Abstract
Four extra early-flowering mutants, named extra early-flowering1 (exe1), exe2, exe3, and exe4, were identified in Triticum monococcum strain KU104-1 following heavy-ion beam mutagenesis. The four exe mutants fell into two groups, namely Type I (moderately extra early-flowering type; exe1 and exe3) and Type II (extremely extra early-flowering type; exe2 and exe4). Analysis of plant development in a growth chamber showed that the speed of leaf emergence was accelerated in exe mutants at the reproductive stage compared to wild-type (WT) plants. The speed of leaf emergence was faster in Type II than Type I plants. Analysis of VERNALIZATION 1 (VRN1), a flowering promoter gene, showed that it was more highly expressed in seedlings at early developmental stages in Type II mutants than Type I mutants. These findings indicate that the difference in earliness between Type I and Type II mutants is associated with the level of VRN1 expression. The original KU104-1 is an einkorn wheat strain that carries a null allele of the VRN2 gene, a repressor of flowering. Thus, our results indicate that the level of VRN1 expression controls earliness in exe mutants independently of VRN2.
Keywords: early-flowering, ion beam mutagenesis, VERNALIZATION 1, wheat, Triticum monococcum
Introduction
Many plant species display an adaptation termed vernalization in which an extended period of low temperature in winter results in competence to flower during the following spring. Interestingly, recent studies have revealed that vernalization systems evolved independently in different plant groups (Ream et al. 2013). In temperate cereals, such as wheat (Triticum aestivum) and barley (Hordeum vulgare), three genes have been found to control the need for vernalization, namely, VERNALIZATION 1 (VRN1), VRN2 and VRN3 (Distelfeld et al. 2009a).
VRN1 encodes an APETALA1/FRUITFULL-like (AP1/FUL-like) MADS-box transcription factor that is up-regulated by vernalization (Danyluk et al. 2003, Murai et al. 2003, Trevaskis et al. 2003, Yan et al. 2003). The expression of VRN1 is epigenetically suppressed in seedlings at early stages of the vegetative growth phase; this suppression is caused by modification of histones at the VRN1 locus (Diallo et al. 2012, Oliver et al. 2009). The repressive histone state is modified by signals induced by vernalization, resulting in the up-regulation of VRN1. A recent study found that rapid increases in histone acetylation levels at the VRN1 locus in response to a 24 hr cold treatment trigger the vernalization effect (Oliver et al. 2013). The level of VRN1 expression gradually increases during the seedling growth stage without the need for vernalization (Kitagawa et al. 2012, Murai et al. 2003), suggesting that the epigenetic status of VRN1 is also modified by internal signals such as aging. Furthermore, VRN1 shows a diurnal expression pattern that is affected by the length of daylight, with a long photoperiod producing up-regulation of its expression level (Shimada et al. 2009). These observations indicate that VRN1 expression is controlled by autonomous and photoperiodic pathways as well as by the vernalization pathway.
The VRN2 locus consists of two linked genes, ZCCT1 and ZCCT2, which encode a protein with a zinc finger motif and a CCT domain (Yan et al. 2004). There are no clear homologs of VRN2 in the Arabidopsis genome. Natural variations have been identified in the VRN2 locus. Simultaneous deletion or non-functional mutations of the two ZCCT genes result in a plant that has a spring habit (Distelfeld et al. 2009b, Dubcovsky et al. 2005, Yan et al. 2004), indicating that VRN2 is a flowering repressor gene. A high level of VRN2 expression is observed in seedlings at the 1-leaf stage, while expression is down-regulated by vernalization and aging. By contrast, VRN1 shows the opposite pattern with low expression in seedlings and up-regulated expression after vernalization (Shimada et al. 2009). It has also been reported that VRN2 shows a diurnal expression pattern and that a long photoperiod up-regulates its expression level (Dubcovsky et al. 2006, Trevaskis et al. 2006). However, as plants encounter a long photoperiod during spring to early summer when they are at the reproductive growth stage, it is unlikely that VRN2 is up-regulated by a long photoperiod in the field.
VRN3 encodes a Raf kinase inhibitor-like protein with a high similarity to the Arabidopsis FLOWERING LOCUS T (FT) protein, which is a florigen (Yan et al. 2006). Therefore, VRN3 is also called FT1 in barley (Hemming et al. 2008, Sasani et al. 2009) and WFT (wheat FT) in wheat (Shimada et al. 2009). Transgenic wheat plants overexpressing VRN3 show an extra early flowering phenotype without the need for vernalization (Shimada et al. 2009, Yan et al. 2006), indicating that VRN3 is a strong flowering promoter. Under long day conditions, VRN3 shows a diurnal expression pattern; however, expression is very low under short day conditions (Kitagawa et al. 2012, Shimada et al. 2009). In this study, we refer to VRN3 as WFT, as in our previous studies (Kitagawa et al. 2012, Shimada et al. 2009).
VRN2 and WFT are preferentially expressed in leaves (Yan et al. 2004, 2006), while VRN1 is expressed in both leaves and shoot apical meristems (SAMs) (Kinjo et al. 2012, Sasani et al. 2009, Yan et al. 2003). These expression patterns suggest that VRN1 and VRN2 might cooperate in the up-regulation of florigenic WFT proteins in leaves. Kinjo et al. (2012) postulated that WFT proteins are transported from the leaves to the SAMs, where they interact with VRN1 to induce flowering.
To further elucidate the mechanisms that control flowering in wheat, especially the relationship between VRN1 and WFT, we are developing a large-scale panel of mutants in diploid einkorn wheat (Triticum monococcum). These mutants were induced by heavy-ion beam mutagenesis and are being systematically screened for effects on flowering time (Murai et al. 2013). Heavy-ion beam irradiation is effective at producing gene deletions (null mutations) (Kazama et al. 2011, 2013). Here we describe four newly identified extra-early flowering mutant lines in diploid einkorn wheat, which have been named extra early-flowering 1 (exe1), exe2, exe3, and exe4. Based on their phenotypes in the field, the four exe mutants were classified into two groups: Type I (moderately extra early-flowering type; exe1 and exe3) and Type II (extremely extra early-flowering type; exe2 and exe4). Our analyses showed that the expression level of VRN1 was correlated with earliness in exe mutants. The parental strain, KU104-1, carries a null allele of VRN2, which is a repressor of flowering. Our expression analyses indicate that the level of VRN1 expression controls earliness in exe mutants independently of VRN2. Here, we present a model for flowering in temperate cereals in which environmental signals, such as cold and long photoperiods, and internal signals, such as aging, are cues for flowering; VRN1 is envisaged as functioning in the acquisition of flowering competency in leaves.
Materials and Methods
Plant material
Seeds of the diploid einkorn wheat (Triticum monococcum, 2n = 2x = 14, genome constitution AmAm) strain KU104-1 were given 50 Gy of carbon-ion irradiation at a liner energy transfer (LET) of 50 keV μm−1 and then sown in the field. It has been demonstrated previously that ion beam is an efficient inducer of mutations in plants and to predominantly cause null mutations (Kazama et al. 2011, 2013). The spikes of M1 plants were bagged and selfed seeds were harvested from each spike and used to produce the M2 lines. From approximately 1,200 M2 lines, we identified 4 mutations that showed an extra early-flowering phenotype; we termed these mutations as extra early-flowering (exe) 1, 2, 3 and 4. Wild-type (WT) strain KU104-1 and the M3 plants of all 4 exe mutations were used in the experiments. The original strain KU104-1 lacks VRN2 locus. Spring einkorn wheat strains are classified into three types in the VRN2 allele: WT, R/W mutant-type and deletion-type (Yan et al. 2004). KU104-1 is the VRN2 deletion-type einkorn wheat.
Field experiments
WT and exe mutant plants were sown in the middle of October in an experimental field at Fukui Prefectural University. The heading dates of each line were scored. WT and exe mutant plants were screened at the maturation stage to examine the following morphological characters: spike number per plant, spike length, and spikelet number per spike.
Growth chamber experiments
To examine leaf emergence speed (plastochron), the total number of leaves and heading times, WT and exe mutant plants were cultivated in a growth chamber under long day (LD; 16 h light/8 h dark) conditions or short day (SD; 10 h light/14 h dark) conditions at 20°C (light intensity ~100 μE m−2 s−1). The phenotypes of 10 WT plants and 10 plants of each exe mutation were assessed. The growth stage with transition from vegetative to reproductive phases was estimated by the initiation timing of stem elongation of the main shoot. Heading time was measured as the number of days from 1-leaf unfolding to the flag leaf unfolding. For the diurnal expression analysis of VRN1, non-vernalized WT plants were grown under LD conditions at 20°C (100 μE m−2 s−1).
Gene expression analysis
Expression of VRN1 and WFT in WT and exe mutant plants at each leaf stage was analyzed in non-vernalized plants grown under LD or SD conditions at 20°C. Seedlings from each line were sampled from the 1-leaf stage to the 6-leaf stage for analysis. Leaves were sampled 2 hr after the start of the light period. For the analysis of the diurnal expression of VRN1, seedlings at the 7-leaf stage were sampled every 2 hr over a 26 hr period. In this study, the 1-leaf stage was assumed to last from the unfolding of the 1-leaf to the unfolding of the 2-leaf.
Total RNAs were extracted from leaves using ISOGEN (Nippon Gene, Japan); cDNAs were synthesized from the total RNAs using an oligo dT primer in accordance with the protocol for the Ready-To-Go T-primed First-Strand Kit (GE Healthcare Life Sciences). Real-time PCR analyses were performed using a LightCycler 2.0 (Roche Diagnostics GmbH) with the following gene-specific primer sets: VRN1, TaMADS#11-545L (5′-GGAGAGGTCACTGCAGGAGGA-3′) and TaMADS#11-698R (5′-GCCGCTGGATGAATGCTG-3′) at an annealing temperature of 65°C; WFT, TaFT-mono3F (5′-GGTACAACTGGTGCCTCGTT-3′) and TaFT-mono3R (5′-GTTGTAGAGCTCGGCGAAGT-3′) at an annealing temperature of 64°C. The relative quantities of the transcripts were determined using a SYBR Green-labelled amplification product from Ubiquitin prepared with the primers Ubi-1L (5′-GCATGCAGATATTTGTGA-3′) and Ubi-1R (5′-GGAGCTTACTGGCCAC-3′) at an annealing temperature of 58°C. The primer sets for VRN1 and Ubiquitin were identical to those used in our previous study (Kitagawa et al. 2012). Two biological replicates with three technical replications were performed. Each biological sample contained leaves from two individual plants.
Results
Classification of the exe mutants
The extra early-flowering (exe) mutants were initially identified during the development of a large-scale mutant panel produced using heavy-ion beam mutagenesis (Murai et al. 2013, Nishiura et al. 2013). For exe1 mutation, two mutant plants were identified out of six M2 plants derived from one ear of M1 plant. One each mutant plant out of ten, nine or seven M2 plants were detected for exe2, exe3 or exe4 mutation, respectively. The M3 plants derived from one M2 mutant plants were uniform and not segregated in earliness (data not shown). These suggest that exe mutations are recessive with relatively small number of locus.
The exe mutants showed extra early heading-time compared to WT plants in the field in seasons 2011/2012 and 2012/2013 (Table 1). In early spring 2013, the plants in the experimental field were damaged by rabbits, which had a particularly adverse effect on early growing tillers. For this reason, heading of the exe mutants was delayed in season 2012/2013 compared to 2011/2012. Nevertheless, the data on heading dates in the field indicated that the exe mutants fell into two distinct groups: a moderately extra early-flowering type (Type I), which included exe1 and exe3; and an extremely extra early-flowering type (Type II), which included exe2 and exe4 (Table 1).
Table 1.
Heading dates of wild-type (WT) and exe mutant plants in the field and the relative earliness (difference) of the mutants
| Line | Season 2011/2012 | Season 2012/2013 | Type | ||
|---|---|---|---|---|---|
|
| |||||
| Heading date | Difference | Heading date | Difference | ||
| WT | 6 Jun | 0 | 8 Jun | 0 | – |
| exe1 | 7 May | −30 | 15 May | −24 | I |
| exe2 | 22 Apr | −45 | 4 May | −35 | II |
| exe3 | 7 May | −30 | 14 May | −25 | I |
| exe4 | 25 Apr | −42 | 4 May | −35 | II |
Sowing dates were Oct 18 for Season 2011/2012 and Oct 16 for Season 2012/2013.
The exe mutant plants tended to have fewer spikes than WT plants (Table 2). Furthermore, compared to WT plants, the spikes were smaller and had fewer spikelets. The Type I and Type II exe mutants clearly differed with respect to these traits: Type I (exe1 and exe3) had spikes of 5.7–5.9 cm length with 26–28 spikelets, whereas Type II (exe2 and exe4) had spikes of 4.7–4.9 cm length with 17–20 spikelets. The smaller spikes in Type II exe mutants are probably associated with their extremely extra early-flowering phenotype.
Table 2.
Mean (±SE) spike length and the number of spikelets per spike in wild-type (WT) and exe mutant plants in the field
| Line | No. of plants examined | No. spikes/plant | Spike length (cm) | No. spikelets/spike | Type |
|---|---|---|---|---|---|
| WT | 3 | 9~17 | 6.9 ± 0.21d | 32.5 ± 0.63d | – |
| exe1 | 7 | 3~11 | 5.9 ± 0.09c | 27.8 ± 0.37c | I |
| exe2 | 6 | 3~11 | 4.9 ± 0.44ab | 19.8 ± 1.22b | II |
| exe3 | 3 | 3~7 | 5.7 ± 0.24bc | 26.0 ± 0.91c | I |
| exe4 | 9 | 5~12 | 4.7 ± 0.19a | 17.1 ± 0.56a | II |
ANOVA (analysis of variance) was performed. The same superscript means no difference significantly (p = 0.05) from each other when Fisher’s LSD (least significant difference) analysis is applied.
Leaf emergence speeds in exe mutants
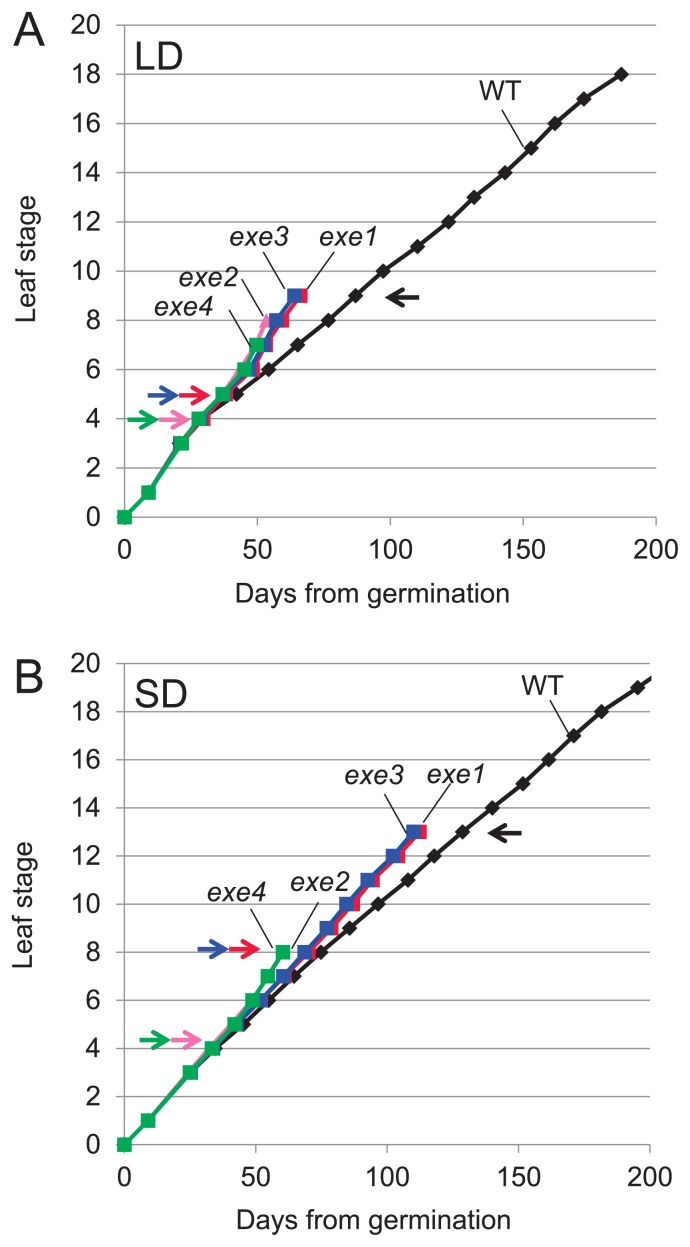
Leaf emergence timing in WT and exe mutant plants was determined by screening leaf unfolding in seedlings cultivated in a growth chamber under LD or SD conditions (Fig. 1). Under LD conditions, WT plants averaged about 180 days from sowing to flag-leaf unfolding. In contrast, Type I exe mutants took about 65 days and Type II about 50 days (Fig. 1A). WT plants transited from the vegetative to reproductive growth phase at the 9-leaf stage. However, exe mutants showed earlier phase transition: at the 5-leaf stage for exe1 and exe3 (Type I), and the 4-leaf stage for exe2 and exe4 (Type II). The production of successive leaves ceased following the initiation of the flag leaf, which could be distinguished from the other leaves by its short blade and the emergence of a spike from its leaf sheath. In WT plants, the flag-leaf occurred at the 18-leaf stage, while in exe1, exe2, exe3, and exe4 the flag leaf appeared at the 9-leaf, 8-leaf, 9-leaf, and 7-leaf stages, respectively.
Fig. 1.
Leaf emergence speed in non-vernalized exe mutants and wild-type (WT) plants grown in a growth chamber. Arrows indicate timing of phase transition: black, WT; red, exe1; pink, exe2; blue, exe3; and green, exe4. (A) Days from germination to leaf unfolding at each leaf stage grown under long day (LD) conditions. The data at the 2-leaf stage are missing. The final leaf of each line indicates flag-leaf. (B) Days from germination to leaf unfolding at each leaf stage under short day (SD) conditions. The data at the 2-leaf stage are missing. The final leaf of each exe line indicates flag-leaf. WT did not head before the 20-leaf stage.
Under SD conditions, WT plants did not unfold the flag-leaf until at least 200 days from sowing. In contrast, Type I exe mutants showed flag leaf unfolding at about 110 days and Type II at about 60 days from sowing (Fig. 1B). WT plants transited from the vegetative to reproductive growth phase at the 13-leaf stage. By contrast, exe1 and exe3 mutants (Type I) transited at the 8-leaf stage, while exe2 and exe4 mutants (Type II) transited at the 4-leaf stage. Interestingly, the timing of phase transition was early (at the 4-leaf stage) in Type II exe mutants under both SD and LD conditions. Flag-leaf emergence occurred at the 13-leaf, 8-leaf, 13-leaf, and 8-leaf stages in exe1, exe2, exe3, and exe4, respectively. The delay in flag-leaf emergence in Type I exe mutants under SD conditions compared to LD conditions indicates that these mutants retained photoperiodic sensitivity. However, Type II exe mutants had lost almost all photoperiodic sensitivity.
The timing of emergence of the first five leaves was very similar in WT and exe mutant plants (Fig. 1); subsequently, however, leaf emergence was more rapid in the exe mutants compared to WT plants. This finding indicates that rapid leaf emergence in the exe mutants was caused by a more rapid rate of leaf initiation (plastochron) in the mutants. Furthermore, the plastochron appeared to be shorter at the reproductive growth phase (after phase transition) in the mutants. Under SD conditions, Type II exe mutants (exe2 and exe4) displayed a shortened plastochron compared to Type I (exe1 and exe3) mutants (Fig. 1B).
Altered photoperiodic sensitivity in exe mutants
To compare photoperiodic sensitivities of WT and exe mutant plants, the timing from 1-leaf unfolding to flag-leaf unfolding (D1f) was determined under LD and SD conditions (Table 3). Relative photoperiodic sensitivity was estimated using the ratio of the averaged D1fs in the LD and SD regimes. WT plants had a ratio greater than 2.60, whereas those of Type I and Type II exe mutants were 1.76 and 1.17–1.24, respectively. The differences in these ratios clearly indicate that the exe mutants have a decreased photoperiod sensitivity compared to WT plants; this difference is in line with the differences in leaf numbers under LD or SD conditions in the three plant groups (Fig. 1). Furthermore, the results also indicate that Type II exe mutants have almost completely lost photoperiodic sensitivity.
Table 3.
Mean (±SE) number of days from 1-leaf unfolding to flag-leaf unfolding (D1f) under long day (LD) or short day (SD) conditions, and relative photoperiod sensitivities
| Line | D1f | Degree of photoperiod sensitivity (SD/LD) | Type | |
|---|---|---|---|---|
|
| ||||
| LD | SD | |||
| WT | 192.5 ± 5.06c | >500.0 | >2.60 | – |
| exe1 | 57.8 ± 0.80b | 101.7 ± 0.99c | 1.76 | I |
| exe2 | 43.9 ± 0.84a | 51.4 ± 0.50a | 1.17 | II |
| exe3 | 54.9 ± 0.31b | 96.9 ± 2.37b | 1.76 | I |
| exe4 | 41.4 ± 0.56a | 51.3 ± 0.68a | 1.24 | II |
ANOVA (analysis of variance) was performed. The same superscript means no difference significantly (p = 0.05) from each other when Fisher’s LSD (least significant difference) analysis is applied.
Patterns of VRN1 expression in exe mutants
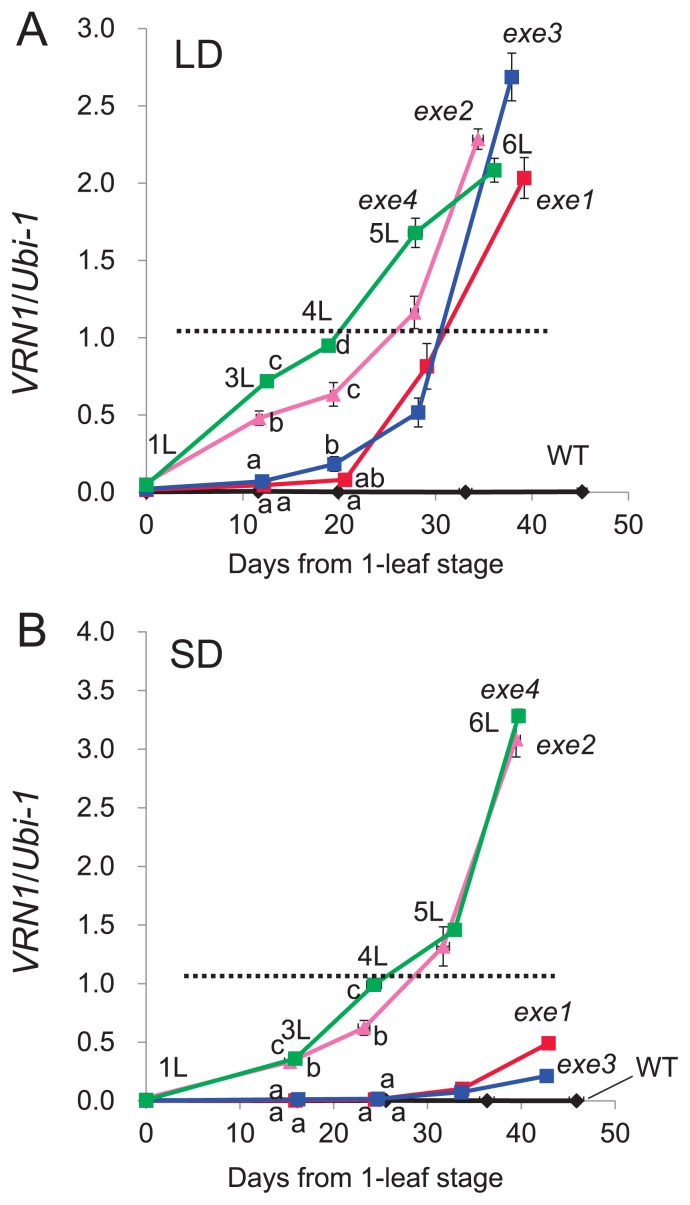
We used real-time PCR analysis to compare the levels of VRN1 expression in WT and exe mutant plants (Fig. 2). Based on the PCR and sequence analyses, it was confirmed that mutations did not occur in VRN1 of exe mutants (data not shown). cDNAs were obtained from leaves of non-vernalized plants at the 1-leaf to 6-leaf stages. Under LD conditions, WT plants showed relatively low VRN1 expression level during these stages, but the level of VRN1 expression increased with time from the 1-leaf stage in the exe mutants. Expression of VRN1 was higher at the 3-leaf to 5-leaf stages in Type II (exe2 and exe4) than Type I (exe1 and exe3) mutant plants (Fig. 2A). Notably, similar levels of VRN1 expression were observed during phase transition in each exe mutant: at the 5-leaf stage for Type I and at the 4-leaf stage for Type II. This suggests that the level of VRN1 expression is associated with phase transition from vegetative to reproductive growth and may be a threshold for flowering competency in the plants.
Fig. 2.
Patterns of VRN1 expression in non-vernalized WT and exe mutant plants at the 1-leaf to 6-leaf stages. The data at the 2-leaf stage are missing. The expression level of VRN1 was determined by real-time PCR. The Ubiquitin (Ubi-1) gene was used as an internal control for calculating the relative levels of expression of the VRN1 gene. Means and standard errors are indicated. The dotted lines indicate the expression level of VRN1 at the phase transition, i.e, the threshold for flowering competence. (A) Expression patterns in seedlings at each leaf stage under LD conditions. Leaf stage numbers (1L, 3L, etc.) are shown for exe4 only. (B) Expression patterns in seedlings at each leaf stage under SD conditions. Leaf stage numbers (1L, 3L, etc.) are shown for exe4 only. ANOVA (analysis of variance) was performed to detect the difference of gene expression level at the 3L and 4L stages. The same superscript means not significantly different (p = 0.05) from each other when Fisher’s LSD (least significant difference) analysis is applied.
The pattern of VRN1 expression under SD conditions was similar in Type II mutants to that observed under LD conditions; thus, the mutants showed a significantly higher level of expression at earlier leaf stages than WT plants (Fig. 2B). By contrast, VRN1 expression under SD conditions was not up-regulated in Type I mutants in a similar manner to that seen under LD conditions; as a consequence, the mutant plants showed a delay in heading times. Under SD conditions, Type II exe mutants (exe2 and exe4) showed phase transition at the 4-leaf stage, whereas Type I exe mutants (exe1 and exe3) were still vegetative at the 6-leaf stage. This indicates that a threshold for flowering competency exists under both SD and LD conditions.
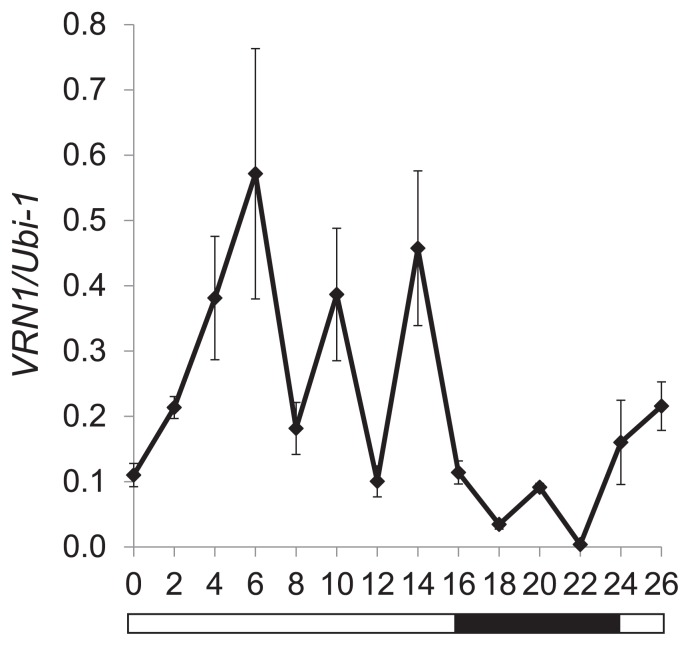
It has been reported that VRN1 shows a diurnal expression pattern in the spring bread wheat cultivar Triple Dirk (Shimada et al. 2009). We therefore examined the WT plants used here (einkorn spring wheat strain KU104-1) for diurnal expression of VRN1 (Fig. 3). At the 7-leaf stage of vegetative growth, the WT plants showed diurnal variation in VRN1 expression under LD conditions. VRN1 expression peaked during the light period and was lower during the dark period. This pattern is consistent with that reported for the Triple Dirk cultivar (Shimada et al. 2009). The occurrence of a diurnal expression pattern (Fig. 3) and the photoperiod-dependent expression of VRN1 (Fig. 2) indicate that this gene is regulated by a light-dark cycle in wheat leaves.
Fig. 3.
Diurnal expression pattern of VRN1 in spring wheat KU104-1 under long day conditions. The level of VRN1 expression was analyzed by real-time PCR using non-vernalized seedlings at the 7-leaf stage. The Ubiquitin (Ubi-1) gene was used as an internal control for calculating the relative levels of VRN1 genes. Each point indicates means and standard errors. The white and black bars along the horizontal axis represent light and dark periods, respectively.
Patterns of WFT expression in exe mutants
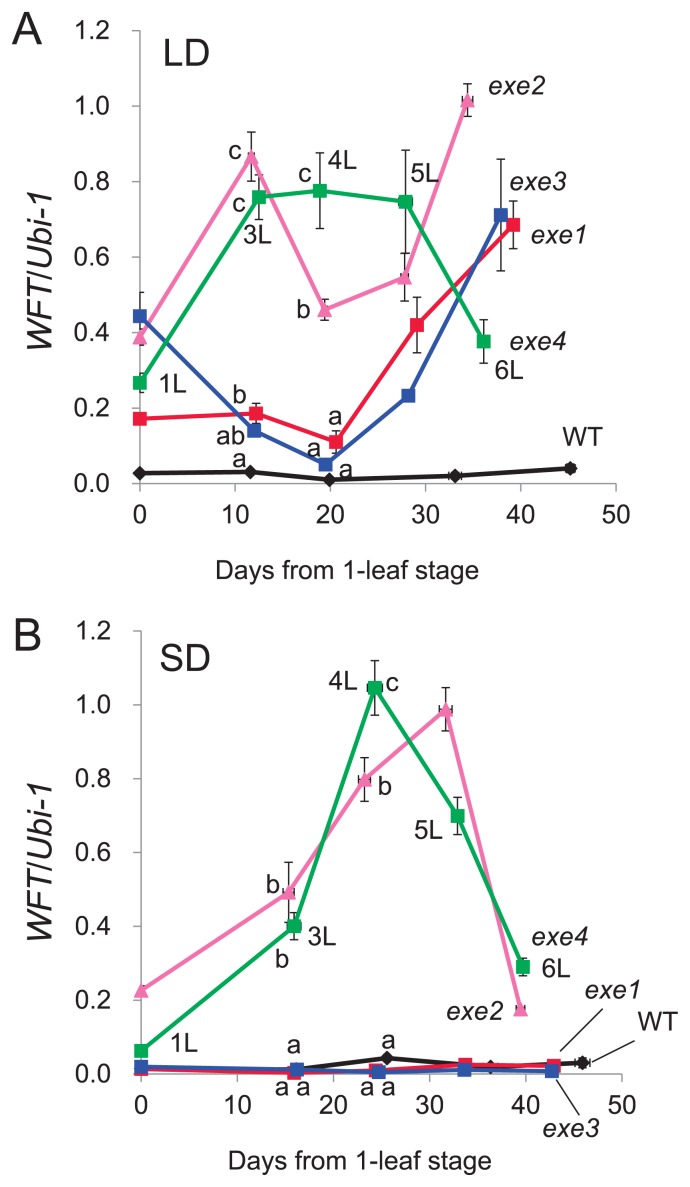
Using PCR analysis and sequence analysis, we confirmed that mutations were not involved in WFT of exe mutants (data not shown). During vegetative growth (1-leaf to 6-leaf stages), WT plants showed a low level of WFT expression under LD and SD conditions (Fig. 4). Under LD conditions, the level of WFT expression level was higher in exe mutants compared to the WT plants; Type II exe mutants (exe2 and exe4) showed a much higher level of expression at the 3-leaf to 5-leaf stages than Type I exe mutants (exe1 and exe3) (Fig. 4A). By contrast, WFT expression in Type I mutants was reduced under SD conditions to a similar extent as in WT plants (Fig. 4B). Significant up-regulation of WFT was observed in Type II mutants, presumably as a consequence of their loss of photoperiodic response which would otherwise suppress WFT expression.
Fig. 4.
Expression patterns of WFT in non-vernalized WT and exe mutants plants at the 1-leaf stage to 6-leaf stages. The data at the 2-leaf stage are missing. The level of WFT expression was determined by real-time PCR. The Ubiquitin (Ubi-1) gene was used as an internal control for calculating the relative levels of expression of the WFT gene. Means and standard errors are indicated. (A) Expression patterns in seedlings at each leaf stage under LD conditions. Leaf stage numbers (1L, 3L, etc.) are shown for exe4 only. (B) Expression patterns in seedlings at each leaf stage under SD conditions. Leaf stage numbers (1L, 3L, etc.) are shown for exe4 only. ANOVA (analysis of variance) was performed to detect the difference of gene expression level at the 3L and 4L stages. The same superscript means not significantly different (p = 0.05) from each other when Fisher’s LSD (least significant difference) analysis is applied.
Discussion
In this study, we compared the characteristics of four extra early-flowering mutants that had been induced by heavy-ion beam irradiation. The mutants fell into two categories, namely Type I exe mutants (exe1 and exe3) that showed a moderately extra early-flowering phenotype in the field and Type II exe mutants (exe2 and exe4) that had an extremely extra early-flowering phenotype (Table 1). Growth chamber analyses indicated that both Type I and II mutants had reduced photoperiodic sensitivity, i.e., the mutants showed a reduced delay in flowering under SD conditions (Table 3). Type II mutants showed a greater loss of photoperiodic sensitivity than Type I mutants and could flower earlier under short day (SD) conditions. The early-flowering phenotype of Type II exe mutants under SD conditions was due to the up-regulation of WFT expression (Fig. 4B). Normally under SD conditions, WFT expression is down-regulated; this indicates that the WFT protein can act as a florigen under LD conditions (Fig. 4B, Kitagawa et al. 2012, Shimada et al. 2009, Yan et al. 2006). The unusual up-regulation of WFT expression in Type II exe mutants under SD conditions might be associated with the higher level of VRN1 expression (Fig. 2B).
The up-regulation of VRN1 expression in exe mutants was associated with earliness in flowering under LD conditions (Fig. 2A). The extremely extra early-flowering in Type II mutants was associated with a more rapid up-regulation of VRN1 expression than in Type I mutants. It is notable that a similar level of VRN1 expression was observed at the timing of phase transition in each exe mutant: at the 5-leaf stage for Type I mutants and at the 4-leaf stage for Type II mutants (Fig. 2A). This finding suggests that the level of VRN1 expression is correlated with the phase transition from vegetative to reproductive growth and may act as a threshold for flowering competency in the plants.
The transition from vegetative to reproductive growth can be divided into two steps: first, the systemic establishment of flowering competency; and second, the determination of floral meristems in the shoot apex (Preston and Kellogg 2008). In a previous study, we analyzed the roles of three novel wheat AP1/FUL-like genes, WFUL1 (identical to VRN1), WFUL2 and WFUL3 during this transition period (Kinjo et al. 2012). We found that WFUL1/VRN1 was expressed in leaves as well as spike primordia of non-vernalized plants at the vegetative stage just before phase transition, while WFUL2 and WFUL3 were not expressed in leaves. This indicated that WFUL1/VRN1 performs a distinct role in leaves before phase transition. The findings in the present study concur with those from the earlier report in suggesting that the VRN1 expression level in leaves confers flowering competency in wheat plants.
Based on data from expression, transgenic and mutant analyses, we previously proposed a gene network model for the interaction of VRN1, VRN2 and WFT/VRN3 in leaves (Shimada et al. 2009). In this model, VRN1 is upstream of WFT and activates WFT expression under LD conditions. Vernalization down-regulates VRN2 and up-regulates VRN1 independently of each other. VRN2 is also suggested to be down-regulated by WFT. The proposed up-regulation of WFT by VRN1 is based on the expression analysis of einkorn wheat (T. monococcum) lines with winter or spring habit and of the maintained vegetative phase (mvp) mutant. The mvp mutant lacks a genomic region including the VRN1 locus, and is unable to flower (Distelfeld and Dubcovsky 2010, Shitsukawa et al. 2007). The proposed activity of WFT in the down-regulation of VRN2 is based on the analysis of transgenic wheat plants with over-expression of WFT (Shimada et al. 2009). Overall, the model can explain why VRN1 is able to repress VRN2 expression (Loukoianov et al. 2005, Trevaskis et al. 2006). Thus, VRN1 is proposed to play a role as an integrator of the vernalization and photoperiodic signals. This model is consistent with our suggestion here that the level of VRN1 expression functions as a threshold for flowering competency in wheat plants.
Trevaskis (2010) put forward an alternative gene network model for VRN1, VRN2 and FT1/VRN3; this model was based on the results of investigations using barley. This alternative model postulates that VRN1 and FT1 mutually up-regulate each other: VRN1 first activates FT1 expression, and then FT1 further activates VRN1. The model was referred to as the “flowering model for temperate cereals” in a recent review paper on flowering in plants (Andrés and Coupland 2012). Our suggestion here that the level of VRN1 expression functions as a threshold for flowering competency is compatible with this model. However, the mechanism for the mutual activation of VRN1 and FT1 is still unknown.
More recently, another model of flowering in wheat and barley was suggested by Chen and Dubcovsky (2012). This model proposes that VRN1 is activated by WFT/VRN3 and then suppresses VRN2 expression. Under this model, VRN1 is not essential for flowering. The latter conclusion was drawn from an analysis of a novel TILLING VRN1 mutant line that could transit to the reproductive growth phase and produce normal flowers (Chen and Dubcovsky 2012). However, it is not certain that this mutant line is a true VRN1 knock-out, because its genetic alteration is a point mutation and VRN1 mRNA is transcribed. In the present study, we showed that the level of VRN1 expression was associated with earliness in exe mutants (Fig. 2). The exe mutants do not have a functional VRN2 locus because the original einkorn strain KU104-1 carried a VRN2 deletion. Thus, our present results indicate that the level of VRN1 expression can control earliness in exe mutants without the need for VRN2 expression. It is not possible to reconcile our conclusion on the function of VRN1 expression in flowering earliness with the expectations of Chen and Dubcovsky’s (2012) model. Furthermore, the WT strain KU104-1 shows a relatively weak vernalization effect that accelerates flowering (data not shown). It has also been reported in barley that vernalization accelerates flowering (spike primordial formation) in lines lacking VRN2 (Hemming et al. 2008, Sasani et al. 2009). Again, the model of Chen and Dubcovsky (2012) does not explain these observations.
VRN1 is transcriptionally activated by prolonged cold during winter to promote flowering. In barley, analysis of transgenic plant having VRN1 promoter fused to GFP (Green Fluorescent Protein) reporter gene revealed that GFP fluorescence increased at the shoot apex and leaves after prolonged cold treatment (Alonso-Peral et al. 2011). The barley cultivar used in this study lacks VRN2, indicating that VRN2 is not involved in the up-regulation of VRN1 and flowering promotion. This observation also rules out the third model of Chen and Dubcovsky (2012).
In bread wheat, it has been known that winter cultivar quantitatively requires prolonged cold temperature to obtain the vernalization saturation. Some winter cultivars require 2–4 weeks of low temperature to reach the maximum vernalization effect on heading, and the others require more than 4 weeks. The positional cloning of the gene for vernalization requirement duration was performed by Li et al. (2013), and they demonstrated that this trait is controlled by recessive VRN1 gene on A genome at the protein level. They also revealed that binding ability of the VRN1 protein to TaHOX1 (the first homeobox protein in wheat) was associated with requirement of low temperature duration to reach vernalization saturation. The present findings support our model of gene network for flowering: VRN1 plays a central role in vernalization pathway (Shimada et al. 2009).
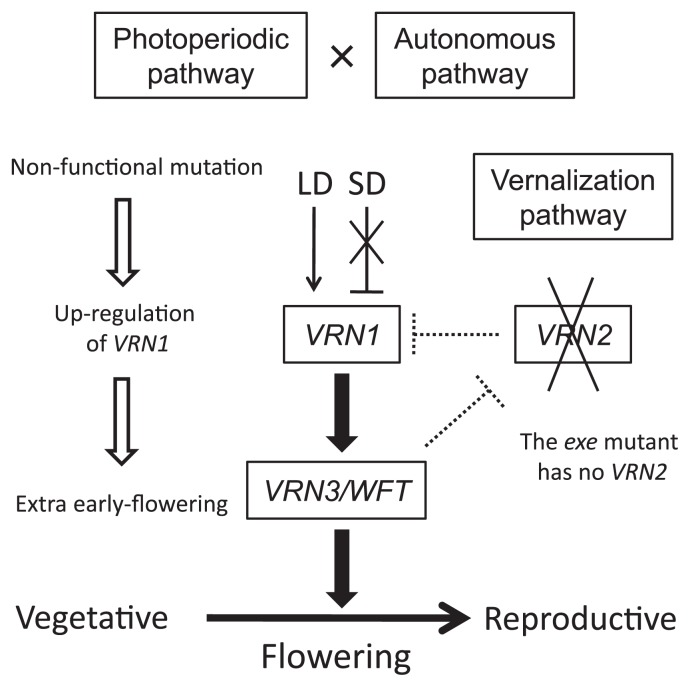
In conclusion, the present expression analyses of VRN1 and WFT in exe mutants indicate that the difference in earliness between Type I and Type II mutants is associated with differences in the patterns of VRN1 expression. Thus, VRN1 is essential for flowering in wheat, and the level of expression of VRN1 determines flowering competency and, therefore, time of flowering (Fig. 5). In Type II exe mutants, the mechanism for down-regulating expression of VRN1 under SD conditions appeared to be disrupted. The accumulation of VRN1 transcripts induced WFT expression, resulting in the extra early-flowering phenotype.
Fig. 5.
Model for the extra early-flowering phenotype in exe mutants. This model is based on that proposed by Shimada et al. (2009). Under this model, VRN1 acts as an integrator of the vernalization and photoperiodic pathways, which are also coordinated with the autonomous pathway. VRN1 up-regulates the florigen gene WFT/VRN3. In exe mutants, the mechanism for suppressing the expression of VRN1 under SD conditions must be disrupted. High levels of accumulation of VRN1 transcripts induce WFT expression, resulting in the extra early-flowering phenotype. Note that exe mutants do not have an active VRN2 allele. Arrows and T-bars indicate promotion and suppression effects, respectively. Arrows with wider lines show stronger effects.
To avoid rainy season for harvesting, early-flowering or early-heading is one of the most important characteristics for bread wheat in East Asia including Japan. The early-heading is closely associated with photoperiodic insensitivity there. The combination of photoperiod-insensitive alleles of Ppd-A1, Ppd-B1 and Ppd-D1, affects heading time and adjustability to each area in Japanese bread wheat cultivars (Seki et al. 2011, 2013). The present study revealed the relationship between photoperiodic insensitivity and VRN1 expression level in wheat. The identification of novel photoperiod-insensitive genes and their natural variations affecting the up-regulation of VRN1 may be useful tools for fine-tuning breeding of heading time. The genes should be identified by the expression QTL analysis of VRN1 with appropriate populations.
Acknowledgements
We are grateful to the National Bioresource Project - Wheat (NBRP-KOMUGI) for providing the WT wheat strain. This work was supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant Number 24113517 to K. Murai.
Literature Cited
- Alonso-Peral, M.M., Oliver, S.N., Casao, M.C., Greenup, A.A. and Trevaskis, B. (2011) The promoter of the cereal VERNALIZATION 1 gene is sufficient for transcriptional induction by prolonged cold. PLoS ONE 6: e29456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés, F. and Coupland, G. (2012) The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Chen, A. and Dubcovsky, J. (2012) Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 8: e1003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk, J., Kane, N.A., Breton, G., Kimin, A.E., Fowler, D.B. and Sarhan, F. (2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol. 132: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo, A.O., Ali-Benali, M.A., Badawi, M., Houde, M. and Sarhan, F. (2012) Expression of vernalization responsive genes in wheat is associated with histone H3 trimethylation. Mol. Genet. Genomics 287: 575–590 [DOI] [PubMed] [Google Scholar]
- Distelfeld, A., Li, C. and Dubcovsky, J. (2009a) Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Distelfeld, A., Tranquilli, G., Li, C., Yan, L. and Dubcovsky, J. (2009b) Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol. 149: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld, A. and Dubcovsky, J. (2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol. Genet. Genomics 283: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky, J., Chen, C. and Yan, L. (2005) Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol. Breed. 15: 395–407 [Google Scholar]
- Dubcovsky, J., Loukoianov, A., Fu, D., Valarik, M., Sanchez, A. and Yan, L. (2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol. Biol. 60: 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming, M.N., Peacock, W.J., Dennis, E.S. and Trevaskis, B. (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol. 147: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama, Y., Hirano, T., Saito, H., Liu, Y., Ohbu, S., Hayashi, Y. and Abe, T. (2011) Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol. 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama, Y., Fujiwara, M.T., Hirano, T., Ohbu, S., Saito, H., Ichida, H., Hayashi, Y. and Abe, T. (2013) Characterization of a heavy-ion induced white flower mutant of allotetraploid Nicotianatabacum. Plant Cell Rep. 32: 11–19 [DOI] [PubMed] [Google Scholar]
- Kinjo, H., Shitsukawa, N., Takumi, S. and Murai, K. (2012) Diversification of three APETALA1/FRUITFULL-like genes in wheat. Mol. Genet. Genomics 287: 283–294 [DOI] [PubMed] [Google Scholar]
- Kitagawa, S., Shimada, S. and Murai, K. (2012) Effect of Ppd-1 on the expression of flowering-time genes in vegetative and reproductive growth stages of wheat. Genes Genet. Syst. 87: 161–168 [DOI] [PubMed] [Google Scholar]
- Li, G., Yu, M., Fang, T., Cao, S., Carver, B.F. and Yan, L. (2013) Vernalization requirement duration in winter wheat is controlled by TaVRN-A1 at the protein level. Plant J. 76: 742–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukoianov, A., Yan, L., Blechl, A., Sanchez, A. and Dubcovsky, J. (2005) Regulation of VRN-1 vernalization genes in normal and transgenic polyploidy wheat. Plant Physiol. 138: 2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai, K., Miyamae, M., Kato, H., Takumi, S. and Ogihara, Y. (2003) WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol. 44: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Murai, K., Nishiura, A., Kazama, Y. and Abe, T. (2013) A large-scale mutant panel in wheat developed using heavy-ion beam mutagenesis and its application to genetic research. Nucl. Instr. Meth. Phys. Res. B 314: 59–62 [Google Scholar]
- Nishiura, A., Kazama, Y., Abe, T., Mizuno, N., Nasuda, S. and Murai, K. (2013) extra early-flowering (exe) mutants in einkorn wheat generated by heavy-ion beam irradiation. Proc. 12th Int. Wheat Genet. Symp. (in press) [Google Scholar]
- Oliver, S.N., Finnegan, E.J., Dennis, E.S., Peacock, W.J. and Trevaskis, B. (2009) Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION 1 gene. Proc. Natl. Acad. Sci. USA 106: 8386–8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, S.N., Deng, W., Casao, M.C. and Trevaskis, B. (2013) Low temperatures induce rapid changes in chromatin state and transcript levels of the cereal VERNALIZATION 1 gene. J. Exp. Bot. 64: 2413–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston, J.C. and Kellogg, E.A. (2008) Discrete developmental roles for temperate cereal grass VERNALIZATION 1/FRUITFULL-like genes in flowering competency and the transition to flowering. Plant Physiol. 146: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream, T.S., Woods, D.P. and Amasino, R.M. (2013) The molecular basis of vernalization in different plant group. Cold Spring Harb. Symp. Quant. Biol. 77: 105–115 [DOI] [PubMed] [Google Scholar]
- Sasani, S., Hemming, M.N., Oliver, S.N., Greenup, A., Tavakkol-Afshari, R., Mahfoozi, S., Poustini, K., Sharifi, H.-R., Dennis, E.S., Peacock, W.J.et al. (2009) The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J. Exp. Bot. 60: 2169–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, M., Chono, M., Matsunaka, H., Fujita, M., Oda, S., Kubo, K., Kiribuchi-Otobe, C., Kojima, H., Nishida, H. and Kato, K. (2011) Distribution of photoperiod-insensitive alleles Ppd-B1a and Ppd-D1a and their effect on heading time in Japanese wheat cultivars. Breed. Sci. 61: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, M., Chono, M., Nishimura, T., Sato, M., Yoshimura, Y., Matsunaka, H., Fujita, M., Oda, S., Kubo, K., Kiribuchi-Otobe, C.et al. (2013) Distribution of photoperiod-insensitive allele Ppd-A1a and its effect on heading time in Japanese wheat cultivars. Breed. Sci. 63: 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, S., Ogawa, T., Kitagawa, S., Suzuki, T., Ikari, C., Shitsukawa, N., Abe, T., Kawahigashi, H., Kikuchi, R., Handa, H.et al. (2009) A genetic network of flowering time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J. 58: 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitsukawa, N., Ikari, C., Shimada, S., Kitagawa, S., Sakamoto, K., Saito, H., Ryuto, H., Fukunishi, N., Abe, T., Takumi, S.et al. (2007) The einkorn wheat (Triticum monococcum) mutant, maintained vegetative phase, is caused by a deletion in the VRN1 gene. Genes Genet. Syst. 82:167–170 [DOI] [PubMed] [Google Scholar]
- Trevaskis, B. (2010) The central role of the VERNALIZATION 1 gene in the vernalization response of cereals. Funct. Plant Biol. 37: 479–487 [Google Scholar]
- Trevaskis, B., Bagnall, D.J., Ellis, M.H., Peacock, W.J. and Dennis, E.S. (2003) MADS box genes control vernalization-induced flowering in cereals. Proc. Natl. Acad. Sci. USA 100: 13099–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis, B., Hemming, M.N., Peacock, W.J. and Dennis, E.S. (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulatd by vernalization and developkental status. Plant Physiol. 140: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L., Loukoianov, A., Tranquilli, G., Helguera, M., Fahima, T. and Dubcovsky, J. (2003) Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L., Loukoianov, A., Blechl, A., Tranquilli, G., Ramakrishna, W., SanMiguel, P., Bennetzen, J.L., Echenique, V. and Dubcovsky, J. (2004) The Wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L., Fu, D., Li, C., Blechl, A., Tranquilli, G., Bonafede, M., Sanchez, A., Valarik, M., Yasuda, S. and Dubcovsky, J. (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]