Abstract
“K2” or “Spice” is an emerging drug of abuse that is laced with psychoactive synthetic cannabinoids JWH-018 and AM2201. Previous studies have identified hydroxylated (OH) and carboxylated (COOH) species as primary human metabolites, and kinetic studies have implicated CYP2C9 and -1A2 as major hepatic P450s involved in JWH-018 and AM2201 oxidation. The present study extends these findings by testing the hypothesis that CYP2C9- and 1A2-selective chemical inhibitors, sulfaphenazole (SFZ) and α-naphthoflavone (ANF), block oxidation of JWH-018 and AM2201 in human liver microsomes (HLM). A concentration-dependent inhibition of JWH-018 and AM2201 oxidation was observed in the presence of increasing concentration of SFZ (0.5 – 50 μM) and ANF (0.1 – 5.0 μM). No metabolic inhibition was observed with omeprazole, quinidine, and ketoconazole. The results presented herein further demonstrate the importance of CYP2C9- and 1A2-mediated oxidation of JWH-018 and AM2201 and the likelihood of adverse toxicity in populations with polymorphic alleles of these enzymes.
Keywords: AM2201, Cytochrome P450, JWH-018, K2/Spice, Synthetic cannabinoids
Introduction
Synthetic cannabinoid laced marijuana alternatives sold under brand names like “K2” and “Spice” have recently emerged as popular drugs of abuse. While Cannabis use is commonly associated with mild symptoms such as appetite stimulation and orthostatic hypotension, K2 users can exhibit severe adverse reactions like hypertension, visual and auditory hallucinations, tachycardia, dysrhythmias, seizures, psychosis, and death 1. It has also been suggested that chronic K2 use, like that of marijuana, can lead to the development of tolerance, dependence, and withdrawal 1.
It is difficult to ascertain the exact number of synthetic cannabinoids being used to manufacture K2-type products, but JWH-018 and AM2201 are consistently linked to severe toxicity and death in humans 1. Since little is known about the toxicology of these substances in humans, there is an urgent public health need to elucidate the underlying molecular mechanisms responsible for the constellation of adverse reactions observed with K2 use.
JWH-018 and AM2201 are biochemically characterized as aminoalkylindoles and pharmacologically characterized as potent, full agonists of cannabinoid CB1 receptors2. In vitro studies using human recombinant P450s identified CYP2C9 and -1A2 as major P450 isoforms 2b responsible for the generation of hydroxylated and carboxylated metabolites. These metabolites can be detected in human blood and are excreted in urine as glucuronic acid conjugates3.
Identification of specific P450 isoforms involved in the metabolism of xenobiotics includes a combination of approaches, such as enzyme kinetics, use of selective reversible or irreversible chemical inhibitors, and use of P450-selective inhibitory mono- or poly-clonal antibodies. In vitro studies using recombinant P450s were previously used to identify CYP2C9 and -1A2 as primary P450s involved in the metabolism of JWH-018 and AM22012b, but confirmation studies are necessary to obtain a higher degree of certainty for the identification of enzyme(s) responsible for synthetic cannabinoid metabolism 4. α-Naphthoflavone (ANF; CYP1A2 inhibitor), sulfaphenazole (SFZ; CYP2C9 inhibitor), omeprazole (OMPZ; CYP2C19 inhibitor), quinidine (QND; CYP2D6 inhibitor), and ketoconazole (KTZ; CYP3A4 inhibitor) are common P450-selective, reversible chemical inhibitors which have been used extensively, in vitro and in vivo, to study the enzymes involved in the metabolism of various drugs 4. To further understand the role of P450 metabolism in the toxicity of JWH-018 and AM2201, the objective of the present study was to study the effects of reversible chemical inhibitors of CYP1A2, -2C9, -2C19, -2D6, and -3A4 on JWH-018 and AM2201 oxidation in pooled human liver microsomes.
Materials and Methods
Materials
Chemicals used in the study were of reagent grade or higher. Analytical standards for JWH-018, AM2201, and their hydroxylated and carboxylated metabolites were provided by Cayman Chemical Co (Ann Arbor, MI). The chemical structures of JWH-018, AM2201, and their metabolites have been previously published 2b. Pooled human liver microsomes (50-donor pool) from 34 male and 16 female donors were purchased from BD Gentest (Woburn, MA). ANF, SFZ, OMPZ, QND, and KTZ were purchased from Sigma Aldrich (St. Louis, MO).
Inhibition of JWH-018 and AM2201 metabolism in human liver microsomes using selective CYP1A2, -2C9, -2C19, -2D6, and -3A4 chemical inhibitors
To examine the effect of P450-selective chemical inhibitors on P450-mediated metabolism of JWH-018 and AM2201, pooled human liver microsomes (0.5 mg/ml) were assayed for activity in the presence of varying concentration of the specific P450 chemical inhibitors as follows: CYP1A2, -2C9, -2C19, -2D6, and -3A4 (ANF: 0.1 – 5 μM; SFZ: 0.5 – 50 μM; OMPZ: 5 – 100 μM; QND: 0.5 – 50 μM; KTZ: 0.1 – 1 μM; respectively). A substrate (JWH-018 and AM2201) concentration equal to the Michaelis-Menten constant (5 μM) for the formation of (ω)-OH metabolite in pooled human liver microsomes was used for all studies 2b. Substrates and inhibitors were added in ethanol and allowed to dry at ambient temperature. Protein (HLM; 0.5 mg/ml) was added in the presence of pH 7.4, 0.1 M KPO4 (final concentration) buffer, and NADPH regeneration system [(20 mM NADP+, 60 mM glucose-6-phosphate (G6P), 60 mM MgCl2, and 100 U/mL glucose 6-phosphate dehydrogenase (G6PDH)] to ensure sufficient NADPH to enable cytochrome P450-mediated oxidation. Chemical inhibitors were also included without substrate under identical assay conditions to ensure that the presence of inhibitors in the incubation would not interfere with the quantification of the metabolites. Total reaction volume was 50 μL and all incubations were performed in triplicate. Reactions were initiated by the addition of the NADPH regenerating system, incubated at 37°C for 20 min and terminated by addition of an equal volume of ethanol prior to LC-MS/MS analysis, as previously described 2b. Assay conditions were previously established to determine that metabolite formation for JWH-018 and AM2201 is linear up to 60 min2b.
Results
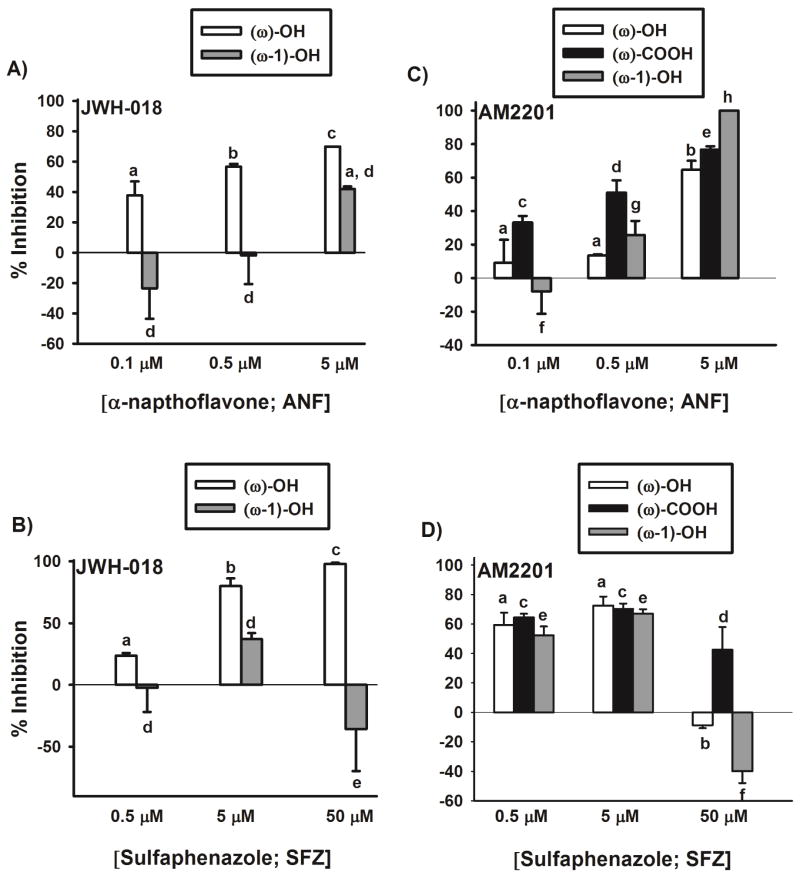
Panels 1A and 1B show the inhibition of JWH-018 oxidation in presence of ANF and SFZ. At ANF concentration of 0.1 μM, 0.5 μM, and 5 μM, JWH-018 ω-oxidation is inhibited by ~40, 60, and 80% while increasing concentration of SFZ (0.5 μM, 5 μM, and 50 μM) inhibits JWH-018 ω-oxidation by ~20, 80, and 100%. Data from figures 1A and 1B tell a different story with respect to inhibition of ω-1 oxidation. Approximately 40% of the ω-1-OH metabolite formation is inhibited by 5 μM ANF (panel 1A) with no inhibition of JWH-018 ω-1 oxidation at lower ANF concentrations (0.1 and 0.5 μM). Similarly, with the CYP2C9 inhibitor (panel 1B), only 40% of the ω-1-OH metabolite formation is inhibited by 5 μM SFZ, with no inhibition of ω-1 oxidation at the other two tested SFZ concentrations (0.5 and 50 μM). This suggests that neither CYP1A2 nor -2C9 are the primary enzymes involved in the ω-1 oxidation of JWH-018. Further studies are required to identify the enzymes involved in the formation of the ω-1-OH metabolite of JWH-018.
Figure 1.
Inhibition of JWH-018 and AM2201 oxidation by P450-selective chemical inhibitors. Concentration dependent inhibition of JWH-018 (Panels A and B) and AM2201 (Panels C and D) oxidation by α-naphthoflavone (ANF; CYP1A2 inhibitor) and sulfaphenazole (SFZ; CYP2C9 inhibitor). Values designated with different letters above the error bars are significantly different (p < 0.05, one-way ANOVA with Newman-Keuls multiple comparison post hoc test, n = 3). No comparisons were made between different metabolites of JWH-018 and AM2201.
Figures 1C and 1D show the inhibition of AM2201 ω- and ω-1 oxidation in presence of ANF and SFZ. Significantly greater inhibition (60–80%) of AM2201 ω- and ω-1 oxidation is observed at lower concentration of SFZ in comparison (10–50%) to ANF. Interestingly, ~60–100% of AM2201 oxidation is inhibited at the highest concentration of ANF, but SFZ, at its highest concentration (50 μM) fails to inhibit ω- and ω-1 oxidation of AM2201. The reason for lack of inhibition of JWH-018 and AM2201 oxidation at the highest SFZ concentration (50 μM) remains unknown.
Figure 2 shows the inhibition of JWH-018 (panel A) and AM2201 (panel B) oxidation in the presence of a single concentration of ANF, SFZ, and a combination of the two inhibitors. A clear additive effect was observed in the inhibition of oxidation when a combination of 5 μM ANF and SFZ was co-incubated with JWH-018 (Figure 2A). Surprisingly, co-incubation of AM2201 with a combination of 5 μM ANF and SFZ did not cause any additional inhibition of AM2201 oxidation when compared to inhibition by ANF or SFZ, independently (Figure 2B). Omeprazole (CYP2C19 inhibitor), quinidine (CYP2D6 inhibitor), and ketoconazole (CYP3A4 inhibitor) had no effect on the oxidation of JWH-018 or AM2201 (data not shown).
Figure 2.
Inhibition of JWH-018 (panel A) and AM2201 (panel B) oxidation in presence of α-naphthoflavone (ANF; CYP1A2 inhibitor), sulfaphenazole (SFZ; CYP2C9 inhibitor), and a combination of the two inhibitors. Values designated with different letters above the error bars are significantly different (p < 0.05, one-way ANOVA with Newman-Keuls multiple comparison post hoc test, n = 3). No comparisons were made between different metabolites of JWH-018 and AM2201.
Discussion
The present study provides additional support for recent kinetic data reporting the role for CYP2C9 and -1A2 as specific P450 isoforms involved in the metabolism of the common psychoactive synthetic cannabinoids JWH-018 and AM2201 2b. The reversible chemical inhibitors ANF and SFZ produced concentration-dependent inhibition of JWH-018 and AM2201 oxidation (Figure 1). However, there was no inhibition of JWH-018 or AM2201 oxidation at the highest concentration of SFZ (50 μM). This significant drawback of using chemical inhibitors for reaction phenotyping studies has been observed previously.5 It has been shown that chemical inhibitors loose P450 specificity when the inhibitor concentration is increased beyond a certain threshold. Specifically, CYP1A2 inhibitor ANF also inhibits -2C9 at concentrations greater than 1 μM.5 Lack of CYP1A2 specificity at higher ANF concentration (causing -2C9 inhibition) used in this study (Figures 1A–D and Figure 2) might be the reason for the apparent discrepancies (from a mathematical perspective) in the inhibition data (Figures 1A–D and Figure 2) of JWH-018 and AM2201. Unfortunately for inhibition studies using chemical inhibitors, maximal inhibition (>80%) of the substrate is usually obtained at high inhibitor concentrations, at which there might be a risk of losing P450 specificity.4, 5b This lack of P450 specificity can be overcome by using non-competitive monoclonal antibody P450 inhibitors which generally show negligible cross-reactivity with other enzymes. Another advantage of this approach is that the degree of inhibition is largely independent of substrate concentration.4
Additive inhibition of CYP1A2 and -2C9 mediated oxidation of JWH-018 was clearly observed (Figure 2A); however, no significant additive effect was observed in the inhibition of AM2201 oxidation with the combination of ANF and SFZ (Figure 2B). This lack of effect could be explained by the involvement of other P450 enzymes in the oxidation of AM2201. Use of inhibitors for CYP2C19, -2D6, and -3A4 had no effect on the metabolism of JWH-018 and AM2201; however, it is possible that other P450s are involved.
CYP2C9 is a major polymorphic enzyme, expressed in liver and intestine6 and is responsible for the metabolism of a number of clinically important drugs such as warfarin, phenytoin, tolbutamide, losartan, and ibuprofen. Over five allelic variants have been identified, including two “loss of function” variants (CYP2C9*4 and CYP2C9*5) 7. It is therefore possible that use of JWH-018 or AM2201 by patients that are homozygous for a particular CYP2C9 variant could contribute to the extreme adverse effects associated with the use of these psychoactive drugs. CYP1A2 is also highly polymorphic in nature and is responsible for the metabolism of caffeine, tricyclic antidepressants, and atypical antipsychotics 8. In addition, CYP1A2 may be inhibited by a number of drugs that are widely used clinically such as ciprofloxacin, fluvoxamine, and verapamil, and therefore, inhibition of CYP1A2 could contribute to JWH-018 and AM2201 mediated-toxicity8. Understanding the relative contribution of specific P450 isoforms in the metabolism of JWH-018 and AM2201 can guide future studies to assess the potential clinical significance of the use of these drugs of abuse in patients receiving drugs that are known CYP2C9 or -1A2 inhibitors.
Conclusion
In conclusion, data from the present study extends and supports previous kinetic studies demonstrating the role of CYP1A2 and -2C9 in the oxidation of designer drugs of abuse JWH-018 and AM2201 and the likelihood of adverse synthetic cannabinoid toxicity due to increased drug exposure in populations with polymorphic alleles of these enzymes.
Acknowledgments
Analytical standards for JWH-018 and AM2201 metabolites were kindly provided by Cayman Chemical Company (Ann Arbor, MI).
This work was funded by a National Institutes of Health Grant to Anna Radominska-Pandya [GM075893] and an Association of Public Health Laboratories Innovation Award to Jeffery Moran, and a grant from the University of Arkansas for Medical Sciences Translational Research Institute, awarded to Curtis Lowery, which is funded by the National Center for the Advancement of Translational Science [UL1TR000039].
List of abbreviations
- JWH-018
[1-naphthalenyl-(1-pentyl-IH-indol-3-yl]-methanone
- AM2201
[1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone
- Δ9-THC
delta-9-tetrahydrocannabinol
- SCs
synthetic cannabinoids
- P450
Cytochrome P450
- HLM
Human Liver Microsomes
Footnotes
Conflict of interest
The authors do not have any conflict of interest.
References
- 1.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: A review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH. Cytochrome P450-mediated Oxidative Metabolism of Abused Synthetic Cannabinoids Found in “K2/Spice”: Identification of Novel Cannabinoid Receptor Ligands. Drug Metab Dispos. 2012 doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, James LP, Moran JH, Radominska-Pandya A. Conjugation of synthetic cannabinoids JWH-018 and JWH-073, metabolites by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39(10):1967–76. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues AD. Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57(5):465–80. doi: 10.1016/s0006-2952(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 5.(a) Newton DJ, Wang RW, Lu AY. Cytochrome P450 inhibitors. Evaluation of specificities in the in vitrometabolism of therapeutic agents by human liver microsomes. Drug Metab Dispos. 1995;23(1):154–8. [PubMed] [Google Scholar]; (b) Rodrigues AD. In: Drug-Drug Interactions: Drugs and the Pharmaceutical Sciences. Rodrigues AD, editor. Vol. 116 Marcel Dekker, Inc; New York: 2002. [Google Scholar]
- 6.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34(5):880–6. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seng KC, Gan G, Sangkar VJ, Phipps ME. Frequency of Cytochrome P450 2C9 (CYP2C9) Alleles in Three Ethnic Groups in Malaysia. Asia Pacific Journal of Molecular Biology and Biotechnology. 2003;11(2):9. [Google Scholar]
- 8.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9(5):625–37. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]