945–949. doi: 10.1097/MCG.0b013e318064e752. [DOI] [PubMed] [Google Scholar]
- 56.Loberg RD, Bradley DA, Tomlins SA, Chinnaiyan AM, Pienta KJ. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J Clin. 2007;57:225–241. doi: 10.3322/canjclin.57.4.225. [DOI] [PubMed] [Google Scholar]
- 57.Akl EA, Gunukula S, Barba M, Yosuico VE, van Doormaal FF, Kuipers S, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011:CD006652. doi: 10.1002/14651858.CD006652.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Akl EA, Vasireddi SR, Gunukula S, Yosuico VE, Barba M, Terrenato I, et al. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011:CD006466. doi: 10.1002/14651858.CD006466.pub3. [DOI] [PubMed] [Google Scholar]