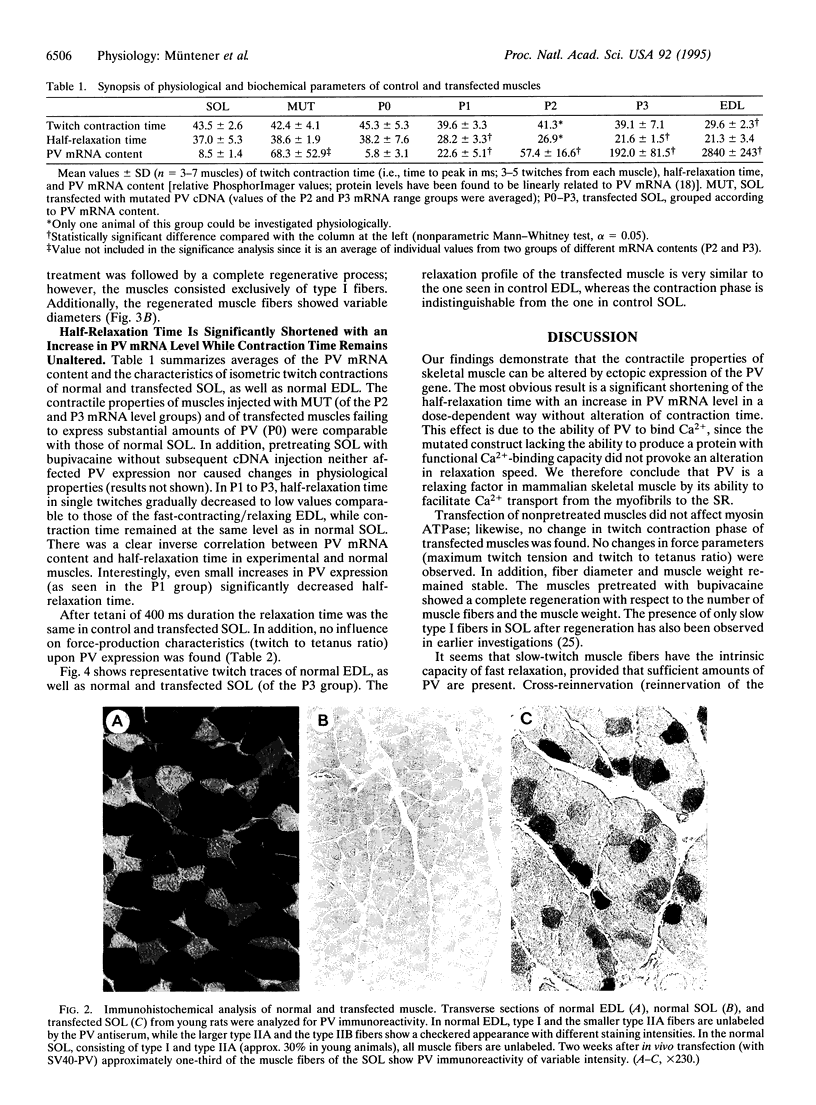
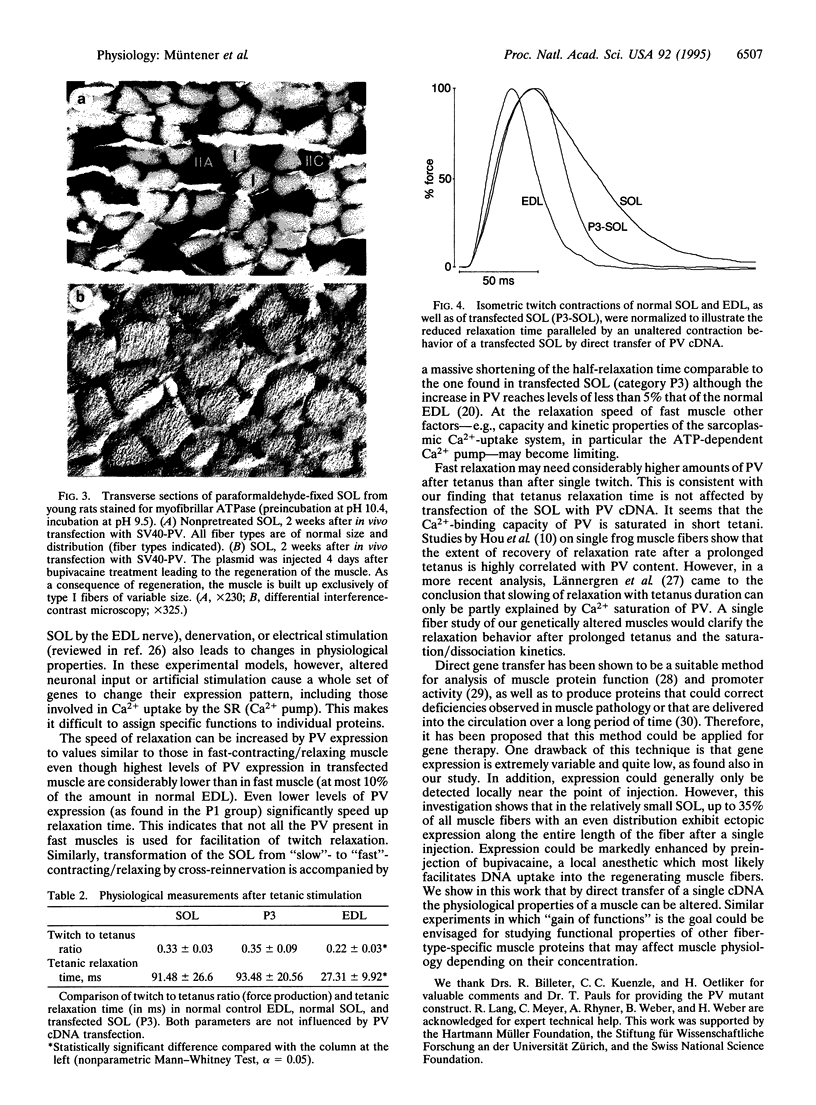
Abstract
Parvalbumin (PV) is a high affinity Ca(2+)-binding protein found at high concentration in fast-contracting/relaxing skeletal muscle fibers of vertebrates. It has been proposed that PV acts in the process of muscle relaxation by facilitating Ca2+ transport from the myofibrils to the sarcoplasmic reticulum. However, on the basis of metal-binding kinetics of PV in vitro, this hypothesis has been challenged. To investigate the function of PV in skeletal muscle fibers, direct gene transfer was applied in normal and regenerating rat soleus muscles which do not synthesize detectable amounts of PV. Two weeks after in vivo transfection with PV cDNA, considerable levels of PV mRNA and protein were detected in normal muscle, and even higher amounts were detected in regenerating muscle. Twitch half-relaxation time was significantly shortened in a dose-dependent way in transfected muscles, while contraction time remained unaltered. The observed shortening of half-relaxation time is due to PV and its ability to bind Ca2+, because a mutant protein lacking Ca(2+)-binding capacity did not promote any change in physiology. These results directly demonstrate the physiological function of PV as a relaxing factor in mammalian skeletal muscle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Dickson G., Love D. R., Jani A., Walsh F. S., Gurusinghe A., Wolff J. A., Davies K. E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991 Aug 29;352(6338):815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- Acsadi G., Dickson G., Love D. R., Jani A., Walsh F. S., Gurusinghe A., Wolff J. A., Davies K. E. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991 Aug 29;352(6338):815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- Benoit P. W., Belt W. D. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine). J Anat. 1970 Nov;107(Pt 3):547–556. [PMC free article] [PubMed] [Google Scholar]
- Berchtold M. W. Structure and expression of genes encoding the three-domain Ca2+-binding proteins parvalbumin and oncomodulin. Biochim Biophys Acta. 1989 Dec 22;1009(3):201–215. doi: 10.1016/0167-4781(89)90104-8. [DOI] [PubMed] [Google Scholar]
- Castillo M. B., Celio M. R., Andressen C., Gotzos V., Rülicke T., Berger M. C., Weber J., Berchtold M. W. Production and analysis of transgenic mice with ectopic expression of parvalbumin. Arch Biochem Biophys. 1995 Feb 20;317(1):292–298. doi: 10.1006/abbi.1995.1165. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Baier W., Schärer L., de Viragh P. A., Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988 Apr;9(2):81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Epstein P., Means A. R., Berchtold M. W. Isolation of a rat parvalbumin gene and full length cDNA. J Biol Chem. 1986 May 5;261(13):5886–5891. [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Haiech J., Derancourt J., Pechère J. F., Demaille J. G. Magnesium and calcium binding to parvalbumins: evidence for differences between parvalbumins and an explanation of their relaxing function. Biochemistry. 1979 Jun 26;18(13):2752–2758. doi: 10.1021/bi00580a010. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Berchtold M. W., Rowlerson A. M. Correlation of parvalbumin concentration with relaxation speed in mammalian muscles. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7243–7247. doi: 10.1073/pnas.79.23.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Elzinga G., Stienen G. J. Force relaxation, labile heat and parvalbumin content of skeletal muscle fibres of Xenopus laevis. J Physiol. 1993 Apr;463:123–140. doi: 10.1113/jphysiol.1993.sp019587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntener M., Rowlerson A. M., Berchtold M. W., Heizmann C. W. Changes in the concentration of the calcium-binding parvalbumin in cross-reinnervated rat muscles. Comparison of biochemical with physiological and histochemical parameters. J Biol Chem. 1987 Jan 5;262(1):465–469. [PubMed] [Google Scholar]
- Pauls T. L., Durussel I., Berchtold M. W., Cox J. A. Inactivation of individual Ca(2+)-binding sites in the paired EF-hand sites of parvalbumin reveals asymmetrical metal-binding properties. Biochemistry. 1994 Aug 30;33(34):10393–10400. doi: 10.1021/bi00200a021. [DOI] [PubMed] [Google Scholar]
- Pauls T. L., Durussel I., Cox J. A., Clark I. D., Szabo A. G., Gagné S. M., Sykes B. D., Berchtold M. W. Metal binding properties of recombinant rat parvalbumin wild-type and F102W mutant. J Biol Chem. 1993 Oct 5;268(28):20897–20903. [PubMed] [Google Scholar]
- Pechère J. F., Derancourt J., Haiech J. The participation of parvalbumins in the activation-relaxation cycle of vertebrate fast skeletal-muscle. FEBS Lett. 1977 Mar 15;75(1):111–114. doi: 10.1016/0014-5793(77)80064-1. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Dedman J. R., Means A. R. Ca2+-dependent regulation of cyclic-AMP phosphodiesterase by parvalbumin. J Biol Chem. 1977 Aug 25;252(16):5609–5611. [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C. K., Gualberto A., Patel C. V., Walsh K. Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol Cell Biol. 1993 Feb;13(2):1264–1272. doi: 10.1128/mcb.13.2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitadello M., Schiaffino M. V., Picard A., Scarpa M., Schiaffino S. Gene transfer in regenerating muscle. Hum Gene Ther. 1994 Jan;5(1):11–18. doi: 10.1089/hum.1994.5.1-11. [DOI] [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Harris J. B., Butler-Browne G. S., Sesodia S. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev Biol. 1990 Sep;141(1):24–40. doi: 10.1016/0012-1606(90)90099-5. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Williams P., Acsadi G., Jiao S., Jani A., Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. Biotechniques. 1991 Oct;11(4):474–485. [PubMed] [Google Scholar]