Abstract
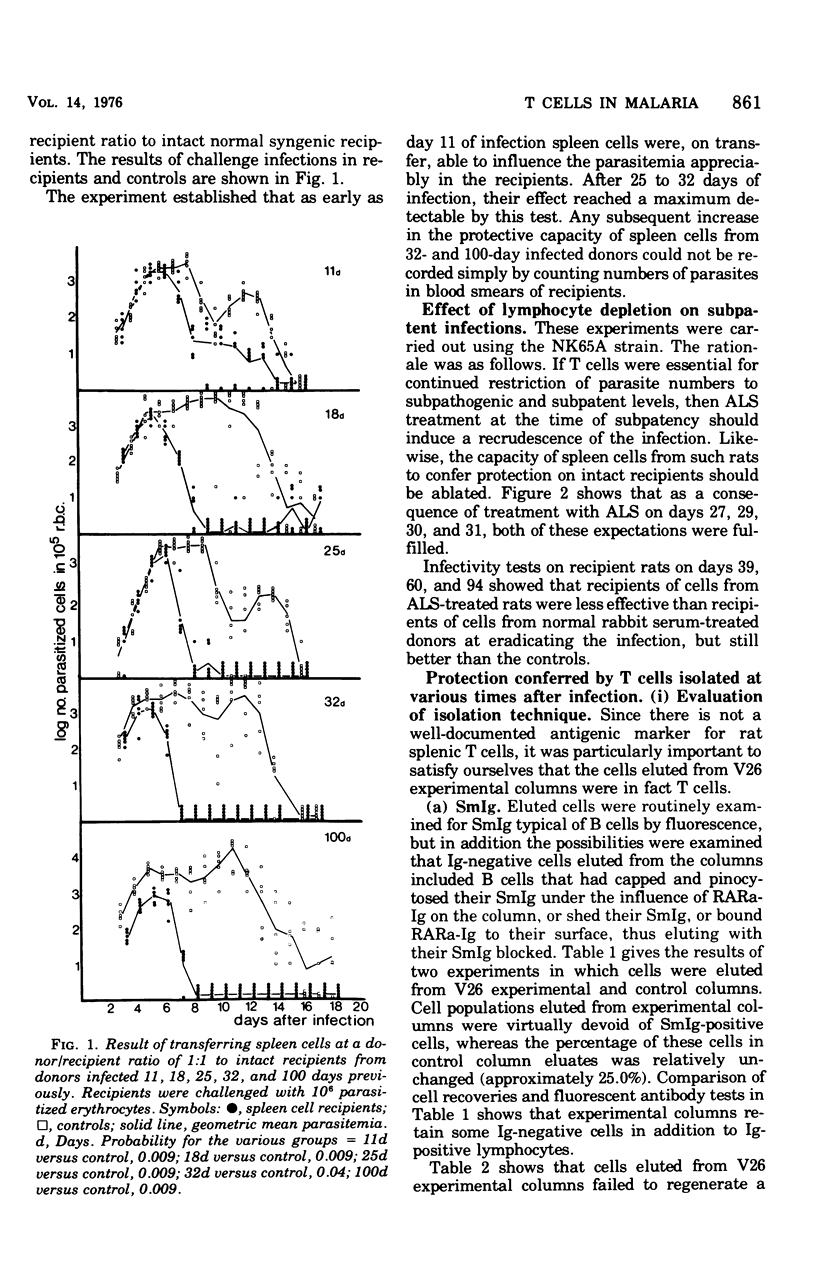
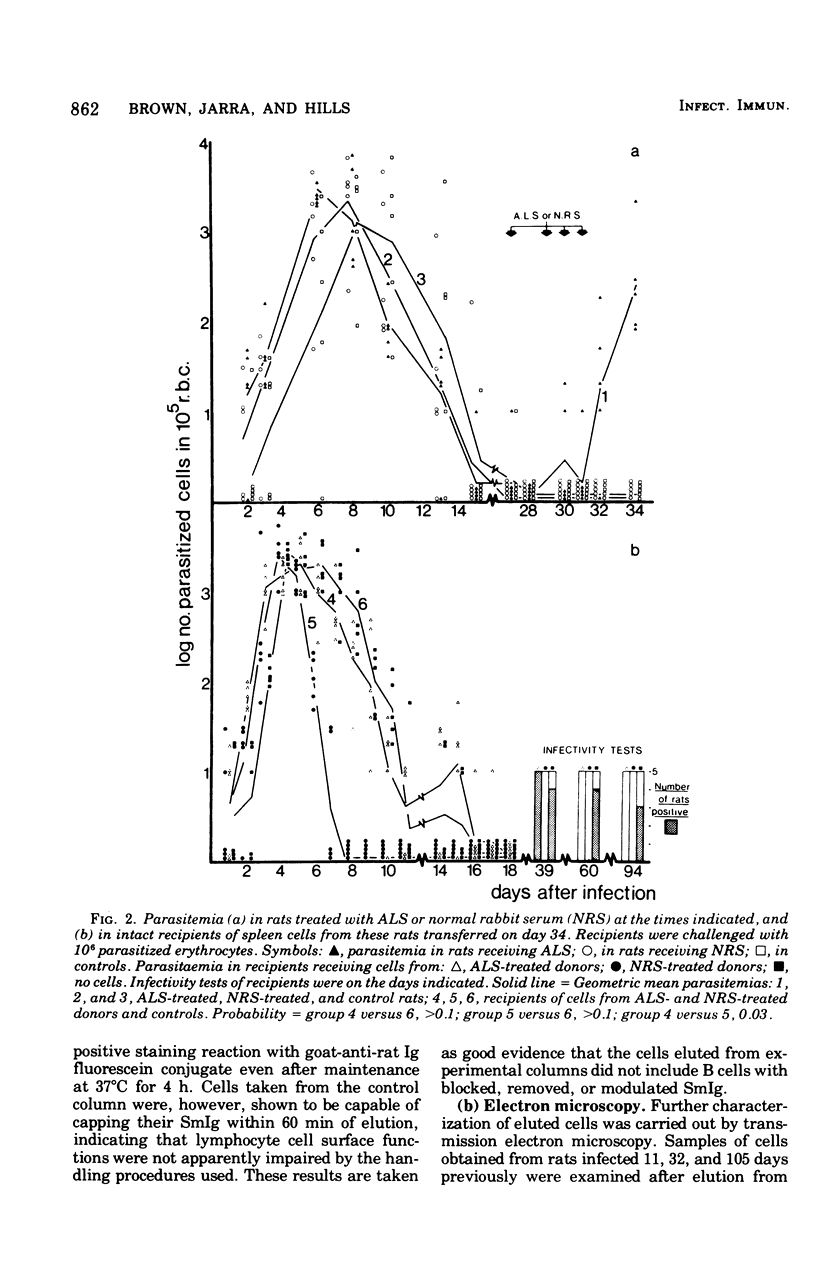
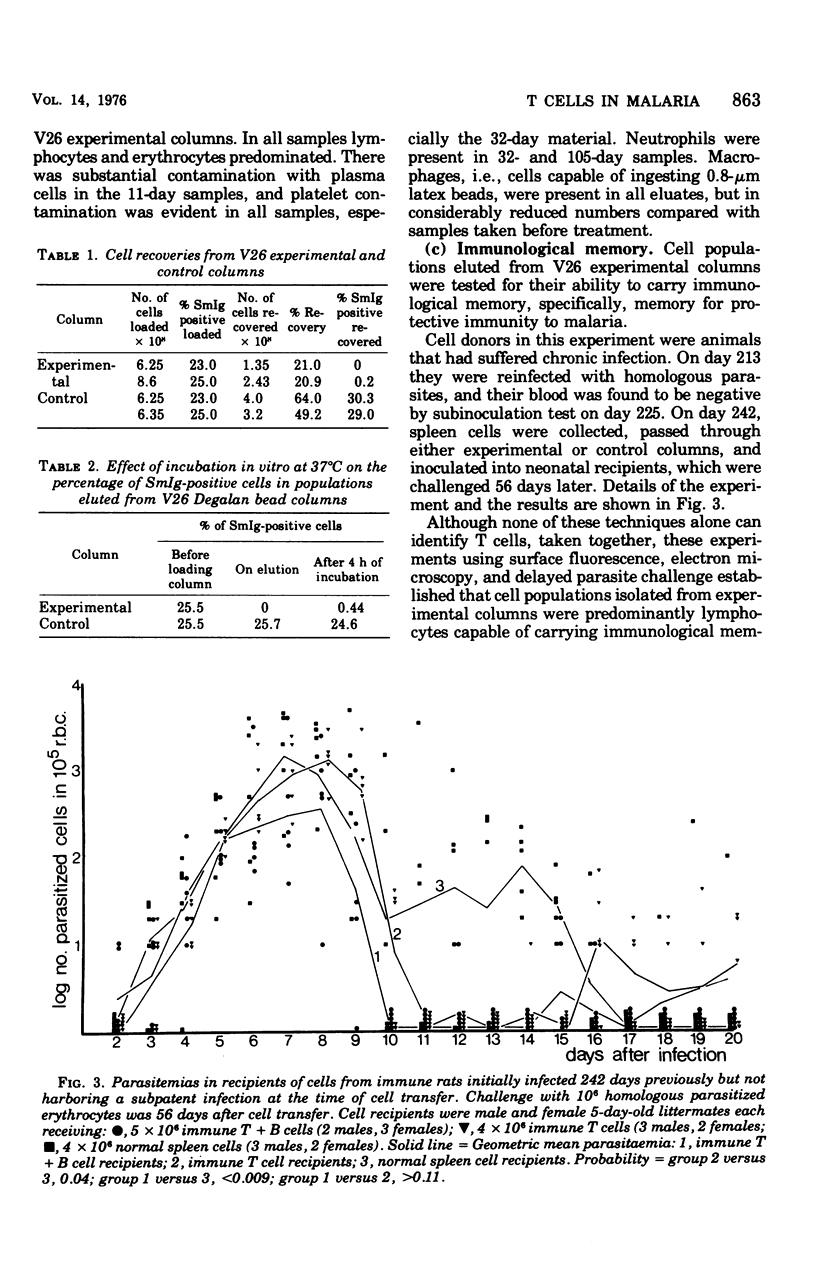
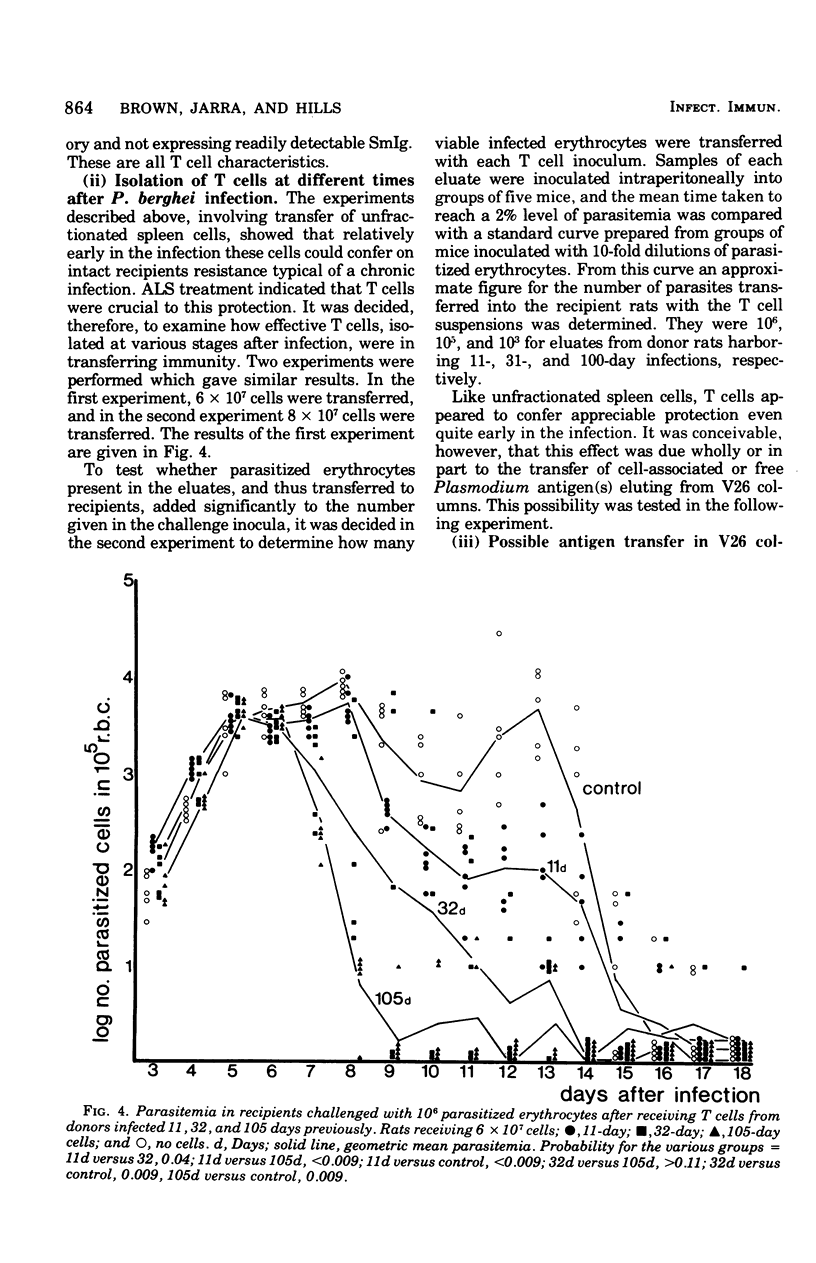
Experiments were carried out in which unfractionated spleen cells, and T lymphocyte subpopulations characterized by certain experimental criteria, were isolated at various times from rats infected with Plasmodium berghei. By adoptive transfer it was shown that unfractionated spleen cells, and T cells alone, could transfer protection to syngenic recipients as early as 11 days after infection of the cell donors. The protection conferred by T cells increased with the duration of the infection in the donors, at least up to 100 days. The additional presence of B cells in transferred lymphocyte populations enhanced their protective capacity over that shown by T cells alone. The role of T cells in protective immunity to malaria is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B. A., Marrack P. C., Kappler J. W. Functional heterogeneity among the T-derived lymphocytes of the mouse. II. Sensitivity of subpopulations to anti-thymocyte serum. J Immunol. 1975 Feb;114(2 Pt 2):747–751. [PubMed] [Google Scholar]
- Ault K. A., Unanue E. R. Events after the binding of antigen to lymphocytes: removal and regeneration of the antigen receptor. J Exp Med. 1974 May 1;139(5):1110–1124. doi: 10.1084/jem.139.5.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Abraham R., Chia E., Gamble J. A subpopulation of T cells bearing Fc receptors. J Immunol. 1975 Oct;115(4):1159–1165. [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- Brown I. N. Immunological aspects of malaria infection. Adv Immunol. 1969;11:267–349. doi: 10.1016/s0065-2776(08)60481-2. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Hills L. A. Antigenic variation and immunity to Plasmodium knowlesi: antibodies which induce antigenic variation and antibodies which destroy parasites. Trans R Soc Trop Med Hyg. 1974;68(2):139–142. doi: 10.1016/0035-9203(74)90187-4. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Serum antibody in acquired malarial immunity. Trans R Soc Trop Med Hyg. 1971;65(2):125–135. doi: 10.1016/0035-9203(71)90210-0. [DOI] [PubMed] [Google Scholar]
- Coleman R. M., Rencricca N. J., Stout J. P., Brissette W. H., Smith D. M. Splenic mediated erythrocyte cytotoxicity in malaria. Immunology. 1975 Jul;29(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Douglas T. C. Occurrence of a theta-like antigen in rats. J Exp Med. 1972 Nov 1;136(5):1054–1062. doi: 10.1084/jem.136.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Cogen R. B. Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J Exp Med. 1973 Jul 1;138(1):163–175. doi: 10.1084/jem.138.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E. A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J Exp Med. 1972 Apr 1;135(4):890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Hudson L., Thantrey N., Roitt I. M. The bursa dependence of chicken thymus-derived lymphocyte surface immunoglobulin. Immunology. 1975 Jan;28(1):151–159. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr Cellular cooperation during in vivo anti-hapten antibody responses. III. The helper cell activity of activated thymocytes, of spleen cells treated with anti-theta serum, and of spleen cells from anti-thymocyte serum-treated or adult thymectomized donors. J Immunol. 1975 Apr;114(4):1408–1414. [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Jokipii A. M., Jokipii L. Progressive increase with time after sensitization in the functional affinity of T lymphocytes. Cell Immunol. 1974 Aug;13(2):241–250. doi: 10.1016/0008-8749(74)90242-1. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Hunter P. C., Jacobs D., Lord E. Functional heterogeneity among the T-derived lymphocytes of the mouse. I. Analysis by adult thymectomy. J Immunol. 1974 Jul;113(1):27–38. [PubMed] [Google Scholar]
- Katz D. H., Osborne D. P., Jr The allogeneic effect in inbred mice. II. Establishment of the cellular interactions required for enhancement of antibody production by the graft-versus-host reaction. J Exp Med. 1972 Sep 1;136(3):455–465. doi: 10.1084/jem.136.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontiainen S., Mitchison N. A. Blocking antigen-antibody complexes on the T-lymphocyte activation during in vitro incubation before adoptive transfer. Immunology. 1975 Mar;28(3):523–533. [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. The oxidation of pyruvate in pigeon breast muscle. Biochem J. 1940 Mar;34(3):442–459. doi: 10.1042/bj0340442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Marrack P. C., Kappler J. W. Antigen-specific and nonspecific mediators of T cell/B cell cooperation. I. Evidence for their production by different T cells. J Immunol. 1975 Mar;114(3):1116–1125. [PubMed] [Google Scholar]
- Möller E. Specificity of hapten-reactive T and B mouse lymphocytes. Affinity and avidity of T- and B-cell receptors and anti-hapten antibodies as factors of dose and time after immunization. Scand J Immunol. 1974;3(3):339–355. doi: 10.1111/j.1365-3083.1974.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. Immunity to infection, allograft immunity and tumour immunity: parallels and contrasts. Transplant Rev. 1974;19(0):226–254. doi: 10.1111/j.1600-065x.1974.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Phillips R. S. Plasmodium berghei: passive transfer of immunity by antisera and cells. Exp Parasitol. 1970 Jun;27(3):479–495. doi: 10.1016/0014-4894(70)90052-4. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Wolstencroft R. A., Brown I. N., Brown K. N., Dumonde D. C. Immunity to malaria. 3. Possible occurrence of a cell-mediated immunity to Plasmodium knowlesi in chronically infected and Freund's complete adjuvant-sensitized monkeys. Exp Parasitol. 1970 Oct;28(2):339–355. doi: 10.1016/0014-4894(70)90103-7. [DOI] [PubMed] [Google Scholar]
- Rubin B., Hertel-Wulff B. Biological significance of Fc receptor-bearing cells among activated T lymphocytes. Scand J Immunol. 1975 Sep;4(5-6):451–462. doi: 10.1111/j.1365-3083.1975.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Hudson L. Specific purification of lymphocyte populations on a digestible immunoabsorbent. J Immunol. 1973 Jan;110(1):313–319. [PubMed] [Google Scholar]
- Spira D. T., Silverman P. H., Gaines C. Anti-thymocyte serum effects on Plasmodium berghei infection in rats. Immunology. 1970 Nov;19(5):759–766. [PMC free article] [PubMed] [Google Scholar]
- Stechschulte D. J. Plasmodium berghei infection in thymectomized rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):748–752. doi: 10.3181/00379727-131-33968. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes: II. Mitogen responsiveness of T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Nov 1;142(5):1041–1051. doi: 10.1084/jem.142.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Yoeli M., Most H. Studies on sporozoite-induced infections of rodent malaria. I. The pre-erythrocytic tissue stage of Plasmodium berghei. Am J Trop Med Hyg. 1965 Sep;14(5):700–714. doi: 10.4269/ajtmh.1965.14.700. [DOI] [PubMed] [Google Scholar]



