Abstract
Background
Mast cells have gained notoriety based on their detrimental contributions to IgE-mediated allergic disorders. Although mast cells express the vitamin D receptor (VDR), it is not clear to what extent 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), or its predominant inactive precursor metabolite in circulation, 25-hydroxyvitamin D3 (25OHD3), can influence IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA) in vivo.
Objective
We sought to assess whether the vitamin D3 metabolites, 25OHD3 and 1α,25(OH)2D3, can repress IgE-dependent mast cell activation via mast cell-CYP27B1 and -vitamin D receptor activity.
Methods
We measured the extent of vitamin D3 suppression of IgE-mediated mast cell degranulation and mediator production in vitro, as well as the vitamin D3-induced curtailment of PCA responses in WBB6F1-KitW/W-v or C57BL/6J-KitW-sh/W-sh mice engrafted with mast cells that did or did not express VDR or CYP27B1.
Results
Here we show that mouse and human mast cells can convert 25OHD3 to 1α,25(OH)2D3 via 25-hydroxyvitamin D-1α–hydroxylase (CYP27B1) activity, and that both of these vitamin D3 metabolites suppressed IgE-induced mast cell-derived pro-inflammatory and vasodilatory mediator production in a VDR-dependent manner in vitro. Furthermore, epicutaneously applied vitamin D3 metabolites significantly reduced the magnitude of skin swelling associated IgE-mediated PCA reactions in vivo; a response that required functional mast cell-VDRs and mast cell-CYP27B1.
Conclusion
Taken together, our findings provide a mechanistic explanation for the anti-inflammatory effects of vitamin D3 on mast cell function by demonstrating that mast cells can actively metabolize 25OHD3 to dampen IgE-mediated mast cell activation in vitro and in vivo.
Keywords: Mast cells, anaphylaxis, inflammation, IgE, vitamin D3, vitamin D receptor, CYP27B1
INTRODUCTION
Mast cells are key pro-inflammatory effector cells that can act as potent initiators and amplifiers of IgE-dependent inflammatory and allergic reactions, such as life-threatening anaphylaxis, allergic rhinitis (hay fever), atopic dermatitis (eczema) and allergic asthma1. Mast cells express the high affinity IgE receptor FcεRI and, upon activation by polyvalent antigen-induced aggregation of FcεRI-bound IgE, they can release a diverse array of preformed cytoplasmic granule-associated mediators (e.g., proteases and vasoactive amines, such as histamine), as well as de novo synthesised pro-inflammatory lipid mediators, cytokines and chemokines1. In certain settings where invading pathogens require elimination, IgE-mediated mast cell activation is beneficial2. However, the detrimental function of unrestrained IgE-driven activity can contribute to excessive inflammation and the severity of pathology associated with allergic disease1, 2. While factors that promote allergic disorders are being investigated intensively (e.g., determinations of genetic predisposition3, 4, there is emerging evidence that mast cells can produce anti-inflammatory (i.e., IL-10) or regulatory mediators (i.e., IL-2), and thereby negatively regulate the magnitude or duration of acute or chronic adverse inflammation in certain settings5–8. These findings raise the possibility that exogenous agents could be employed to engage regulatory pathways in mast cells which in turn can dampen allergic inflammation via steroid independent approaches.
In recent years the secosteroid hormone vitamin D3 has emerged as a crucial immunoregulatory agent, exerting broad anti-inflammatory actions via the nuclear VDR that is widely expressed in the immune system9, 10. Vitamin D3 (cholecalciferol) is predominantly generated in the epidermis when pre-vitamin D3, the photochemical product derived from 7-dehydrocholesterol in response to ultraviolet-B (UVB) irradiation of the skin, undergoes spontaneous thermal isomerisation. The conversion of vitamin D3 to its active metabolite 1α,25(OH)2D3, is carried out in a series of hydroxylation events, firstly by liver cytochrome P450 proteins (e.g., CYP27A1, CYP2DII, CYP3A4, CYP2R1, CYP2D25) to generate the intermediate metabolite, 25OHD3, and then by 25-hydroxyvitamin 1α-hydroxylase (CYP27B1) in the proximal tubule of the kidney to form 1α,25(OH)2D311. 1α,25(OH)2D3 exerts its transcriptional activity by binding to the VDR, which leads to the recruitment of its preferred dimerization partner, the retinoid X receptor, to form a heterodimeric complex that targets vitamin D response elements in the promoter regions of genes. Depending on the simultaneous binding of either nuclear co-activators or co-repressors, the DNA-bound complex can function as a ligand-dependent activator or repressor of gene transcription11–13.
Epidemiological and experimental data suggest that vitamin D3 insufficiency and suboptimally low levels of circulating 25OHD3 are linked to the pathogenesis of allergic disorders, particularly asthma and eczema in children and infants, respectively14–16. At the molecular level, 1α,25(OH)2D3 modifies immune cell functions, including macrophage differentiation, dendritic cell antigen presentation, enhancement of regulatory T cell numbers and activity, and also dampens T helper 17 differentiation9, 17. Surprisingly, it is not known to what extent any potential effect of the vitamin D3 metabolites, 1α,25(OH)2D3 or its precursor, 25OHD3, reflects its action on mast cells versus other cell populations during IgE-mediated cutaneous anaphylactic responses in vivo. We recently reported that 1α,25(OH)2D3 can induce skin mast cells to produce the anti-inflammatory cytokine IL-10 and thereby curtail IgE-independent inflammation associated with chronic UVB exposure of the skin7. In this study, we investigated firstly if 1α,25(OH)2D3 can VDR-dependently suppress the extent of IgE-mediated mast cell activation both in vitro and during IgE-induced PCA in vivo; secondly, we determined whether mast cells express CYP27B1 and whether its ability to synthesise 1α,25(OH)2D3 is required to mediate 25OHD3-induced negative regulation of IgE-mediated function in vitro and in vivo; and finally we tested whether human mast cells respond like mouse mast cells to the immunoregulatory properties of vitamin D3.
RESULTS
Vitamin D3 down-regulates IgE-mediated mast cell degranulation and cytokine production in a VDR-dependent manner in vitro
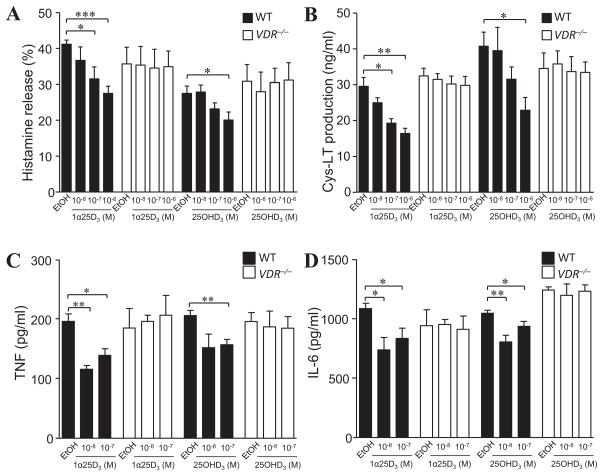
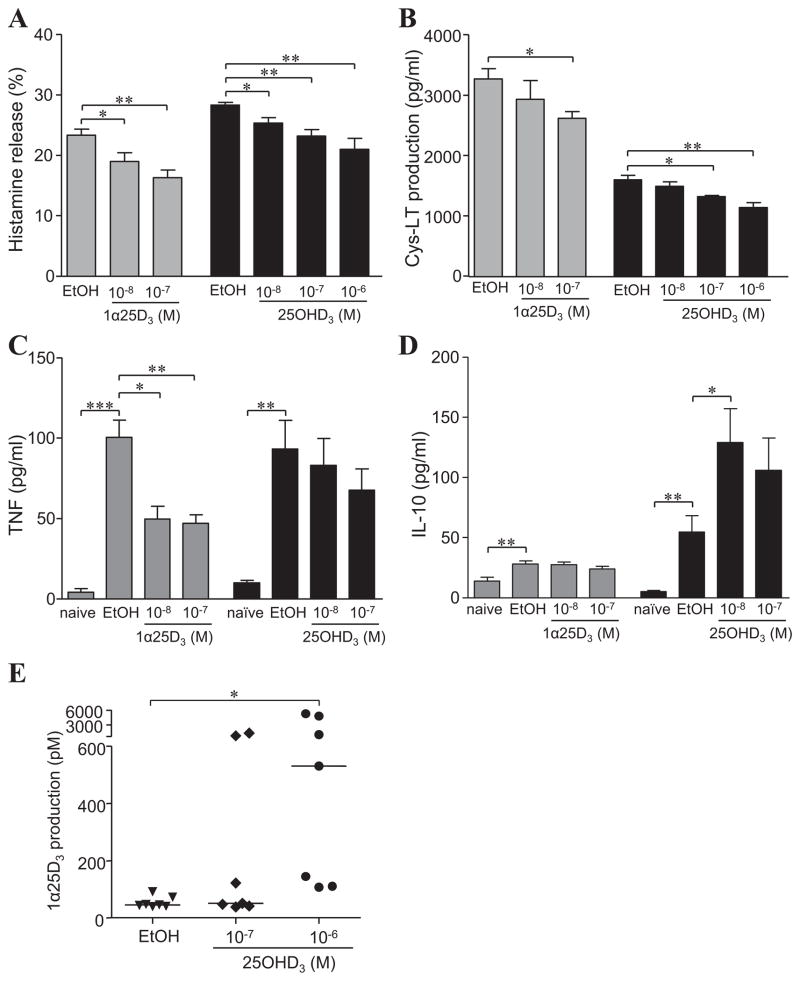
Having shown that mast cells can limit inflammation associated with contact hypersensitivity reactions5, and that they are responsive to 1α,25(OH)2D3 in a VDR-dependent manner7, 19, we tested whether 1α,25(OH)2D3, as well as its inactive precursor metabolite 25OHD3, can modify IgE + specific antigen-induced activation of BMCMCs in vitro. Exposure to 1α,25(OH)2D3 for 24 h reduced IgE-mediated histamine release (by 23–34%; Fig 1, A) and cysteinyl leukotriene (Cys-LT) production (by 34–44%; Fig 1, B) at the highest concentrations (10−7 and 10−6 M) tested in wild-type (WT) C57BL/6J (B6) mouse BMCMCs. Reduction of de novo TNF (Fig 1, C) and IL-6 (Fig 1, D) production was significant for both 10−8 M and 10−7 M 1α,25(OH)2D3 in B6-WT (i.e., VDR+/+) BMCMCs. The curtailed release of these vasodilatory and pro-inflammatory mediators was not due to reduced cell viability, or repression of the key signalling elements, phospho (p)Erk1/2, p38, pJNK or pNF-κB-p65, downstream of IgE-FcεRI activation, or altered cell surface expression of the high affinity IgE receptor, FcεRI, or of reduced c-Kit receptor expression (see Fig E1 in this article’s Online Repository at www.jacionline.org); a finding previously reported to occur in BALB/c BMCMCs with long term 30 to 40 d 1α,25(OH)2D3 (10−7 M) incubation that causes mast cell apoptosis and inhibits maturation/differentiation of mast cell progenitors19. Importantly, we found that functional VDRs were necessary to mediate the negative regulation of mast cells in this setting (Figs 1, A to D). Interestingly, the inactive 25OHD3 metabolite, like its biologically active form, required functional VDR expression to exert similar immunosuppressive activity on IgE-mediated BMCMC function, particularly at the highest concentrations of 10−7 M and 10−6 M tested (Fig 1, A to D).
Figure 1. Mast cell-VDRs are required for optimal 1α,25(OH)2D3 or 25OHD3 impairment of IgE-mediated mast cell activation in vitro.
WT (B6J) and VDR−/− BMCMCs incubated with 1α,25(OH)2D3 (1α25D3) or vehicle (EtOH) 16 h or 24 h prior to (for 25OHD3), and during IgE + DNP-HSA stimulation and release of (A) histamine (30 min), (B) Cys-LT (30 min), (C) TNF (6 h), and (D) IL-6 (6 h). Data: 3 to 5 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons.
CYP27B1 hydroxylase activity is required for 25OHD3-induced suppression of IgE-mediated mast cell activation
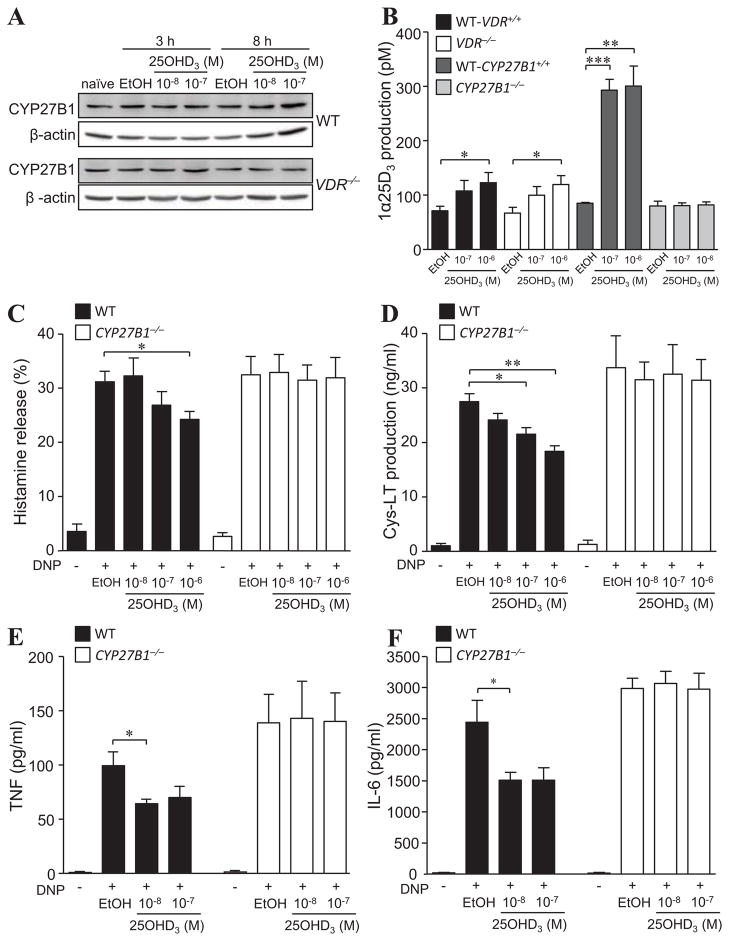
It is unclear whether mast cells exhibit CYP27B1 activity and can convert 25OHD3 to 1α,25(OH)2D3. Therefore, we first analysed CYP27B1 expression in BMCMCs by immunoblot (Fig 2, A) as well as immunofluorescence and flow cytometric analysis (see Fig E2 in this article’s Online Repository). Both B6-VDR+/+ and VDR−/− BMCMCs constitutively expressed CYP27B1 and although no change in CYP27B1 protein levels were observed up to 8 h following incubation with either 10−8 M or 10−7 M 25OHD3 (Fig 2, A), VDR+/+, VDR−/− and to a greater extent CYP27B1+/+ BMCMCs (possibly due to the more diverse genetic background of this mouse colony) all had the ability to convert 10−7 and 10−6 M 25OHD3 to 1α,25(OH)2D3 (Fig 2, B). Importantly, CYP27B1−/− BMCMCs were unable to produce 1α,25(OH)2D3, showing that 25-hydroxyvitamin D-1α-hydroxylase is essential. Interestingly, unlike in vivo findings in the proximal tubule of the kidney where CYP27B1 activity can be inhibited by 1α,25(OH)2D320, 1α,25(OH)2D3 lacked the ability to VDR-dependently trans-repress CYP27B1 mRNA (up to 6 h; Fig E3 in this article’s Online Repository) or reduce protein expression (up to 8 h) in WT BMCMCs (Fig 2, A).
Figure 2. 25OHD3 driven inhibition of IgE-mediated activation functions via mast cell-CYP27B1 catalytic activity.
A) CYP27B1 and β-actin protein expression in WT and VDR−/− BMCMCs cultured for 3 or 8 h with 25OHD3 at indicated concentrations or vehicle (EtOH). (B) WT, VDR−/−, or CYP27B1−/− BMCMC production of 1α,25(OH)2D3 (1α25D3) incubated with 25OHD3 for 6 h. (C to F) WT and CYP27B1−/− BMCMCs pre-treated with 25OHD3 24 h prior to IgE + DNP-HSA stimulation and release of (C) histamine (30 min), (D) Cys-LT (30 min), (E) TNF (6 h), and (F) IL-6 (6 h) into supernatants. Data: 3 to 4 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons.
Notably, as determined for VDR−/− BMCMCs (Fig 1, A to D), CYP27B1−/− BMCMCs were also unresponsive to the suppressive properties of 10−7 M or 10−6 M 25OHD3 compared to WT CYP27B1-active BMCMCs which exhibited significantly reduced histamine release (Fig 2, C), Cys-LT (Fig 2, D), TNF (Fig 2, E) and IL-6 (Fig 2, F) production. Thus, although these findings do not exclude some suppressive effects mediated by directly bound 25OHD3-VDR, our in vitro data provide evidence that mast cell-CYP27B1 hydroxylase is required for mast cells to generate 1α,25(OH)2D3, which in turn, can repress IgE-mediated BMCMC activation in a VDR-dependent manner.
Mast cell VDRs are essential for optimal curtailment of IgE-dependent PCA reactions by epicutaneous 1α,25(OH)2D3 treatment in vivo
Our finding that the vitamin D3 metabolites 1α,25(OH)2D3 and 25OHD3 can restrain IgE-induced mast cell activation in vitro, and that this activity requires mast cell-VDRs, raised the possibility that topical application of these secosteroid derivatives could ameliorate mast cell-specific skin reactions associated with PCA in vivo. To test this, we used genetic and cell transfer approaches in two types of c-kit mutant mice, (i.e., WBB6F1-KitW/W-v and C57BL/6J-KitW-sh/W-sh mice) which are profoundly mast cell-deficient and can be selectively engrafted with in vitro-derived mast cells from WT mice or mice lacking specific mast cell-associated receptors (i.e., VDR−/−) or enzyme activity (i.e., CYP27B1−/−). This “mast cell knock-in”-like approach helps to reveal the extent to which the mast cell-dependent effects in mutant mice can be separated from those due to other abnormalities18, 21 associated with their mutation(s), as only the mast cell deficiency is repaired by engraftment.
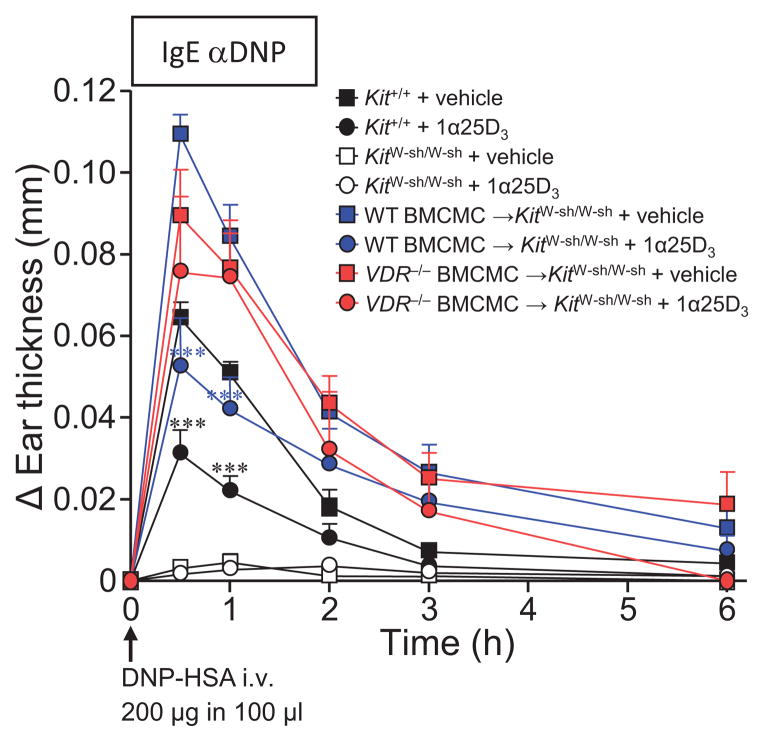
A single topical application of 1α,25(OH)2D3 at a dose of 0.06 nmol per ear (3 μM), 16 h prior to i.v. challenge with specific-antigen (DNP-HSA), significantly reduced the immediate phase (0.5–1 h) of IgE-induced PCA-associated ear swelling (Fig 3) and corresponded with attenuated transcription of histidine decarboxylase (HDC) and leukotriene C4 synthase (LTC4S) mRNA compared to untreated PCA ears in WT C57BL/6J (Kit+/+) mice (see Fig E4 in this article’s Online Repository). In contrast, due to their lack of mast cells, KitW-sh/W-sh mice did not exhibit PCA reactions in IgE-sensitized ears (ear swelling was indistinguishable from that observed in their vehicle-injected ear pinnae, regardless of 1α,25(OH)2D3 application) (Fig 3 and see Fig E5 in this article’s Online Repository). However, despite implications that mast cells might be a target of 1α,25(OH)2D322, it remained to be definitively clarified whether in the course of a PCA reaction, 1α,25(OH)2D3 could exert direct restraint of mast cell function, or acted independently of this, perhaps by altering vascular endothelial permeability.
Figure 3. Epicutaneous application of 1α,25(OH)2D3 suppresses IgE-mediated PCA reactions in a mast cell-VDR-dependent manner.
Changes (Δ) in ear thickness 0–6 h after i.v. injection of 200 μg of DNP-HSA into mice and 16 h after pretreated with topical application of 0.06 nmol/ear 1α,25(OH)2D3 (1α25D3; circles) or vehicle (EPGW; squares) that occurred concurrent with i.d. injection of 20 ng IgE anti-DNP in Kit+/+ (WT) mice, KitW-sh/W-sh, WT BMCMC→KitW-sh/W-sh, or VDR−/− BMCMC→KitW-sh/W-sh mice. Data; n = 9 to 12 mice/group, 3 independent experiments. ***; P < 0.001 for comparisons of 1α,25(OH)2D3 versus vehicle-treated ears within the same group of mice.
To address this question, we assessed KitW-sh/W-sh mice engrafted with WT BMCMCs (WT BMCMC→KitW-sh/W-sh mice). Like WT littermates, they exhibited a marked reduction in the magnitude of the IgE-PCA reaction following 1α,25(OH)2D3 application (Fig 3). To evaluate whether mast cell-VDRs were required to mediate these suppressive effects of 1α,25(OH)2D3, we also tested VDR−/− BMCMC→KitW-sh/W-sh mice and found that these animals failed to respond to topical 1α,25(OH)2D3 application, exhibiting comparable levels of ear swelling versus corresponding vehicle-treated IgE-PCA mice (Fig 3). Importantly, we also noted that our findings in KitW-sh/W-sh mice were recapitulated in WBB6F1-Kit+/+, mast cell-deficient KitW/W-v and WT or VDR−/− BMCMC→KitW/W-v mice (see Fig E6 in this article’s Online Repository). This confirmed that notwithstanding the particular range of abnormalities carried by KitW/W-v and KitW-sh/W-sh mice, selective repair of their mast cell deficiency was sufficient to demonstrate the requirement for mast cell-VDRs for the immunoregulatory function of 1α,25(OH)2D3 we observed in this experimental setting. Furthermore, the differences in ear thickness between the groups of IgE-Ag-challenged WT or VDR−/− BMCMC→KitW-sh/W-sh mice were unlikely to be related to disparities in the extent of mast cell engraftment, as similar numbers of ear pinna mast cells were present in the two groups irrespective of IgE-sensitization or 1α,25(OH)2D3 treatment (see Fig E7 in this article’s Online Repository).
Skin irritation or heightened ear swelling responses were not observed in any of the HMEM-Pipes vehicle-injected ears from the groups of mice tested with a single epicutaneous application of 0.06 nmol/ear 1α,25(OH)2D3 (see Fig E5 in this article’s Online Repository); nor was there evidence of elevated ear swelling after 9 applications every 2 d of 0.06 nmol/ear or 0.25 nmol/ear dose of 1α,25(OH)2D3 in C57BL/6J WT mice (see Fig E8, A in this article’s Online Repository). In contrast, multiple exposures of 1α,25(OH)2D3 significantly elevated thymic stromal lymphopoietin (TSLP) mRNA levels only in the mice receiving the higher amount tested (0.25 nmol/ear dose) (see Fig E8, B in this article’s Online Repository). Notably, although a single (see Fig E9 in this article’s Online Repository) or multiple application of 1α,25(OH)2D3 (0.25 nmol/ear or 0.06 nmol/ear dose) markedly curtailed ear swelling responses, each to a similar extent, in the first 30 min of the PCA reaction, the extent of the repression was dampened over the 1 h to 6 h time course in C57BL/6J WT mice that received multiple applications of the high dose 1α,25(OH)2D3 compared with the lower dose group (see Fig E8, C in this article’s Online Repository). These findings indicate that the induction of TSLP might reduce the regulatory effect of 1α,25(OH)2D3 to a limited extent in C57BL/6J WT mice and that the lower dose of 1α,25(OH)2D3 was required for optimal attenuation of IgE-mediated PCA reactions in vivo.
Mast cell-CYP27B1 activity is required for 25OHD3-induced attenuation of PCA
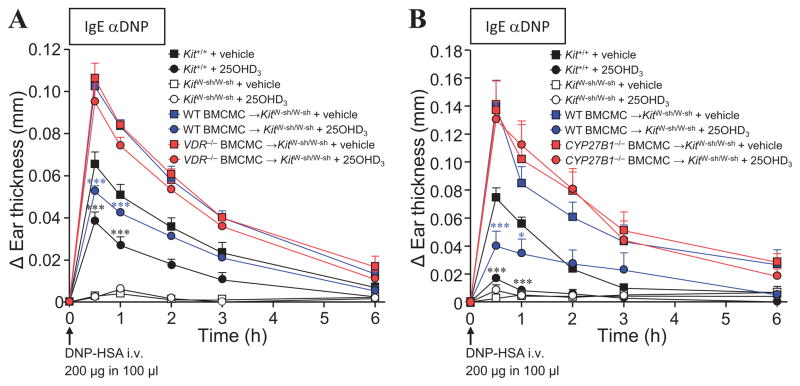
Based on our in vitro data (Fig 1, A to D), we next assessed whether epicutaneous 25OHD3 could reduce IgE- and mast cell-dependent PCA reactions in vivo. 25OHD3 pre-treatment mirrored the effects of 1α,25(OH)2D3, causing a significant reduction in IgE-dependent ear swelling in WT mice and WT BMCMC→KitW-sh/W-sh mice, but not in VDR−/− BMCMC→KitW-sh/W-sh mice (Fig 4, A). Notably, mast cell-CYP27B1 activity was necessary for reduced tissue swelling in this setting (Fig 4, B). Absence of mast cell-CYP27B1 rendered the BMCMC-engrafted groups of KitW-sh/W-sh (Fig 4, B) and KitW/W-v mice (see Fig E10 in this article’s Online Repository) unresponsive to 25OHD3 compared to WT littermates and WT BMCMC-engrafted groups. Ear swelling in vehicle-injected control ears was minimal, indistinguishable among the groups of mice tested and remained unchanged with 25OHD3 treatment (see Figs E10 and E11 in this article’s Online Repository). No significant disparities were evident in the numbers of mast cells in ear pinnae among the engrafted groups of KitW-sh/W-sh and KitW/W-v mice (data not shown). Thus, our data show that mast cell-VDRs are required to mediate the activity of the vitamin D3 metabolites, 1α,25(OH)2D3 and 25OHD3 and strongly suggest that autogenous mast cell 1α,25(OH)2D3 production through the activity of CYP27B1 contributed to the response to 25OHD3 (even though other potential extra-renal sources of 1α,25(OH)2D3 derived from the administered 25OHD3 might have been available to interact with CYP27B1−/− BMCMC-VDRs in the treated ear).
Figure 4. Mast cell-VDR and –CYP27B1 activity are required for epicutaneous 25OHD3 dampening of IgE-mediated PCA reactions.
Changes (Δ) in ear thickness 0–6 h after i.v. injection of 200 μg of DNP-HSA into mice and 24 h after topical application of 0.06 nmol/ear 25OHD3 (circles) or vehicle (EPGW; squares) and 16 h after i.d. injection of 20 ng IgE anti-DNP antibody (right ears) in Kit+/+ (WT) mice, KitW-sh/W-sh, WT BMCMC→KitW-sh/W-sh, (A) VDR−/− BMCMC→KitW-sh/W-sh, or (B) CYP27B1−/− BMCMC→KitW-sh/W-sh mice. Data are from 3 (A, n = 2 to 4 mice/group/experiment), or 4 (B, n = 3 to 4 mice/group/experiment) independent experiments. *, P < 0.05; **, P < 0.01; ***; P < 0.001 for comparisons of 25OHD3 versus vehicle-treated ears within the same group of mice.
IgE-mediated activation of human mast cells can be negatively regulated by vitamin D3 metabolites
Due to the substantial immunosuppressive ability of both 1α,25(OH)2D3 and 25OHD3 on mouse BMCMCs in vitro and in vivo, we decided to examine the potential clinical relevance of these findings by testing human CBMCs or PBMCs. Like their mouse counterparts, CBMCs and PBMCs exhibited a dose-dependent reduction in histamine release and Cys-LT production in response to either of the vitamin D3 metabolites (Fig 5, A and B; see Fig E12 in this article’s Online Repository). Although TNF production was significantly impaired by 10−8M and 10−7 M 1α,25(OH)2D3 and to a lesser extent by 25OHD3 (Fig 5, C), IgE-dependent CBMC-IL-10 production remained unchanged by 1α,25(OH)2D3 and was even significantly enhanced in the presence of 10−8 M 25OHD3 (Fig 5, D). As in mouse BMCMCs7 (Fig 2, A), these vitamin D3 driven immunomodulatory effects are likely mediated via CBMC-VDRs (see Fig E12 in this article’s Online Repository) and –CYP27B1 hydroxylation of 25OHD3 to biologically active 1α,25(OH)2D3 (Fig 5, E and see Fig E12 in this article’s Online Repository).
Figure 5. Vitamin D3 metabolites can impair IgE-mediated human mast cell activation.
CBMCs pre-treated with 1α,25(OH)2D3 (1α25D3) or 25OHD3 or vehicle (EtOH) at the time of, or 8 h prior to (for 25OHD3), sensitization with human myeloma IgE for 16 h, followed by challenge with anti-human IgE antibody and release of (A) histamine (30 min), (B) Cys-LT (30 min), (C) TNF (16 h), or (D) IL-10 (16 h) into supernatants. Data: 3 to 5 different cord blood donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons. (E) CBMC production of 1α,25(OH)2D3 (1α25D3) following incubation with 25OHD3 for 6–7 h. Data: 7 independent cord blood donors. *, P < 0.05 for the indicated comparison, as analysed by Wilcoxon t-test.
DISCUSSION
In this study, we have identified that mouse and human mast cells express 25-hydroxyvitamin D-1α–hydroxylase which enables them to convert inactive 25OHD3 to biologically active 1α,25(OH)2D3. Mast cell-CYP27B1 activity and mast cell-VDRs represent important mechanisms by which the vitamin D3 metabolites, 25OHD3 and 1α,25(OH)2D3, can repress overt IgE-mediated mast cell activation in vitro and in vivo. The intrinsic ability to endogenously produce 1α,25(OH)2D3 is a previously unrecognised function of mast cells and is likely to have clinical implications in settings of 25OHD3 insufficiency.
Interestingly, we observed that the inactive 25OHD3 metabolite, like its biologically active form, required functional VDR expression to exert similar immunosuppressive activity on IgE-mediated BMCMC function, particularly at the highest concentrations of 10−7 and 10−6 M tested (Fig 1, A to D). 25OHD3 can bind to VDR with low affinity and has been described as a weak genomic agonist at this concentration23. It is therefore possible that 25OHD3-bound VDR exerted its regulatory effects in a manner similar to that of its more biologically efficacious metabolite 1α,25(OH)2D3, albeit at higher concentrations. However, our data indicates that the most likely mechanistic explanation for 25OHD3-VDR repression of IgE-mediated mast cell function is via the mast cell’s intrinsic ability to convert 25OHD3 to 1α,25(OH)2D3 in a CYP27B1-dependent manner.
Although our in vitro investigations provided evidence that treatment of IgE-activated mast cells with 25OHD3 or 1α,25(OH)2D3 appeared to cause a modest reduction in the release of each individual mediator tested, it is conceivable that the cumulative effect of these individual changes could result in a substantial diminished response in the setting of mast cell-dependent IgE-mediated PCA in vivo. However, additional repressive effects on G protein coupled receptors, such as the anaphylatoxin receptors24 or sphingosine-1-phosphate receptors25, 26, which can enhance IgE-dependent skin inflammation in vivo cannot be excluded and are yet to be explored. Importantly, our data show that mast cell-VDRs at the skin site of topical application are required to mediate the activity of the vitamin D3 metabolites, 1α,25(OH)2D3 and 25OHD3, and strongly suggest that endogenous mast cell 1α,25(OH)2D3 production through the catalytic activity of CYP27B1 contributed to the response to 25OHD3 (even though other potential extra-renal sources of 1α,25(OH)2D3 –derived from the administered 25OHD3 might have been available to interact with CYP27B1−/ −BMCMC-VDRs in the treated ear).
In other settings, application of 1α,25(OH)2D3 to the skin can induce a regulatory-type of environment by enhancing CD4+CD25+ T regulatory cell suppressive activity27 and down regulating immune responses in models of contact hypersensitivity22, 28. Recently, Li et al.29 reported that CD1 WT mice treated every other day for 18 d with a 0.25 nmol/ear dose of 1α,25(OH)2D3 developed atopic dermatitis-like skin inflammation. We found that multiple exposures of 1α,25(OH)2D3 at only the higher amount tested (0.25 nmol/ear dose compared to 0.06 nmol/ear) significantly elevated TSLP mRNA levels in the treated skin but was not coupled with skin irritation and exacerbated ear swelling responses in the C57BL/6J WT mice tested; a disparity which might be due to the different genetic backgrounds of the mice. Importantly, the lower dose of 0.06 nmol 1α,25(OH)2D3/ear used throughout our in vivo studies which elicited marked negative regulatory effects on IgE-dependent PCA reactions, could be applied multiple times without enhancing TSLP expression or skin irritation and was able to attenuate PCA reactions to a similar extent irrespective of whether applied as a single or multiple (9×) application; a finding which suggests that low-calcemic VDR-agonists might have beneficial anti-inflammatory functions when topically applied at much lower amounts than those reported to induce TSLP and trigger atopic dermatitis in mice29.
The observation that IgE-activated human CBMCs can potentially be altered from a pro-inflammatory to an IL-10-expressing anti-inflammatory state when exposed to sufficient levels of vitamin D3 is of interest, and may even be clinically relevant. Allergic disorders have dramatically increased in prevalence in industrialized countries. The pathogenesis of such disorders is likely to be multi-factorial but there is growing speculation of a link with vitamin D3 insufficiency, defined by most experts as below 20 ng/mL (i.e. 48 nM) and optimal >30 ng/mL (72 nM) of 25OHD310, 16; a concentration range that is similar to those tested in our human CBMCs (10−8 to 10−6 M; Fig 5, A to D; see Fig E12 in this article’s Online Repository). Maternal 25OHD3 insufficiency during pregnancy has been linked to an increased risk of eczema via alterations in skin barrier function and changes in microbial defence in the first 12 months of infancy15, and levels of serum vitamin D3 have been shown to inversely correlate with serum IgE levels in children with asthma14. Based on our in vitro and in vivo findings, it is plausible that suboptimal levels of circulating 25OHD3 could unshackle an intrinsic mechanism of mast cell restraint, resulting in heightened detrimental contributions of mast cells during IgE-mediated disorders.
In conclusion, our study has provided evidence that the vitamin D3 metabolites, 1α,25(OH)2D3 and 25OHD3 can attenuate the generation of pro-inflammatory signals from IgE-activated mouse and human mast cells. Essential to this negative regulatory function of vitamin D3 is mast cell expression of VDRs, and for 25OHD3, the hydroxylase activity of CYP27B1. Our results in C57BL/6J-KitW-sh/W-sh and WBB6F1-KitW/W-v mice show that epicutaneously applied vitamin D3 metabolites, working via mast cell-associated VDR and CYP27B1 activity, can restrain mast cell-driven passive cutaneous anaphylaxis in vivo. The nature of the mast cell’s responses to vitamin D3 may vary in different species and depend on metabolite concentrations readily available, not only in terms of serum levels in circulation but also the local levels of biologically active 1α,25(OH)2D3 in tissues, derived from either external or mast cell-intrinsic sources. However, our findings suggest that optimal levels of vitamin D3 may contribute to the homeostatic regulation of mast cells, and that a deficiency in vitamin D3 may result in dysregulation of this vitamin D:mast cell regulatory axis. These findings also highlight the possibility of employing certain low-calcemic agonists of VDRs, or perhaps even dietary supplementation with 25OHD3 itself, for the management of mast cell-driven allergic responses and perhaps other inflammatory disorders in which mast cells are implicated.
ONLINE REPOSITORY METHODS
Mice
B6.129S4-Vdrtm1Mbd/J mice were backcrossed to C57BL/6 mice for greater than nine generations. As previously reported, adult KitW-sh/Wsh and KitW/W-v mice have a profound deficiency of mast cells, including <1.0% the WT level of mast cells in the dermisE1–3. All mice (including VDR−/− mice) with the exception of CYP27B1−/− mice, were provided commercial mouse chow containing Vitamin D3 (cholecalciferol) at >2,000 IU/kg ad libitum. Derivation of parental strain was undertaken by homologous recombination in embryonic stems where a neomycin resistance gene was inserted in place of exons VI, VII and VIII of the mouse CYP27B1 gene, replacing both the ligand binding and heme binding domains, as previously describedE4. These mice were originally maintained on a mixed genetic background with B6 and BALB/c strains and then backcrossed for an additional 3 generations with C57BL/6 mice in house in Adelaide. CYP27B1−/− mice were maintained on a high calcium diet containing 1.5% calcium in drinking water and chow containing 1% calcium, 0.85% phosphorus, 0% lactose and 2200 IU/kg Vitamin D3 (Specialty Feeds). Experiments were performed in compliance with the ethical guidelines of the National Health and Medical Research Council of Australia, with approval from the Institute of Medical and Veterinary Science Animal Ethics Committee (Australia).
Generation of BMCMCs
As previously describedE2, 5, BMCMCs were obtained by culturing bone marrow cells from femurs and tibias of mice in DMEM (Life Technologies) supplemented with 10% fetal calf serum (FCS; Bovogen) and 20% WEHI-3 conditioned medium (containing 3–4 ng/mL IL-3) for 4–6 wk, at which time > 95% of the cells were identified as mast cells by May Grünwald-Giemsa staining and by flow cytometric analysis (c-Kit+, FcεRI+).
Preparation of vitamin D3
1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) and 25-hydroxyvitamin D3 (25OHD3) (Sigma-Aldrich) were reconstituted at 1 or 10 mM with 100% absolute ethanol (EtOH; Sigma-Aldrich) and stored, shielded from light, in an airtight tube at −80°C. The chemical integrity of both metabolites was regularly verified using a scanning spectrophotometer.
Multiple epicutaneous applications of vitamin D3 with or without IgE-dependent PCA
For experiments where 1α,25(OH)2D3 was epicutaneously applied a total of 9 times every 2 d in the absence of PCA, female C57BL/6J mice received per application a dose of 0.06 nmol/ear (3 μM in 20 μL of EPGW vehicle) or 0.25 nmol/ear (12.5 μM in 20 μL of 100% EtOH vehicle) to the right ear or vehicle alone as indicated to the left ear and change in ear thickness from baseline measured prior to each application. For some experiments on the same day as the final application of 1α,25(OH)2D3, mice were i.d. injected with 20 ng IgE anti-DNP in the right ear, or vehicle HMEM-Pipes in the left ear. 16h later, mice were i.v. injected with 200 μg of DNP-HSA and changes (Δ) in ear thickness 0–6 h were measured and calculated from baseline measured at day 0 prior to first application of 1α,25(OH)2D3. For all experiments, ear pinnae were collected for histological analysis and gene expression analysis 24 h after the final application of 1α,25(OH)2D3 or 6 h after induction of the PCA reaction.
Histology and quantification of mast cell numbers
Mice were killed by CO2 inhalation and samples of ear pinna were fixed in 10% buffered formalin, embedded in paraffin (with care to ensure a cross-section orientation), and 4-μm sections were cut. Ear sections were stained with 0.1% Toluidine Blue (pH 1.0) for the detection of mast cells (cytoplasmic granules appear purple). Ear pinna mast cells were counted in 6–9 consecutive fixed fields of 870 μm width using a 20× microscope objective (200× final magnification), and mast cell numbers were expressed per horizontal ear cartilage field length (millimeter), using computer-generated image analysis (NIH Image J software, version 1.46r). The entire length of a strip of skin extending from the base to the tip of the ear pinna (~5.4–8.1 mm) was quantified. After i.d. engraftment of BMCMCs, KitW-sh/W-sh or KitW/W-v mice exhibited mast cells from the base to the tip of the ear pinnae, in an anatomical distribution similar to that of the native mast cell populations in the corresponding WT mice.
Immunofluoresence
For CYP27B1 immunofluorescence in BMCMCs, cells were centrifuged at 500 rpm for 5 min onto Polysine™ slides (Menzel-Glaser), fixed with 150 μL IC Fixation buffer (Fixation & Permeabilisation Kit; eBioscience) for 20 min at room temperature before washing in Permeabilization Buffer (eBioscience) for 5 min. Cells were then incubated with 3 μg/mL rabbit anti-CYP27B1 Ab (Santa Cruz Biotechnology) or 3 μg/mL rabbit polyclonal IgG isotype control Ab (Dako) for 16 h at 4°C. Slides were then rinsed three times in Permeabilization Buffer and incubated with Alexa 594-conjugated goat anti-rabbit Ab (1:200 dilution; Molecular Probes) for 1 h at room temperature in the dark. Following three additional washes in Permeabilization Buffer, cells were incubated with 1 μg/mL DAPI (Roche) for 2 min at room temperature, rinsed in Permeabilization Buffer, mounted with Fluorescence Mounting Medium (Dako) and imaged using a Nikon Spectral Imaging Confocal Microscope Digital Eclipse C1si and EZ-C1 software (version 3.20).
Preparation of human mast cells
Mature cord blood-derived mast cells (CBMCs) or peripheral blood-derived mast cells (PBMCs) were generated by first isolating CD34+ progenitor cells from human umbilical cord blood or human buffy coat provided by Australian Red Cross, respectively. Briefly, blood was diluted with sterile phosphate buffered saline (PBS) at a ratio of 1:1, layered gently over Histopaque®-1077 (1.77 g/L; Sigma-Aldrich) and after centrifugation (600 g, 30 min), the interface containing mononuclear cells was harvested and the remaining red blood cells were disrupted with haemolytic solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA 2Na). CD34+ progenitor cells were enriched by positive immunomagnetic selection using CD34 MicroBeads and an autoMACS Separator (Miltenyi Biotec) according to the manufacturer’s instructions. The isolated CD34+ cells were then transferred into 12-well plates at a density of 5 × 106 cells/mL in IMDM medium (Life Technologies) supplemented with 1% insulin-transferrin-selenium (Life Technologies), 5 × 10−5 M 2-mercaptoethanol (Life Technologies), 1% penicillin-streptomycin (Life Technologies), 0.1% bovine serum albumin (BSA; Sigma-Aldrich), 100 ng/ml recombinant human (rh) SCF, 50 ng/ml rhIL-6 and 1 ng/ml rhIL-3 (all rh cytokines from Shenandoah Biotechnology INC.) and placed in a CO2 incubator at 37°C. The cytokine-supplemented medium was replaced weekly and rhIL-3 was omitted from the medium after the first 2 wk of culture. From 6 wk, 10% FCS was added to the medium, and CBMCs or PBMCs used at 10 wk of culture. At that time, the populations contained 96% mast cells as determined by May Grünwald-Giemsa staining and by flow cytometric analysis (tryptase+; 10 μg/mL; Millipore).
Measurement of histamine and cysteinyl leukotriene
BMCMCs and CBMCs or PBMCs were pre-incubated in 10% charcoal-stripped-FCS complete medium for 72 h, supplemented with the vitamin D3 metabolites and sensitized with IgE as outlined above for mast cell activation in vitro. Following the 16 h IgE-sensitization BMCMCs or CBMCs or PBMCs (106 cells/mL) were re-suspended in Tyrodes buffer and then activated with DNP-HSA (10 ng/ml; BMCMCs) or anti-human IgE Ab (1 μg/mL; CBMCs or PBMCs) for 30 min at 37°C in the presence of 1α,25(OH)2D3 (10−8 – 10−6 M) or 25OHD3 (10−8 – 10−6 M) or EtOH (0.03%). Histamine or Cys-LT levels in supernatants and corresponding cell lysates (histamine only) were measured using histamine (Beckman Coulter) or Cys-LT EIA (Cayman Chemical) kits according to manufacturers’ instructions.
Immunoblotting
BMCMCs and CBMCs were pre-incubated in 10% charcoal-stripped-FCS complete medium for 72 h, then treated with 25OHD3 (10−8 or 10−7 M) for 3 and 8 h, and lysed in ice-cold lysis buffer (50 mM Tris-base, 100 mM NaCl, 5 mM EDTA, 67 mM Na4P2O7, 0.01% Triton X-100 and complete protease inhibitors cocktail [Roche]). Proteins were separated with SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline that contained 0.1% Tween-20; they were then probed with an rabbit anti-CYP27B1 antibody (600 ng/mL; Santa Cruz Biotechnology) or rabbit anti-β-actin antibody (1:1000 dilution; Cell Signaling) overnight at 4°C. Membranes were then probed with horseradish peroxidase–conjugated antibody against rabbit IgG (1:2000 dilution; Cell Signaling) and bands visualized using ECL reagent (Amersham) with a LAS4000 imaging system (Fujifilm).
For cells that were sensitized with IgE anti-DNP mAb (SPE-7; 2mg/ml), IgE was added to the cells at the same time as administration of 1α,25(OH)2D3 (10−7 M) or vehicle (0.03% EtOH) and incubated for 16 h at 37° C in a CO2 incubator. Cells were centrifuged 180 × g for 5 min, resuspended in Tyrode’s buffer (129 mM NaCl, 8.4 mM glucose, 10 mM HEPES, 5 mM KCl, 1 mM MgCl2, 1.4 mM CaCl2 and 1% BSA at pH 7.4), centrifuged again and then resuspended with Tyrode’s buffer at 4 × 106 cells/mL. Cells were activated with 10 ng/mL of DNP-HSA–specific antigen for 2 or 15 min at 37 °C in the presence of 1α,25(OH)2D3 (10−7 M) or EtOH (0.03%). The reaction was quenched by the addition of ice-cold buffer followed immediately by centrifugation at 180 × g for 5 min at 4 °C. Cells were lysed in ice-cold lysis buffer, electrophoretically transferred and bands visualized using the same protocol as detailed above with the exception that membranes were probed with rabbit anti-phospho-Erk1/2, anti-phospho-p-38, anti-phospho-JNK1/2 and anti-phospho-NF-κB-p65. Stripped membranes were then probed with total form of anti-Erk1/2, anti-p-38, anti-JNK1/2 and anti-NF-κB-p65 (all including phosphor-antibodies were used at 1:1000 dilution; Cell Signaling). Immunoblots presented in figures are representative of 2 or 3 similar independent experiments.
Flow cytometric analysis
BMCMCs were incubated with 1α,25(OH)2D3 (10−8 – 10−7 M) and IgE anti-DNP Ab (2 μg/ml) for 16 h before cell surface FcεRI and c-kit expression determination. BMCMCs were washed in FACS buffer (PBS with 2% FCS) and incubated with anti-mouse CD16/CD32 mAb (1 μg/mL) on ice for 15 min. After FcR blocking, BMCMCs were incubated with anti-FcεRIα-FITC (2.5 μg/mL; eBioscience) or anti-c-kit-PE (2 μg/mL) antibodies or isotype control American hamster IgG-FITC (2.5 μg/mL) and rat IgG2b-PE (2.5 μg/mL) antibody for 30 min on ice and then analysed on a Beckman Coulter Cytomics FC500 and using CXP Cytometry List Mode Data Acquisition and Analysis Software version 2.2 (Beckman Coulter). All antibodies were obtained from eBioscience. For BMCMC-CYP27B1 expression, cells were incubated with rabbit anti-CYP27B1 Ab (3 μg/mL; Santa Cruz Biotechnology) or isotype control rabbit IgG Ab (3 μg/mL; Dako) in Permeabilization Buffer (eBioscience) for 30 min on ice, then incubated with Alexa 594-conjugated goat anti-rabbit Ab (1:100 dilution; Molecular Probes) for 30 min on ice, and finally analysed by flow cytometry. For determination of CBMC-VDR expression, cells were fixed in IC fixation buffer (eBioscience) for 20 min at room temperature, incubated with rat anti-VDR antibody (10 μg/mL; Millipore) or isotype control rat IgG2a antibody (2 μg/mL; eBioscience) in Permeabilization buffer (eBioscience) for 30 min on ice. Cells were washed, incubated with goat anti-rat FITC-conjugated antibody (1:100 dilution; Life Technologies) for 30 min on ice and then analysed by flow cytometry.
RNA extraction and real-time PCR
Ear pinnae were finely sliced, sonicated in 500 μl TRIzol reagent (Life Technologies) from which RNA was extracted according to the manufacturer’s instructions. For mRNA analysis, 0.5 μg of RNA was used for complementary DNA (cDNA) synthesis using the QuantiTect reverse transcription kit (QIAGEN). Quantitative real-time PCR was performed using a 1:4 dilution of cDNA with the QuantiTect SYBR Green PCR System (QIAGEN) on a Rotor-Gene 6000 PCR machine (QIAGEN). PCR assays were performed for 45 cycles (95° C for 15 s, 55° C for 20 s, and 72 °C for 20 s). Relative expression levels of TSLP mRNA was normalised to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control using the Rotor-Gene Series 6000 Software (QIAGEN). The following oligonucleotide sequences were used: TSLP, forward 5′-AGCTTGTCTCCTGAAAATCGAG-3′, reverse 5′-AGGTTTGATTCAGGCAGATGTT-3′; LTC4S, forward 5′-ATGAAGGACGAAGTGGCTCTT-3′, reverse 5′-CCTGTAGGGAGAAGTAGGCTTG-3′; HDC, forward 5′-AGGAGCAATCCAAGGGAGAT-3′, reverse 5′-GGTATCCAGGCTGCACATTT-3′,and GAPDH forward 5′-ACATCATCCCTGCATCCACT-3′, reverse 5′-ACTTGGCAGGTTTCTCCAG-3′. CYP27B1 cDNA was primed using the Mm_Cyp27b1_1_SG QuantiTect primer assay (QIAGEN) and PCR was conducted according to the manufacturer’s instructions.
Measurement of 1a,25(OH)2D3 in BMCMCs and CBMCs
Five wk old BMCMCs (WT [VDR mouse colony] or VDR−/− or WT [CYP27B1 mouse colony] or CYP27B1−/− or 10 wk old CBMCs (2 × 106 cells/ml) were pre-incubated in 10% charcoal-stripped-FCS complete medium (DMEM or IMDM, respectively) for 72 h in a CO2 incubator at 37°C. Cells were then replenished with the charcoal-stripped-FCS DMEM (supplemented with 3 ng/mL rmIL-3 for BMCMCs) and IMDM (supplemented with 100 ng/mL rhSCF, 50 ng/mL rhIL-6 for CBMCs). For CBMCs, 2 × 106 cells/mL were incubated with 25OHD3 (10−7 or 10−6 M) or EtOH (0.03%) for 6–7 h, whereas BMCMCs (2 × 106 cells/mL) were incubated for 24 h and the supernatant replaced with new medium containing 25OHD3 (10−7 or 10−6 M) for a further 6 h incubation. Culture supernatants and cell lysates were collected and snap-frozen in liquid nitrogen. Samples were stored, shielded from light, at −80° C until analysis. Levels of 1α,25(OH)2D3 supernatants and corresponding cell lysates were measured using a radioimmunoassay kit (Immunodiagnostic Systems) according to the manufacturer’s instructions. Mast cell production of 1α,25(OH)2D3 was determined as the amount measured in the cell lysate + the supernatant and expressed as pM.
Supplementary Material
Key Messages.
Mouse and human mast cells can convert 25OHD3 to 1α,25(OH)2D3 via CYP27B1 catalytic activity.
The vitamin D3 metabolites, 25OHD3 and 1α,25(OH)2D3, repress IgE-mediated mast cell-derived proinflammatory and vasodilatory mediator release in a VDR-dependent manner in vitro.
Epicutaneously applied vitamin D3 metabolites significantly reduce the magnitude of IgE-mediated PCA reactions in vivo; a response that requires functional mast cell-VDRs and mast cell-CYP27B1 at the affected skin site.
Acknowledgments
We thank Boris Fedoric and Zhen Liu for technical help, Zelig Eshar (Weizmann Institute of Science, Israel) for providing IgE anti-DNP mAb-producing mouse SPE-7 hybridoma cells and Tim Hercus for IgE anti-DNP mAb purification.
Declaration of funding sources for the research reported in the manuscript:
This work was supported by an Australian National Health and Medical Research Council (NHMRC) Career Development Fellowship (to M.A.G.) and NHMRC project grants (to M.A.G., P.A.G., M.S.S and A.F.L.), by a grant from the Canadian Institutes of Health Research (to D. Goltzman), and by grants from the U.S. National Institutes of Health AI070813, AI023990, and CA072074 to S.J.G.
Abbreviations
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 25OHD3
25-hydroxyvitamin D3
- BMCMC
Bone marrow-derived cultured mast cell
- CBMC
Cord blood-derived mast cell
- CYP27B1
25-hydroxyvitamin D-1α-hydroxylase
- Cys-LT
Cysteinyl leukotriene
- DNP
2,4-Dinitrophenol
- EtOH
Ethanol
- EPGW
Ethanol, propylene glycol, and water
- HDC
histidine decarboxylase
- i.d
intradermal
- i.v
intravenous
- HSA
Human serum albumin
- PBMC
peripheral blood-derived mast cell
- PCA
Passive cutaneous anaphylaxis
- TSLP
Thymic stromal lymphopoietin
- UVB
Ultraviolet-B
- VDR
Vitamin D receptor
- WT
Wild-type
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242:128–43. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 4.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Sakashita M, et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat Genet. 2012;44:1222–6. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 5.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. Epub 2007 Sep 2. [DOI] [PubMed] [Google Scholar]
- 6.Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, et al. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am J Pathol. 2008;172:1638–49. doi: 10.2353/ajpath.2008.070559. Epub 2008 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med. 2010;207:455–63. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, et al. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–71. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523:95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–84. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. Epub 2008 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike JW. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol Cell Endocrinol. 2011;347:3–10. doi: 10.1016/j.mce.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129:1243–51. doi: 10.1016/j.jaci.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–35. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D deficiency in 2010: health benefits of vitamin D and sunlight: a D-bate. Nat Rev Endocrinol. 2011;7:73–5. doi: 10.1038/nrendo.2010.234. [DOI] [PubMed] [Google Scholar]
- 17.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11:584–96. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 18.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam S-Y, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baroni E, Biffi M, Benigni F, Monno A, Carlucci D, Carmeliet G, et al. VDR-dependent regulation of mast cell maturation mediated by 1,25-dihydroxyvitamin D3. J Leukoc Biol. 2007;81:250–62. doi: 10.1189/jlb.0506322. Epub 2006 Oct 11. [DOI] [PubMed] [Google Scholar]
- 20.Takeyama K, Kato S. The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3. Biosci Biotechnol Biochem. 2011;75:208–13. doi: 10.1271/bbb.100684. [DOI] [PubMed] [Google Scholar]
- 21.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama I, Minatohara K, Yokozeki H, Nishioka K. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71–6. doi: 10.1159/000237348. [DOI] [PubMed] [Google Scholar]
- 23.Menegaz D, Mizwicki MT, Barrientos-Duran A, Chen N, Henry HL, Norman AW. Vitamin D receptor (VDR) regulation of voltage-gated chloride channels by ligands preferring a VDR-alternative pocket (VDR-AP) Mol Endocrinol. 2011;25:1289–300. doi: 10.1210/me.2010-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;131:541–8. e1–9. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–74. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–83. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 28.Guo Z, Okamoto H, Imamura S. The effect of 1,25(OH)2-vitamin D3 on Langerhans cells and contact hypersensitivity in mice. Arch Dermatol Res. 1992;284:368–70. doi: 10.1007/BF00372042. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- E1.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam S-Y, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. Epub 2007 Sep 2. [DOI] [PubMed] [Google Scholar]
- E3.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA. Evidence that vitamin D3 promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med. 2010;207:455–63. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.