Summary
The nexus of information concerning the CD4-binding site and its recognition by human antibodies capable of effective neutralization has expanded remarkably in the last few years. While barriers are substantial, new insights from donor-serum responses, atomic-level structures of antibody-Env complexes, and next-generation sequencing of B cell transcripts are invigorating vaccine-design efforts to elicit effective CD4-binding-site antibodies.
Keywords: Antibody template, B cell ontogeny, gp120 envelope glycoprotein, HIV-1 vaccine, Structure-based design
Introduction
The viral spike (Env) of human immunodeficiency virus type 1 (HIV-1) is a metastable trimer comprised of three gp120 and three gp41 subunits and the target of known virus-neutralizing antibodies [1]. Despite sustained global efforts and partially successful results from the RV144 vaccine trial [2-4], spike mechanisms of immune evasion continue to frustrated vaccine efforts. One prominent site of Env vulnerability to antibody is the CD4-binding site, the surface that the HIV-1 gp120 envelope glycoprotein uses to bind the CD4 receptor on the surface of host cells as the first step of virus-cell entry (Fig. 1). A number of factors make this site attractive as a vaccine target. First, soluble versions of the CD4 receptor – which consists of four unpaired immunoglobulin domains [18] that extend from the cell surface in tandem head-to-tail fashion with the membrane-distal domain containing the site for HIV-1 recognition [17] – neutralize HIV-1 reasonably well: a two domain-version of CD4 is capable of neutralizing 92% of a panel of 84 diverse HIV-1 isolates [19]•• and, when placed in an immunoglobulin context, CD4-IgG1 neutralizes 93% and other versions such as a dodecameric IgM context are even more effective [20,21]. Second, analysis of sera from HIV-1-infected donors indicates that a substantial fraction have potent broadly neutralizing antibodies that target the CD4-binding site [22]. Third, isolation of antibodies from select HIV-1-infected donors has allowed for the identification and characterization of a number of human antibodies that target the CD4-binding site and effectively neutralize HIV-1 [6,23-25],[19]••[26]••.
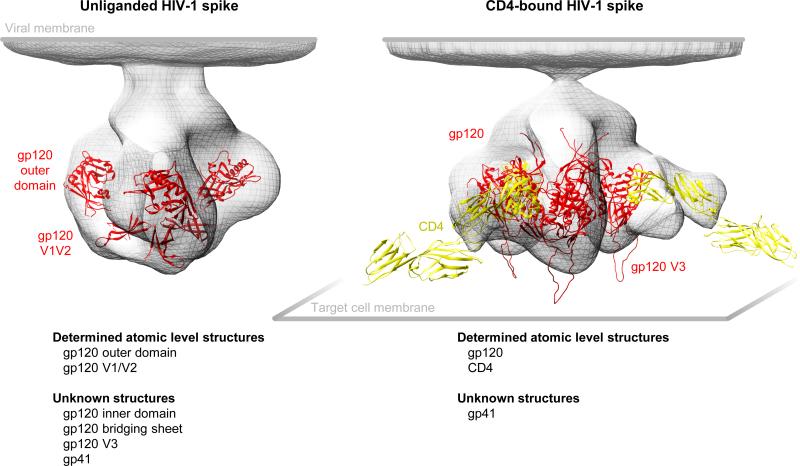
Figure 1. Structural details of the HIV-1 viral spike and its interaction with the CD4 receptor.
Although substantial effort has been expended to determine the structure of the HIV-1 spike, unliganded or in complex with CD4, surprisingly few atomic-level details are known. The overall architecture of the unliganded and CD4-bound forms of the HIV-1 spike were determined by cryo-electron tomography at ~20 A resolution by Subramanian and colleagues [5] and are shown here, fitted with known atomic-level structures. On the unliganded spike (left image), only the outer domain and the V1/V2 regions are known [6-9], as the inner domain and bridging sheet regions likely assume conformations differ from those determined in currently available crystal structures [10-12]. On the CD4-bound spike (right), the entire gp120 has been determined in various complexes with 2-domain CD4 [13-16], and these have been placed in the context of the entire 4-domain extracellular portion of CD4 [17].
Despite these attractive CD4-binding site features, no CD4-binding site-directed monoclonal antibody has yet been elicited by vaccination that is capable of effective HIV-1 neutralization. Moreover, analysis of seroconverter cohorts indicates that broadly neutralizing CD4-binding site antibodies are either not commonly elicited or not elicited in high titers during the first two years of infection [27,28]. Why is it so difficult to elicit broadly neutralizing antibodies that target the CD4-binding site? And are there redeeming qualities to the CD4-binding site that compensate for the relative paucity of effective CD4-binding site antibodies elicited in the first two years by natural infection? In this review, we attempt to address these questions and their implications for vaccine design. In the first section of this review, we describe effective CD4-binding site antibodies and their mechanisms for broad HIV-1 recognition. The second section of this review describes epitopes recognized by effective CD4-binding site neutralizers and attempts to convert these epitopes into epitope-based immunogens. Finally, several of the most effective CD4-binding site antibodies appear to be generated through similar B cell ontogenies in multiple people, and in the last section of this review we describe the vaccine implications of “reproducible” B cell ontogenies and class-based immunogen design.
Effective broadly neutralizing CD4-binding-site antibodies
In general, CD4-binding-site antibodies – defined as those antibodies that compete with CD4 for recognition of gp120 – can be separated into two categories: effective neutralizers, capable of neutralizing tier II isolates of HIV-1(Table 1), and non-potent neutralizers, only capable of neutralizing laboratory-adapted isolates. Non-potent neutralizers such as antibodies b13 and F105 generally show broad reactivity with diverse strains of monomeric gp120, but bind to conformations of the gp120 that are incompatible with the functional viral spike (Fig. 2) [33-35]. These antibodies are readily elicited by natural infection; by some estimates a quarter of non-potent neutralizers in infected donors target the CD4-binding site. By contrast, effective neutralizers are rarely found. The first effective CD4-binding site antibody, named b12 [29], was identified in the early 1990s by phage display from an HIV-1-infected donor and is able to neutralize 30-40% of circulating HIV-1. Despite substantial effort, an additional effective CD4-binding site antibody was not identified for 15 years, the next being antibody HJ16, which was not found until early 2010, through use of single-cell sorting and immunoglobulin sequencing (Table 1)[23].
Table 1.
CD4-binding-site antibodies that effectively neutralize HIV-1.
| Antibody type |
Representative mAb |
Donor | IGHV | IGK/LV | CDR H3 length |
CDR L3 length |
Maturation* (HV/LV, %) |
Breadth* (%) |
Potency# (μg/mi) |
Identification% | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Canonical CDR H3-utilizer | b12 | ND | 1-3*01 | 3-20*01 | 18 | 9 | 13/13 | 45 | 9.0 | Phage | [29] |
| HJ16 | 242315 | 3-30*18 | 4-1*01 | 19 | 8 | 28/20 | 30 | 12.8 | EBV | [23] | |
| CH103 | CHAVI 505 | 4-61*01 | 3-1*01 | 13 | 10 | 16/12 | 54 | 13.8 | RSC3-probe | [30]•• | |
| VH1-2-derived VRC01 class | VRC01 | NIAID 45 | 1-2*02 | 3-20*01 | 12 | 5 | 32/39 | 89 | 0.5 | RSC3-probe | [24] |
| VRC-PG04 | IAVI 74 | 1-2*02 | 3-20*01 | 14 | 5 | 30/19 | 78 | 0.8 | RSC3-probe | [6] | |
| VRC-CH31 | CHAVI 0219 | 1-2*02 | 1-33*01 | 13 | 5 | 23/15 | 81 | 0.6 | RSC3-probe | [6] | |
| VRC-PG20 | IAVI 23 | 1-2*02 | L2-14*01 | 13 | 5 | 24/15 | 77 | 0.8 | RSC3-probe | [26]•• | |
| 3BNC117 | RU 3 | 1-2*02 | 1-33*01 | 10 | 5 | 27/17 | 84 | 0.4 | 2CC-probe | [25] | |
| 12A12 | IAVI 57 | 1-2*02 | 1-33*01 | 13 | 5 | 22/18 | 82 | 0.1 | 2CC-probe | [25] | |
| VRC23 | 127/C | 1-2*02 | 3-15*01 | 12 | 5 | 21/14 | 65 | 7.4 | RSC3-probe | [19]•• | |
| VH1-46-derived | 1B2530 | RU1 | 1-46*02 | 1-47 | 16 | 11 | 27/16 | 42 | 12.3 | 2CC-probe | [25] |
| 8ANC131 | RU8 | 1-46*02 | 3-11 | 16 | 9 | 26/19 | 77 | 4.1 | 2CC-probe | [25] | |
Affinity maturation rates of both heavy chain and light chain variable domains are calculated on IgBLAST based on nucleotide sequences except those for antibodies b12 which were calculated with amino acid sequences.
Average potency values are based on IC50 values with breadth defined at a maximum of 50μg/ml as reported in cited references or [19]••.
“Phage” refers to phage display,“ EBV” to Epstein-Barr virus immortalization, and “RSC3-probe” and“ 2CC-probe” to the different probes used in B cell selection.
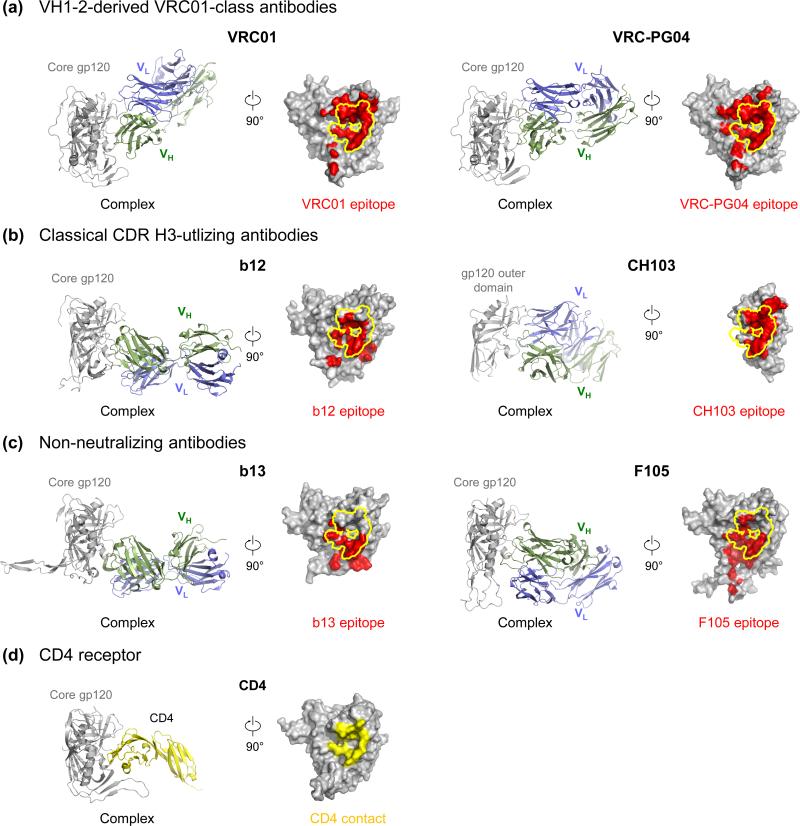
Figure 2. Antibody recognition at the CD4-binding site of HIV-1 gp120.
The structures of gp120 in complex with CD4-binding site antibodies (a-c) or CD4 (d) are shown in cartoon diagrams with HIV-1 gp120 colored in gray, antibody-heavy chain colored in green, antibody-light chain colored in blue, and CD4 colored in yellow. The epitopes of antibodies are highlighted in red on gp120 surface with the outer domain-contact site for CD4 outlined in yellow (a-c). The site of vulnerability defined as the outer domain contact of CD4 on HIV-1 gp120 is shown as a yellow surface (d). Effective neutralizers, either VH1-2-derived VRC01-class antibodies [6,31] or canonical CDR H3-utilizing antibodies [30,32], target this vulnerable site with high precision. In contrast, non-neutralizing CD4-binding site antibodies, such as b13 and F105 [33], only partially contact the site of vulnerability and induce significant conformational changes on monomeric gp120.
The atomic-level structure of the antibody b12-gp120 complex [32] inspired the design of epitope-specific probes that selectively bind to neutralizing CD4-binding site antibodies. One of these, resurfaced stabilized core 3, or RSC3, involved a disulfide stabilized core gp120 with roughly 30% of its surface residues modified to reduce antigenic recognition at all regions other than the outer domain-contact site for CD4 [24]. Use of the RSC3 probe allowed for the identification of broad and potent antibodies from donor NIAID 45, including antibody VRC01, capable of neutralizing over 90% of circulating HIV-1 isolates at an average 50% inhibitory concentration (IC50) of ~0.3 μg/ml [24]. Over the last three years, subsequent use of the RSC3 probe or of a 2-disulfide stabilized core gp120, called Ds12F123 [32] or 2CC [36], has allowed for the identification of dozens of broadly neutralizing antibodies from over 10 donors (Table 1). About half of the identified antibodies share VH1-2-gene origin with antibody VRC01 [6,23-25],[19]••[26]••. Two others derive from the closely related VH1-46 gene [25]. The remainder share diverse VH-gene origins (Table 1)[23,35],[30]••.
VH1-2-derived antibodies such as antibody VRC01 form the majority of highly effective HIV-1 neutralizing antibodies directed towards the CD4-binding site that have been identified thus far (Table 1). Structural studies of these antibodies in complex with HIV-1 gp120 [6,31],[26]•• reveal a common mode of recognition, focused on the outer domain-contact site of CD4 (Fig. 2). These antibodies mimic the CD4-receptor in the manner by which their heavy chains recognize gp120 [31]. Their light chains also share similarity, with a characteristic 5-residue light chain 3rd complementary determining region (CDR L3) comprising a hydrophobic-Glu or a hydrophobic-Gln residue pair at its 3rd and 4th position [6],[37]•[26]•• , and both heavy and light chains show substantial somatic hypermutation. Two recent developments may speed the identification of additional VRC01-class antibodies: accurate prediction of serum antibodies coupled to RSC3-probe identification of antibodies from identified donors [19]•• and bioinformatics identification of VH1-2-derived broadly neutralizing antibodies directly from the next-generation sequencing of B cell transcripts [6],[26]••. Because of their similarity in recognition of HIV-1 and B cell ontogeny, these VH1-2 derived antibodies appear to form a reproducible “class” – the “VRC01-class” of CD4-binding site antibodies [26]••.
VH1-46-derived antibodies such as 1B2530 and 8ANC131 have been identified from two HIV-1-infected donors (donors RU1 and RU8, respectively), through use of the 2CC probe [25]. Because of the close similarity between VH1-2 and VH1-46 germlines, it seems likely that the VH1-46-derived antibodies recognize gp120 in a manner similar to that of the VRC01-class, but specific differences must exist as conserved VRC01-class features such as a 5-residue CDR L3 are not observed with VH1-46-derived antibodies [25],[37]•. Sequence and structural analysis of these antibodies should reveal details of their recognition and B cell ontogeny, and this is currently being undertaken [38].
Other broadly neutralizing antibodies such as b12, HJ16, and CH103 derive from diverse VH-genes [23,29],[30]••. Structural analysis of b12 and CH103 antibodies in complex with HIV-1 gp120 [31],[30]•• indicates that these antibodies utilize classic loop-based mechanisms of recognition, with the CDR H3 contributing ~50% of the paratope interface (Fig.2). Since CDR H3s are products of V(D)J gene recombination, this type of recognition can be considered V(D)J-encoded, as opposed to the VRC01-class which appears to be substantially VH-gene encoded.
The CD4-binding site of vulnerability
The binding site for the CD4 receptor on gp120 spans the outer domain and bridging sheet regions of gp120 [13]. On the outer domain, the recognition surface involves a number of discontinuous segments, which include the CD4-binding loop, the base of the V5 loop, Loop D, and the outer domain-entry and -exit loops (Fig. 2). Analysis of the epitopes recognized by CD4-binding antibodies reveals substantial similarity in the recognized region of gp120 (Fig. 2). The non-potent b13 and F105 antibodies induce conformations of the β21-β22 region (b13) or of the bridging sheet (F105), which are only present on monomeric gp120 and are incompatible with the functional viral spike [33]. Antibody b12 is able to bind to functional viral spikes, inducing only small conformational changes [5]; b12 also binds to a region on the outer domain that is outside of the site of CD4-binding, and viral escape occurs by mutations outside of the conserved site of CD4 binding [39]. Antibody CH103 also targets the outer domain of gp120; its epitope however overlaps substantially with the V5 and D loops, with viral escape occurring by alterations in these regions [30]••. By contrast, the VRC01-class of antibodies recognizes a surface on the outer domain which corresponds precisely to the CD4 attachment site.
Interestingly, increased neutralization breadth is observed with the NIH45-46 somatic variant of VRC01, which contains a 4-amino acid insertion in the CDR H3, which extends beyond the region recognized by CD4 onto the gp120-inner domain (Fig. 2) [25]. A G54W variant of NIH45-46, meanwhile, shows even greater effectiveness [40], by interaction with the vestibule of the Phe43 cavity, a hotspot of CD4 interaction [13].
Env immunogens (Fig. 3) have thus far failed to elicit potent broadly neutralizing antibodies against the CD4-binding site [51]. Analysis of Env immunizations of rhesus macaque shows that while antibodies which can target the CD4-binding site are readily developed, they do not neutralize HIV-1 [52-54]. This is thought to be due to a failure to recognize the site of vulnerability on viruses with bespoke precision, a precision which is required because of conformational masking of the CD4-binding site [10,21],[55]•. Immunodominant surfaces (such as that targeted by the F105 antibody, beneath the bridging sheet) are also thought to contribute to subdominance of CD4-binding-site-directed antibodies that neutralize HIV-1. Physical stabilization of the variant gp120 molecule with structure-designed cavity-filling mutations and disulfide bonds that lock the bridging sheet to the outer domain yields a substantial increase in elicitation of antibodies against CD4-induced epitopes, but only a minor increase in neutralization [36]. Because a majority of the CD4-binding-site epitope spans the outer domain of HIV-1 gp120, substantial interest has focused on the use of outer domain molecules as immunogens [56]. An immunogenic bonus is that these molecules inherently encode a reduced amount of off-target regions for the immune system. Initial trials with a membrane-bound form of the outer domain did not yield increased titers of neutralizing antibodies over core gp120 [47], and other outer domain formats also did not lead to improved elicitation [48]•. A number of recent studies using various immunization schemes have led to encouraging neutralizing titres. These include a novel cross-immunization strategy utilizing HIV-1, SIV and FIV gp140 molecules in a DNA-prime and protein-boost strategy [57] and a recently described 100-amino acid molecule encompassing 70% of the b12 epitope. The latter was used as a priming immunogen followed by a gp120 boost, and this led to the elicitation of antibodies capable of Tier 2-virus neutralization after one year [50]••. When transmitted/founder gp140s were used, immunization studies with gp140 immunogens from a broad spectrum of viruses led to the elicitation of CD4-binding-site-directed antibodies with reasonable breadth, though neutralization potency was low [58]. Further examination of these studies may allow for an understanding of the antibodies elicited and their mechanisms of neutralization; thus far, however, none of the reported elicited responses includes a vaccine-elicited broadly neutralizing monoclonal antibody. Additional immunogens which hold potential to elicit broadly neutralizing antibodies include a designed b12-epitope scaffold [49] as well as a stabilized trimeric fully cleaved gp140 (BG505 664.SOSIP) [8]. The ability to produce stable trimers with diminished affinity for non-neutralizing antibodies while maintaining affinity to potent neutralizing antibodies may also lead to effective antibody induction [59]. The very precise recognition required to avoid off-target binding may explain difficulties thus far encountered with eliciting broadly neutralizing antibodies. Perhaps relevant to this, immunization with soluble trimeric gp140s – which had failed to elicited broadly neutralizing antibodies in small mammals and non-human primates – succeeded in eliciting a very broadly neutralizing single-domain VHH antibody in llamas [60]. Modulation of the immune response through use of adjuvants [61-63] or immunogen multimerization [64] may facilitate development of antibodies targeting the CD4-binding site. Highly-tailored immunization protocols utilizing optimal vaccination schema and diverse immunogens may be critical to eliciting antibodies capable of broad and potent neutralization at the CD4-binding site.
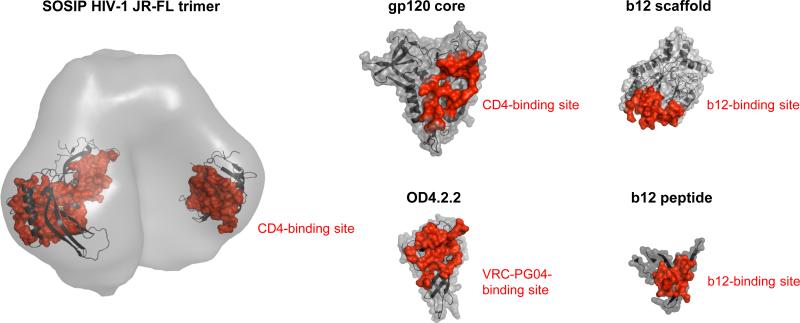
Figure 3. Immunogens to elicit CD4-binding site–directed neutralizing antibodies.
A number of HIV-1 envelope glycoprotein oligomers have been tested for their ability to elicit neutralizing antibodies (as reviewed in [41]), and we highlight here a particularly promising soluble HIV-1 gp140 “SOSIP” variant [8,9,42-44], which is displayed with electron microscopy-determined density and modeled gp120-outer domain. Other promising HIV-1 immunogens include core HIV-1 gp120 ([45,46], outer domain gp120 (OD4.2.2) [47,48], b12-epitope scaffold [49],and b12-epitope peptide [50]••, which are shown in cartoon and semi-transparent surface representation (grey), with CD4-, VRC-PG04-, or b12-sites of binding highlighted in red. While vaccination with HIV-1 Env immunogens containing the CD4-binding site and capable of binding broadly neutralizing antibodies have in select cases elicited encouraging titers of HIV-1 neutralizing antibodies (for example, [50]••), such neutralization remains to be confirmed at the monoclonal antibody level.
B cell ontogeny and structure-based immunogen design
Difficulties with eliciting CD4-binding site antibodies have prompted investigation of the B cell ontogenies of broadly neutralizing antibodies in HIV-1-infected donors. That is, in addition to understanding the epitopes broadly neutralizing antibodies recognize on HIV-1, it may be helpful to understand the developmental pathways that generate such antibodies [6,65-68],[26]••. Progress in this vein includes longitudinal analysis of the development of antibody CH103 in donor CH505, who was followed from time of infection to development of broadly neutralizing serum response [30]••. Sequencing of both virus and B cell transcripts indicate co-evolution to drive the maturation of the CH103 antibody. Because CH103 uses a CDR H3-dominated mode of recognition dependent on specific V(D)J recombination, this type of recognition may be difficult to reproduce. Nonetheless, attempts to use insights from the CH103 lineage to replicate the elicitation of antibodies like CH103 are now being undertaken.
Thus far, however, the only broadly neutralizing responses observed in multiple donors and derived from related B cell ontogenies are for the VH1-2-derived and VH1-46-derived antibodies of the VRC01 and 8ANC131 classes, respectively [6,25],[26]••. Antibodies in both of these classes are characterized by extraordinary degrees of somatic hypermutation of 25-35% [6,24,25],[26]••. Moreover, these levels of somatic hypermutation, both in complementary-determining regions as well as variable framework regions, have been found to be generally required for broad and potent neutralization [31],[69]••. Because of these increased mutation rates, a single immunogen may not be able to directly elicit antibodies of the VRC01 or 8ANC131 classes. In fact, several studies have shown that circulating HIV-1 strains generally do not bind, cannot be neutralized by, and do not engage germline-reverted variants of these antibodies [31],[70]•[71]•.
Several recent efforts have thus focused on designing immunogens that can explicitly engage VRC01-class germline antibodies [72]•[73]••. Constructs that successfully engaged germline variants of a subset of VRC01-class antibodies were designed in different contexts, including trimeric gp140 and nanoparticle multimerization of gp120 outer domain, although the immunogenicity of these constructs is yet to be ascertained. Specifically, a procedure for immunogen design targeting a particular class of antibodies, such as the VRC01 class, may involve the following steps (Fig. 4). By utilizing similarities between serum neutralization patterns and antibody neutralization fingerprints, donor sera can be interrogated for VRC01-like antibody specificities [26]••. Antibody ontogenies for selected sera can then be analyzed to define germline precursors and maturation intermediates. Knowledge of the ontogeny of VRC01-class antibodies from a larger set of donors may assist in the development of more general immunogens. The process of immunogen design for VRC01-class antibodies can thus be divided into three steps: (1) Germline engagement; (2) Guided affinity maturation; and (3) Clonal expansion. In all steps, in addition to optimizing binding and/or engagement of the target antibody variants, designs generally should be optimized for immunogenicity by silencing undesired epitopes. A potential way of such immune-focusing is the addition of glycans to cover immunogen surfaces other than the target epitope and/or the rearrangement of native glycans to improve epitope accessibility [62,74-77], for which recent methodological advances could be of utility [78]•. The question of immunogen presentation is also of importance and an essential part of immunogenicity optimization. In current studies, multimerization of the antigen constructs has been used as a way to improve engagement of germline and mature B cells [73]••, and gp140 constructs appear to be better candidates for broadly neutralizing antibody engagement than Env spikes on pseudoviruses [79]. Testing of candidate immunogens in appropriate animal models must specifically be considered since the germline genes characteristic of the VRC01-class antibodies may not have sufficiently close analogs in traditional animal models, such as mouse, rabbit, or rhesus macaque [37]•[73]••.
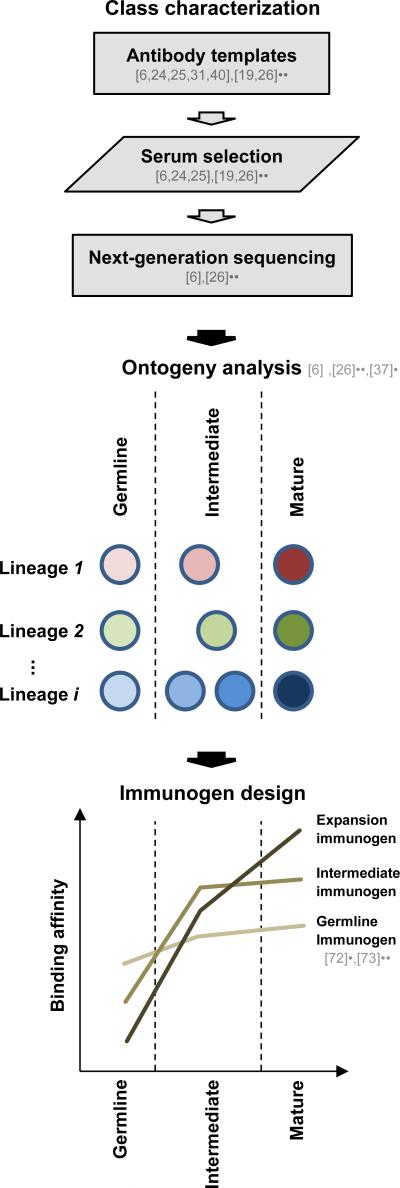
Figure 4. Schematic of a general procedure for immunogen design for a target antibody class, and current progress for the VRC01 class.
(top) Initial definition of target antibody class. By using known antibody templates (based on both identified antibodies and their structures in complex with antigen), sera can be interrogated for antibodies from the given class can and selected for next-generation sequencing analysis of the antibodyome. (middle) Analysis of antibody ontogeny for delineation of germline, intermediate, and mature antibodies. (bottom) Design of immunogens for elicitation (germline immunogen), guided affinity maturation (intermediate immunogen), and clonal expansion (expansion immunogen) of the class antibodies. Germline immunogens should possess sufficient affinity to allow antibody elicitation, while the increased affinity of the intermediate and expansion immunogens for their target antibodies versus less mutated variants can effect maturation and clonal expansion.
Conclusion
Overall, the field is embarked on an information-based process of vaccine development, involving atomic-level structures of antibodies, their HIV-1 epitopes, and structure-based immunogens informed by B cell ontogeny. Over the last three years, the explosive growth of newly identified CD4-binding-site antibodies [6,23-25],[19]••[26]•• – and of their atomic-level structure with HIV-1 Env [6,31],[26]•• –has invigorated vaccine design, by providing multiple templates for effective HIV-1 neutralization. Meanwhile next-generation sequencing of B cell transcripts and longitudinal studies are providing unprecedented insight into the developmental processes by which highly effective CD4-binding site neutralizers are generated and mature [6],[30]••[26]••. Structure-based design is now creating immunogens capable of specific interactions and of requisite affinity [73]••. New ways to analyze serum responses allows accurate delineation of antibody specificity in polyclonal serum, providing clues from natural human responses [26]••. We cannot say with certainty that the combination of these recent developments will allow for the creation of immunogens capable of eliciting broadly neutralizing antibodies against the site of CD4 binding: nevertheless, the next few years promise to be exciting times in HIV-1 vaccine development.
Keypoints.
The site of binding for the CD4 receptor is an attractive vaccine target on HIV-1.
Over the last three years, dozens of highly effective HIV-1 neutralizing antibodies have been identified that target the CD4-binding site.
Atomic-level structures of effective CD4-binding site antibodies with HIV-1 gp120 reveal the requirement for precise targeting of the CD4-binding site of vulnerability as well as the expansion of the site of vulnerability to include regions on the conserved inner domain that do not interact with CD4.
Definition of the B cell ontogeny of broadly neutralizing CD4-binding-site antibodies is providing insight into roadblocks in the development or maturation of these antibodies.
Vaccine designers are harnessing information from broadly neutralizing antibodies, their sites of recognition, and their B cell ontogeny, to create immunogens that overcome specific developmental roadblocks.
Acknowledgements
We thank J. Stuckey for assistance with figures and members of the Structural Biology Section and Structural Bioinformatics Core, Vaccine Research Center, for discussions or comments on the manuscript. We thank J. Mascola and the Humoral Immunology Section for providing neutralization data cited in Table 1, and J. Baalwa, D. Ellenberger, D. Gabuzda, F. Gao, B. Hahn, K. Hong, J. Kim, F. McCutchan, D. Montefiori, L. Morris, J. Overbaugh, E. Sanders-Buell, G. Shaw, R. Swanstrom, M. Thomson, S. Tovanabutra, C. Williamson, and L. Zhang for contributing the HIV-1 envelope plasmids used in neutralization panels. Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Purpose of review
The HIV-1 site of binding for the CD4 receptor has long attracted attention as a potential site of vulnerability to antibody-mediated neutralization. We review recent findings related to effective CD4-binding-site antibodies isolated from HIV-1-infected individuals and discuss implications for immunogen design targeting the CD4-binding site.
Recent findings
Highly effective CD4-binding-site antibodies such as antibody VRC01 have the ability to neutralize over 90% of circulating HIV-1 strains. Sequence and structural analysis of these antibodies from over half a dozen HIV-1-infected donors reveal remarkable similarity in their ontogenies and their modes of recognition, all of which involve mimicry of CD4 receptor by antibody-heavy chain. Meanwhile, other effective neutralizers such as antibody CH103 have been shown to utilize a different mode of recognition, with next-generation sequencing of both virus and antibody suggesting co-evolution to drive the development of antibody-neutralization breadth.
References and recommended reading
Papers of particular interest, published within the annual period of review, (18 months/ 2012-2013) have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1884-1888;1998280 doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran EE, Borgnia MJ, Kuybeda O, Schauder DM, Bartesaghi A, Frank GA, Sapiro G, Milne JL, Subramaniam S. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS Pathog. 2012;8:e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myszka DG, Sweet RW, Hensley P, Brigham-Burke M, Kwong PD, Hendrickson WA, Wyatt R, Sodroski J, Doyle ML. Energetics of the HIV gp120-CD4 binding reaction. Proc Natl Acad Sci U S A. 2000;97:9026–9031. doi: 10.1073/pnas.97.16.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433:834–841. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 12.Guttman M, Kahn M, Garcia NK, Hu SL, Lee KK. Solution structure, conformational dynamics, and CD4-induced activation in full-length, glycosylated, monomeric HIV gp120. J Virol. 2012;86:8750–8764. doi: 10.1128/JVI.07224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, et al. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A. 2004;101:2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pancera M, Majeed S, Ban YE, Chen L, Huang CC, Kong L, Kwon YD, Stuckey J, Zhou T, Robinson JE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc Natl Acad Sci U S A. 2010;107:1166–1171. doi: 10.1073/pnas.0911004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Kwong PD, Hendrickson WA. Dimeric association and segmental variability in the structure of human CD4. Nature. 1997;387:527–530. doi: 10.1038/387527a0. [DOI] [PubMed] [Google Scholar]
- 18.Maddon PJ, Molineaux SM, Maddon DE, Zimmerman KA, Godfrey M, Alt FW, Chess L, Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987;84:9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang GY, Louder MK, Schmidt SD, Altae-Tran HR, et al. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013;340:751–756. doi: 10.1126/science.1233989. [This paper provides a means to predict the specificity of HIV-1-neutralizing antibodies in serum, from the pattern of neutralization of diverse HIV-1 isolates.] [DOI] [PubMed] [Google Scholar]
- 20.Arthos J, Cicala C, Steenbeke TD, Chun TW, Dela Cruz C, Hanback DB, Khazanie P, Nam D, Schuck P, Selig SM, et al. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. J Biol Chem. 2002;277:11456–11464. doi: 10.1074/jbc.M111191200. [DOI] [PubMed] [Google Scholar]
- 21.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, et al. B cell ontogeny of a highly effective class of HIV-1-neutralizing antibodies. Immunity. 2013 In press. [Structures and next-generation sequencing of B cell transcripts are used to define the recognition and development of broadly neutralizing antibodies of the VRC01 class in six donors. The remarkable developmental similarity of these antibodies indicates this type of antibody is in principal “reproducible” in the general human population.] [Google Scholar]
- 27.Lynch RM, Tran L, Louder MK, Schmidt SD, Cohen M, Members CCT, Dersimonian R, Euler Z, Gray ES, Abdool Karim S, et al. The development of CD4 binding site antibodies during HIV-1 infection. J Virol. 2012;86:7588–7595. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis E, Simek M, Lakhi S, Karita E, Kamali A, Inambao M, Sanders EJ, Wrin T, Cormier E, Price MA, et al. Keystone Symposia on HIV Vaccines. X2. Keystone; Colorado: 2013. Development of broadly neutralizing antibody responses in a large Sub-Sharan HIV primary infection cohort. p. 2017. [Google Scholar]
- 29.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 30••.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [Virus and developing antibody are followed in an HIV-1-infected donor from time of infection, providing unprecedented insight into the maturation pathway of a broadly neutralizing CD4-binding site antibody.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid J, Shi W, Xu L, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Kwon YD, Zhou T, Wu X, O'Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009;326:1123–1127. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posner MR, Cavacini LA, Emes CL, Power J, Byrn R. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquir Immune Defic Syndr. 1993;6:7–14. [PubMed] [Google Scholar]
- 35.Barbas CF, Collet TA, Amberg W, Roben P, Binley JM, Hoekstra D, Cababa D, Jones TM, Williamson RA, Pilkington GR, et al. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 36.Dey B, Svehla K, Xu L, Wycuff D, Zhou T, Voss G, Phogat A, Chakrabarti BK, Li Y, Shaw G, et al. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS Pathog. 2009;5:e1000445. doi: 10.1371/journal.ppat.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.West AP, Jr., Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2083–2090. doi: 10.1073/pnas.1208984109. [Bioinformatics analysis of VRC01-class antibodies defines sequence-based requirements.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acharya P, Zhou T, et al. Keystone Symposia on HIV Vaccines. X2. Keystone; Colorado: 2013. Nussenzweig MC, Kwong PD: VH1-46 derived effective neutralizers. p. 1001. [Google Scholar]
- 39.Wu X, Zhou T, O'Dell S, Wyatt RT, Kwong PD, Mascola JR. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. J Virol. 2009;83:10892–10907. doi: 10.1128/JVI.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diskin R, Scheid JF, Marcovecchio PM, West AP, Jr., Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsell MN, Schief WR, Wyatt RT. Immunogenicity of HIV-1 envelope glycoprotein oligomers. Curr Opin HIV AIDS. 2009;4:380–387. doi: 10.1097/COH.0b013e32832edc19. [DOI] [PubMed] [Google Scholar]
- 42.Kang YK, Andjelic S, Binley JM, Crooks ET, Franti M, Iyer SP, Donovan GP, Dey AK, Zhu P, Roux KH, et al. Structural and immunogenicity studies of a cleaved, stabilized envelope trimer derived from subtype A HIV-1. Vaccine. 2009;27:5120–5132. doi: 10.1016/j.vaccine.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris A, Borgnia MJ, Shi D, Bartesaghi A, He H, Pejchal R, Kang YK, Depetris R, Marozsan AJ, Sanders RW, et al. Trimeric HIV-1 glycoprotein gp140 immunogens and native HIV-1 envelope glycoproteins display the same closed and open quaternary molecular architectures. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11440–11445. doi: 10.1073/pnas.1101414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundling C, O'Dell S, Douagi I, Forsell MN, Morner A, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian- human immunodeficiency virus. J Virol. 2010;84:9086–9095. doi: 10.1128/JVI.01015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Y, McKee K, Tran K, O'Dell S, Schmidt SD, Phogat A, Forsell MN, Karlsson Hedestam GB, Mascola JR, Wyatt RT. Biochemically defined HIV-1 envelope glycoprotein variant immunogens display differential binding and neutralizing specificities to the CD4-binding site. J Biol Chem. 2012;287:5673–5686. doi: 10.1074/jbc.M111.317776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moody MA, Yates NL, Amos JD, Drinker MS, Eudailey JA, Gurley TC, Marshall DJ, Whitesides JF, Chen X, Foulger A, et al. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol. 2012;86:7496–7507. doi: 10.1128/JVI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, Zhou T, Yang ZY, Svehla K, O'Dell S, Louder MK, Xu L, Mascola JR, Burton DR, Hoxie JA, et al. Enhanced exposure of the CD4-binding site to neutralizing antibodies by structural design of a membrane-anchored human immunodeficiency virus type 1 gp120 domain. J Virol. 2009;83:5077–5086. doi: 10.1128/JVI.02600-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Joyce MG, Kanekiyo M, Xu L, Biertumpfel C, Boyington JC, Moquin S, Shi W, Wu X, Yang Y, Yang ZY, et al. Outer domain of HIV-1 gp120: Antigenic optimization, structural malleability, and crystal structure with antibody VRC-PG04. J Virol. 2013;87:2294–2306. doi: 10.1128/JVI.02717-12. [An antigenically optimized outer domain construct is shown to deviate structurally from outer domain in the context of core gp120.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azoitei ML, Correia BE, Ban YE, Carrico C, Kalyuzhniy O, Chen L, Schroeter A, Huang PS, McLellan JS, Kwong PD, et al. Computation-guided backbone grafting of a discontinuous motif onto a protein scaffold. Science. 2011;334:373–376. doi: 10.1126/science.1209368. [DOI] [PubMed] [Google Scholar]
- 50••.Bhattacharyya S, Singh P, Rathore U, Purwar M, Wagner D, Arendt H, Destefano J, Labranche CC, Montefiori DC, Phogat S, et al. Design of an Escherichia coli Expressed HIV-1 gp120 Fragment Immunogen That Binds to b12 and Induces Broad and Potent Neutralizing Antibodies. J Biol Chem. 2013;288:9815–9825. doi: 10.1074/jbc.M112.425959. [In one of the first papers to show potential elicitation of effective HIV-1 antibodies, rabbits were primed with two fragments of gp120 then boosted with full length gp120; elicited antibodies show broad neutralization of HIV-1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlsson Hedestam GB. Nature Rev. Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 52.Morner A, Douagi I, Forsell MN, Sundling C, Dosenovic P, O'Dell S, Dey B, Kwong PD, Voss G, Thorstensson R, et al. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J Virol. 2009;83:540–551. doi: 10.1128/JVI.01102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundling C, Forsell MN, O'Dell S, Feng Y, Chakrabarti B, Rao SS, Lore K, Mascola JR, Wyatt RT, Douagi I, et al. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med. 2010;207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundling C, Li Y, Huynh N, Poulsen C, Wilson R, O'Dell S, Feng Y, Mascola JR, Wyatt RT, Karlsson Hedestam GB. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci Transl Med. 2012;4:142ra196. doi: 10.1126/scitranslmed.3003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee LK, Moore LR, Schmidt SD, Stuckey J, Yang Y, Zhou T, et al. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5663–5668. doi: 10.1073/pnas.1112391109. [Unliganded core gp120 is shown to “snap” into the CD4-bound conformation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Tomov V, Kurteva S, Wang L, Ren X, Gorny MK, Zolla-Pazner S, Sodroski J. Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1. J Virol. 2004;78:12975–12986. doi: 10.1128/JVI.78.23.12975-12986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Z, Ren L, Zheng Y, Qi Z, Liang H, Liu Y, Hong K, Shao Y. Eliciting broad neutralizing antibody to HIV-1: envelopes of different lentivirus cross immunization by prime-boost vaccination. Vaccine. 2012;30:5316–5323. doi: 10.1016/j.vaccine.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 58.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, et al. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J Virol. 2013;87:4185–4201. doi: 10.1128/JVI.02297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong T, Crooks ET, Osawa K, Binley JM. HIV-1 virus-like particles bearing pure env trimers expose neutralizing epitopes but occlude nonneutralizing epitopes. J Virol. 2012;86:3574–3587. doi: 10.1128/JVI.06938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCoy LE, Quigley AF, Strokappe NM, Bulmer-Thomas B, Seaman MS, Mortier D, Rutten L, Chander N, Edwards CJ, Ketteler R, et al. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. J Exp Med. 2012;209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Hannaman D, Reed SG, Felber BK, Pavlakis GN. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.037. Epub 25 April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed FK, Clark BE, Burton DR, Pantophlet R. An engineered mutant of HIV-1 gp120 formulated with adjuvant Quil A promotes elicitation of antibody responses overlapping the CD4-binding site. Vaccine. 2012;30:922–930. doi: 10.1016/j.vaccine.2011.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melchers M, Matthews K, de Vries RP, Eggink D, van Montfort T, Bontjer I, van de Sandt C, David K, Berkhout B, Moore JP, et al. A stabilized HIV-1 envelope glycoprotein trimer fused to CD40 ligand targets and activates dendritic cells. Retrovirology. 2011;8:48. doi: 10.1186/1742-4690-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, Waagmeester G, Sartorius R, D'Apice L, Caivano A, et al. Co-Immunization with Multimeric Scaffolds and DNA Rapidly Induces Potent Autologous HIV-1 Neutralizing Antibodies and CD8 T Cells. PLoS One. 2012;7:e31464. doi: 10.1371/journal.pone.0031464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: leading B cells down the right path. Nat Struct Mol Biol. 2011;18:1317–1321. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- 67.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mouquet H, Nussenzweig MC. HIV: Roadmaps to a vaccine. Nature. 2013;496:441–442. doi: 10.1038/nature12091. [DOI] [PubMed] [Google Scholar]
- 69••.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev I, Pancera M, Zhou T, Incesu RB, Fu BZ, et al. Somatic mutations of the immunoglobulin framework are generaly required for broad and potent HIV-1 neutralizing activity. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [A mechanistic explanation is presented for the observation that HIV-1 neutralizing antibodies generally show increased levels of framework mutations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Scharf L, West AP, Jr., Gao H, Lee T, Scheid JF, Nussenzweig MC, Bjorkman PJ, Diskin R. Structural basis for HIV-1 gp120 recognition by a germ-line version of a broadly neutralizing antibody. Proc Natl Acad Sci U S A. 2013;110:6049–6054. doi: 10.1073/pnas.1303682110. [Germline antibody structures provide insight into the initial interactions with HIV-1 Env required to activate naïve mature B cells of the VRC01 class.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F, Diskin R, Scheid JF, Sather DN, Burton DR, et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 2013;9:e1003106. doi: 10.1371/journal.ppat.1003106. [A large panel of HIV-1 Envs is shown to lack interaction with germline versions of the broadly neutralizing CD4-binding site antibodies b12, NIH45-46 and 3BNC60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, Jardine J, Menis S, Scheid JF, West AP, et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. The Journal of experimental medicine. 2013;210:655–663. doi: 10.1084/jem.20122824. [Specific modifications to HIV-1 Env at three sites of N-linked glycosylation are shown to allow germline activation of antibodies of the VRC01 class.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73••.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [A multimeric structure-based immunogen is shown to trigger B cell signaling for germline version of VRC01-class antibodies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai K, Boyington J, Shi W, Schmidt S, Georgiev I, Lingwood D, Kwong P, Mascola J, Yang Z, Nabel G. Hyperglycosylated resurfaced stabilized GP120 core as an immunogen elicits antibodies targeted at the CD4-binding site. Retrovirology. 2012;9:P23. [Google Scholar]
- 75.Tobin GJ, Trujillo JD, Bushnell RV, Lin G, Chaudhuri AR, Long J, Barrera J, Pena L, Grubman MJ, Nara PL. Deceptive imprinting and immune refocusing in vaccine design. Vaccine. 2008;26:6189–6199. doi: 10.1016/j.vaccine.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 76.Kumar R, Tuen M, Li H, Tse DB, Hioe CE. Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation. Vaccine. 2011;29:9064–9074. doi: 10.1016/j.vaccine.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, Montefiori D, Polacino P, Hu SL. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.Chuang GY, Boyington JC, Joyce MG, Zhu J, Nabel GJ, Kwong PD, Georgiev I. Computational prediction of N-linked glycosylation incorporating structural properties and patterns. Bioinformatics. 2012;28:2249–2255. doi: 10.1093/bioinformatics/bts426. [Glycan addition to immunogens can help to focus the immune response away from unwanted sites; this paper provides methodology to predict glycan occupancy, a step towards the prediction of sites where glycans can be added.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ota T, Doyle-Cooper C, Cooper AB, Huber M, Falkowska E, Doores KJ, Hangartner L, Le K, Sok D, Jardine J, et al. Anti-HIV B Cell lines as candidate vaccine biosensors. J Immunol. 2012;189:4816–4824. doi: 10.4049/jimmunol.1202165. [DOI] [PMC free article] [PubMed] [Google Scholar]