Abstract
Background
The reservoir and mode of transmission of Mycobacterium ulcerans, the causative agent of Buruli ulcer, still remain a mystery. It has been suggested that M. ulcerans persists with difficulty as a free-living organism due to its natural fragility and inability to withstand exposure to direct sunlight, and thus probably persists within a protective host environment.
Methodology/Principal Findings
We investigated the role of free-living amoebae as a reservoir of M. ulcerans by screening the bacterium in free-living amoebae (FLA) cultures isolated from environmental specimens using real-time PCR. We also followed the survival of M. ulcerans expressing green fluorescence protein (GFP) in Acanthameoba castellanii by flow cytometry and observed the infected cells using confocal and transmission electron microscopy for four weeks in vitro.
IS2404 was detected by quantitative PCR in 4.64% of FLA cultures isolated from water, biofilms, detritus and aerosols. While we could not isolate M. ulcerans, 23 other species of mycobacteria were cultivated from inside FLA and/or other phagocytic microorganisms. Laboratory experiments with GFP-expressing M. ulcerans in A. castellani trophozoites for 28 days indicated the bacteria did not replicate inside amoebae, but they could remain viable at low levels in cysts. Transmission electron microscopy of infected A. castellani confirmed the presence of bacteria within both trophozoite vacuoles and cysts. There was no correlation of BU notification rate with detection of the IS2404 in FLA (r = 0.07, n = 539, p = 0.127).
Conclusion/Significance
This study shows that FLA in the environment are positive for the M. ulcerans insertion sequence IS2404. However, the detection frequency and signal strength of IS2404 positive amoabae was low and no link with the occurrence of BU was observed. We conclude that FLA may host M. ulcerans at low levels in the environment without being directly involved in the transmission to humans.
Author Summary
Mycobacterium ulcerans, the causative agent of Buruli ulcer (BU) is an environmental pathogen known to reside in aquatic habitat. However, the reservoir and modes of transmission to humans still remain unknown. M. ulcerans can probably not live freely due to its natural fragility and inability to withstand exposure to direct sunlight. This study investigated the hypothesis that free-living amoebae (FLA) can serve as a reservoir of M. ulcerans by testing for its presence in amoebae isolated from water bodies in BU endemic and non-endemic communities and whether the pathogen can remain viable when experimentally infected in amoebae in the laboratory. We detected only one (IS2404) of the three (IS2606 and KRB) targets for the presence of M. ulcerans in amoebae cultures and found no correlation between its presence in the environment and BU notification rate. M. ulcerans remained viable at low levels in amoebae for 28 days in vitro. We therefore conclude that FLA may host M. ulcerans at low levels in the environment without being directly involved in the transmission to humans.
Introduction
Mycobacterium ulcerans is a slow growing environmental pathogen responsible for a necrotizing cutaneous infection called Buruli ulcer (BU). The disease has been reported in over 30 countries worldwide mainly in tropical and subtropical climates and emerged as an increasing cause of morbidity in endemic rural communities in some West and Central African countries with Benin, Côte d'Ivoire and Ghana bearing the highest burden of disease [1].
Most BU endemic areas are found close to slow flowing or stagnant water bodies and it is therefore assumed that the aquatic ecosystem may be a source of M. ulcerans from which the bacterium is transmitted to humans. This is supported by several studies that have detected M. ulcerans DNA sequences in a variety of environmental specimens including fish, snails, detritus, biofilms, soil, water filtrands, insects and protozoa [2]–[5]. Recently in Australia, M. ulcerans DNA has been detected in mosquitoes, faecal matter and skin lesions of small terrestrial mammals (ringtail and brushtail possums) that are thought to harbor and vector the bacterium [6], [7]. However, the main reservoir and modes of transmission of BU outside Australia still remain unknown.
Since the discovery that Legionella pneumophila is able to infect and replicate in free-living amoebae (FLA) [8], there has been an increasing number of studies on the role of FLA in the survival of pathogenic organisms [9]. Also, several species of mycobacteria (M. shottsii, M. pseudoshottsii, M. tuberculosis, M. leprae, M. ulcerans, M. marinum, M. bovis, M. avium subsp paratuberculosis and M. avium) have been shown to survive within protozoa [4], [10]–[16]. M. ulcerans bears characteristic genomic signatures that are typical of host restricted pathogens suggesting that M. ulcerans is unlikely to be free-living in the environment but is instead undergoing or has undergone adaptation to a specific ecological niche [17]. Internalization of infectious agents inside other parasites is a recurring theme in biology and represents an evolutionary strategy for survival that may sometimes enhance pathogenesis or transmissibility [18]: Bacteria “hidden” in their protozoan hosts may more easily infect vertebrate end hosts, multiplying within protozoans to escape immune reactions [15], [18].
Water bodies in areas of high BU endemicity have been reported to contain significantly more FLA than in low endemic areas [19]. Recently, we demonstrated that M. ulcerans can be phagocytosed in vitro by Acanthamoeba polyphaga and persist for at least 2 weeks [4]. This study also showed a higher detection frequency of the IS2404 target in FLA cultures as compared to crude samples from the environment. The aim of the present study was to further explore FLA as a reservoir for M. ulcerans by screening M. ulcerans in FLA from aquatic environment sampled for 10 months and relating this to the BU notification rate in the same endemic area. Furthermore, we experimentally investigated the ability of M. ulcerans to survive and replicate within A. castellanii by infecting these amoebae with M. ulcerans expressing green fluorescence protein (GFP).
Materials and Methods
Study sites and specimen collection
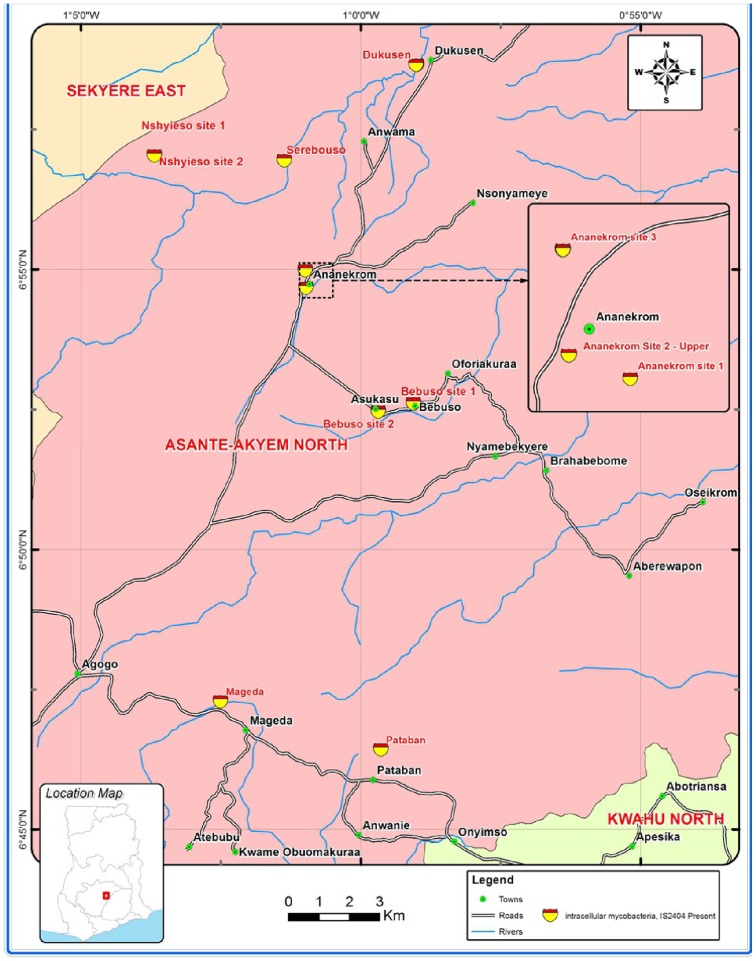
The study was carried out in five endemic communities (with recorded human BU cases): Ananekrom, Nshyieso, Serebouso, Dukusen and Bebuso, and two non-endemic communities (no recorded human BU cases): Mageda and Pataban in the Asante Akim North Municipal of Ghana (Table 1, Fig. 1). These communities are on average 18 km apart and were selected based on number of BU cases reported at the Agogo Presbyterian Hospital (APH), the Municipal health facility serving all communities (Fig. 1). Week-long monthly field visits were made for 10 months between October 2008 and July 2009 to randomly collect environmental specimens: water, biofilm from plants and tree trunks, detritus and aerosols. The specimens were taken between 6:00 am and 8:00 am, the peak period of human contact activities in the water bodies. Biofilms (n = 428) were taken by scraping surfaces of tree trunks, floating logs and tree stumps with sterile scalpels and cotton swabs into 50 mL sterile Falcon tubes. Detritus (n = 45) were scooped by hand into 50 mL sterile Falcon tubes. Water specimens (n = 53) were taken from mid column with buckets of which 3–10 liters were concentrated via 0.45 µm membrane filters (Sartorius Stedim Biotech GmbH, Germany) depending on the turbidity of the water. During the last two months of sampling, non-nutrient agar (NNA) plates (n = 13) seeded with Escherichia coli were exposed for 30 minutes for isolation of FLA from aerosols generated next to the water bodies.
Table 1. Information on sampling sites.
| Clinically diagnosed human cases positive by IS2404 PCR | |||||||
| Communities | Water body | Activities | Population size | 2008 (%) | 2009 (%) | 2010 (%) | 2011 (%) |
| Ananekrom | Egyaah River upper and lower, Betom River | Fishing, recreation, pathway for humans and animals, *domestic activities | 1951 | 28 (1.44) | 31 (1.59) | 39 (2.00) | 33 (1.69) |
| Nshyieso | Esuo-Efi River (stagnant water) | *Domestic activities | 1429 | 8 (0.56) | 10 (0.70) | 5 (0.35) | 17 (1.19) |
| Serebouso | Onwamtifi River | *Domestic activities | 1275 | 7 (0.55) | 16 (1.25) | 11 (0.86) | 6 (0.47) |
| Dukusen | Onwam River | *Domestic activities | 675 | 4 (0.59) | 2 (0.30) | 7 (1.04) | 2 (0.30) |
| Bebuso | Pupunasoe River (stagnant water) | *Domestic activities, human and animal crossing, fishing | 966 | 6 (0.62) | 4 (0.41) | 5 (0.52) | 4 (0.41) |
| Mageda | Abena Supuni River | Market traders drink from it | 773 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pataban | Pataban River | Drinking | 1421 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
*Domestic activities: bathing, washing, cooking and sometimes drinking.
Figure 1. Distribution of the IS2404 target and intracellular mycobacteria from the sampling sites.
Specimen processing
Seven milliliters phosphate buffered saline (PBS) were added to the membrane filters (cut into smaller pieces), swabs and scalpels contained in 50 mL Falcon tubes and shaken vigorously to dislodge the substrate and biofilms from the surface. For detritus, specimens were processed as described by Gryseels et al. [4].
Isolation of FLA
A piece of the membrane filter and 2 to 3 drops of the suspensions (biofilms and detritus) were inoculated at the centre of 1.5% NNA plates seeded with E. coli for cultivation of FLA [20] at 28.5°C. The inoculated NNA plates were examined daily for the presence of trophozoites and cysts using the 10× objective of a bright field microscope. When FLA were observed, they were subcultured on new NNA plates seeded with E. coli [21]. After 3 or 4 subcultures, the FLA were harvested by scraping the surface with an inoculating loop and suspending them in 1.5 mL sterile distilled water.
DNA preparation
The modified Boom method was used for the extraction of DNA from FLA cultures as described previously [22], [23].
Detection of M. ulcerans DNA
Two multiplex real-time PCR assays were performed on the DNA extracts to test the presence of three distinct sequences: IS2404, IS2606 and the ketoreductase B (KRB) domain in the M. ulcerans genome as described by Fyfe et al. [24]. All DNA extracts were first screened for the IS2404 target multiplexed with an internal positive control to check for PCR inhibitors such as humic and fulvic acids (commonly found in environmental specimens) [24]. The second PCR assay for detecting the presence of IS2606 and KRB was done on FLA cultures that turned out positive for the IS2404 target. Amplification and detection was carried out using the 7500 real-time PCR system (Applied Biosystems).
Identification of FLA
The identification of FLA of the genera Acanthamoeba, Naegleria and Vahlkampfiidae was confirmed using the primer sets JDP1/JDP2, ITSfw/ITSrv and JITSfw/JITSrv respectively as described by Gryseels et al. [4] and Eddyani et al. [19].
Isolation of M. ulcerans and other intracellular mycobacteria from specimens
Specimens were processed to kill extracellular mycobacteria as described by Gryseels et al. [4], decontaminated using the oxalic acid method [25], inoculated on LJ medium, and incubated at 30°C with weekly examination for a year. Supernatants containing extracellular mycobacteria were cultured for week at the same temperature to monitor the effectiveness of the isolation method.
Identification of intracellular mycobacteria
Direct smear examination (DSE) by ZN staining for acid fast bacilli (AFB) was performed on single colonies grown on LJ media. Cultures positive for AFB were subjected to a nested PCR specific for the mycobacterial 16S rRNA gene [26], [27] after heat-inactivation of mycobacterial suspensions in TE for 10 minutes. Amplicons were sequenced by the VIB Genetic Service Facility (Antwerp, Belgium). The sequenced data were compared with known sequences in the GenBank database and interpreted using the BlastN algorithm (available on http://www.ncbi.nlm.nih.gov/BLAST/). The 16S rRNA sequences were also matched against entries in the RIDOM (Ribosomal Differentiation of Medical Microorganisms) database (http://www.ridom-rdna.de/). Direct detection of mycobacterial DNA in FLA cultures was done using the same 16S rRNA PCR assay.
Infection of A. castellanii with M. ulcerans
M. ulcerans strains JKD8083 (which expresses GFP) and 04126204 (which does not express GFP) were grown in 7H9 broth or 7H10 agar supplemented with OADC (Difco). Real-time PCR was performed on M. ulcerans strains to estimate cell numbers as previously described [28]. Primers targeting the 16S rRNA gene were used for detection of mycobacteria. Known amounts of M. ulcerans Agy99 genomic DNA were used to construct a standard curve and cell numbers were estimated based on the predicted mass of an M. ulcerans chromosome [24].
A. castellanii was cultured in peptone-yeast extract-glucose (PYG) medium at 22°C in the dark as described by Moffat and Tompkins [29]. Trophozoites were harvested at 400× g for 10 minutes (Eppendorf, 5810R) and adjusted to a final concentration of 106 cells ml−1 in Acanthamoebae (AC) buffer as described [29].
Bacterial strains were concentrated by centrifugation at 6000× g for 15 minutes at room temperature and then resuspended in AC buffer. A preparation of 1×106 cells of A. castellanii was mixed with 1×106 cells of either M. ulcerans (JKD8083) or (04126204) in 20 mL PYG broth and incubated at 22°C for 30 minutes. Co-cultures were washed three times in AC buffer and treated with amikacin (150 µg/ml) as described [30] to kill extracellular bacteria. Trophozoites were then washed and resuspended in 50 mL AC buffer. Three milliliters samples were taken at 1, 2, 7, 14, 21 and 28 days for analysis.
Flow cytometry
At each time point, samples were washed with FACS buffer six times before a final resuspension in 500 µl of FACS buffer. FACS was carried out using uninfected amoebae to identify trophozoite populations. The subsequent infected samples were gated only on these relevant populations. All samples, including the controls (uninfected amoebae and bacteria only) were analyzed with a FACS (Becton Dickinson) equipped with a 488 nm argon laser. At each time point 50,000 events were counted. Background fluorescence was determined by using infections of A. castellanii with non-fluorescent M. ulcerans (04126204). Percentages of fluorescing amoebae were then calculated using Flowjo (v8.7).
Confocal and electron microscopy
Aliquots of infected amoebae in AC buffer were again pelleted and washed in 1× PBS before DAPI staining according to the manufacturer's instructions (Invitrogen). Samples were imaged using a LAS700 confocal microscope (Zeiss) with a 100× oil immersion lens.
At different time points 1 mL aliquots were used to examine for M. ulcerans within A. castelanii as described previously [31] using the electron microscope.
Statistical analysis
Statistical analyses were performed in SPSS 18.0 (SPSS Inc., Chicago, IL) software. Standard multiple regression was used to investigate whether the isolation frequency of FLA in communities (water bodies) was related to the waterbody-specific prevalence of the IS2404 target and mycobacterial DNA (in those FLA). Hierarchical multiple regression was also used to assess the ability of some parameters (detection of IS2404 positive FLA, detection of mycobacterial DNA in FLA, and isolation of intracellular mycobacteria) to predict BU notifications (number of reported cases/number of inhabitants/month), after controlling for the influence of time. Logistic regression was performed to assess the influence of time (months) on the detection of IS2404 in FLA, isolation of intracellular mycobacteria and frequency of isolated FLA. The relationship between the isolation of FLA and the type of specimen and the communities sampled was investigated using the Pearson product-moment and Spearman Rank Order correlation (rho) coefficient. Kruskal-Wallis and Mann-Whitney U Tests were used to compare the isolation frequency of FLA and detection frequency of IS2404 between the different types of specimen and communities. P values <0.05 were considered significant.
Results
Isolation and identification of FLA
Five hundred and thirty nine environmental specimens were collected from October 2008 to July 2009. FLA were cultured from 405 (75.10%) specimens. Confirmation using three different PCR primer sets permitted the classification into three genera of FLA from 370 (68.65%) specimens with some cultures harboring more than one genus of FLA (Acanthamoeba [n = 157], Vahlkamfiidae [n = 306] and Naegleria [n = 118]) (Table S2). FLA were isolated from all specimen types (Table 2), and showed a statistically significant difference across the specimen types, (χ2 (4, n = 539) = 14.532, p = 0.006) with aerosols recording the highest mean rank (354.50) compared to the other specimen types. A Mann-Whitney U Test also showed that FLA were more frequently isolated from aerosols than plant biofilm (p = 0.006) (Bonferroni adjustment alpha level = 0.008). FLA were isolated from all communities, the isolation frequency showed a significant difference across communities ((χ2 (6, n = 539) = 14.955, p = 0.021) with Nshyieso recording the highest mean rank (308.22). A Mann-Whitney U Test showed no significant difference in FLA isolation between Mageda and Nshyieso (p = 0.878) but showed a difference between Nshyieso and the communities Dukusen, Ananekrom and Serebouso (p = 0.000, p = 0.002, p = 0.006).
Table 2. Isolation of FLA per type of specimen and sampling site.
| Sampling sites | Aerosols (%) | Biofilm (%) | Detritus (%) | Water (%) | Total (%) |
| Ananekrom site 1 | 1/1 (100) | 41/70 (58.57) | 4/4 (100) | 9/11 (81.82) | 55/86 (63.95) |
| Ananekrom site 2-upper | 0/0 (0) | 1/2 (50.0) | 1/1 (100) | 0/0 (0) | 2/3 (66.67) |
| Ananekrom site 2-lower | 0/0 (0) | 0/2 (0) | 1/1 (100) | 0/0 (0) | 1/3 (33.33) |
| Ananekrom site 3 | 0/0 (0) | 3/4 (75) | 2/2 (100) | 0/1 (0) | 5/7 (71.43) |
| Bebuso site 1 | 1/1 (100) | 33/54 (61.11) | 2/3 (66.67) | 5/7 (71.43) | 41/65 (63.08) |
| Bebuso site 2 | 2/2 (100) | 19/24 (79.17) | 0/0 (0) | 2/2 (100) | 23/28 (82.14) |
| Dukusen | 4/4 (100) | 52/88 (59.09) | 6/8 (75) | 5/10 (50.0) | 67/110 (60.91) |
| Mageda | 0/0 (0) | 8/10 (80.0) | 5/5 (100) | 0/1 (0) | 13/16 (81.25) |
| Nshyieso site 1 | 0/0 (0) | 2/3 (66.67) | 2/2 (100) | 0/1 (0) | 4/6 (66.67) |
| Nshyieso site 2 | 1/1 (100) | 64/77 (83.12) | 4/6 (66.67) | 9/9 (100) | 78/93 (83.87) |
| Pataban | 0/0 (0) | 7/10 (70.0) | 4/5 (80.0) | 0/1 (0) | 11/16 (68.75) |
| Serebouso | 4/4 (100) | 51/84 (60.71) | 6/8 (75.0) | 9/10 (90.0) | 70/106 (66.04) |
| Total | 13/13 (100) | 281/428 (65.65) | 37/45 (82.22) | 39/53 (75.58) | 370/539 (68.65) |
Detection of M. ulcerans DNA in FLA cultures
Twenty five out of 370 FLA cultures obtained from 539 specimens (4.64%) tested positive for the IS2404 target (Table S1). CT values ranged from 29.46 to 38.05 corresponding to ≤1–10 genomes µl−1 DNA extract of M. ulcerans. All IS2404 positive FLA cultures tested negative for the IS2606 and KRB targets. The IS2404 target was detected significantly more often in Acanthamoeba and Vahlkampfiidae (χ2 (1, n = 539) = 5.532 p = 0.019, phi = 0.111 and χ2 (1, n = 539) = 4.814 p = 0.028, phi = 0.103) than in Naegleria (χ2 (1, n = 539) = 0.259, p = 0.611, phi = 0.033). None of the mycobacterial cultures isolated intracellularly from six of these specimens tested positive for IS2404. IS2404 positive FLA were detected in all endemic and non-endemic communities (Table 3, Fig. 1). There was no significant difference in detection of the IS2404 target from FLA across the specimen types (χ2 (4, n = 539) = 6.715, p = 0.152).
Table 3. Detection of IS2404 target in FLA cultures per type of specimen and sampling site.
| Sampling site | Type of specimen | Total | ||||
| Aerosols | Biofilm plant | Biofilm trunk | Detritus | Water | ||
| Ananekrom site 1 | 0/1 (0) | 2/38 (5.26) | 0/32 (0) | 0/4 (0) | 1/11 (9.09) | 3/86 (3.49) |
| Ananekrom site 2-upper | 1/1 (100) | 0/1 (0) | 0/1 (0) | 1/3 (33.33) | ||
| Ananekrom site 2-lower | 0/1 (0) | 0/1 (0) | 0/1 (0) | 0/3 (0) | ||
| Ananekrom site 3 | 0/2 (0) | 0/2 (0) | 1/2 (50) | 0/1 (0) | 1/7 (14.29) | |
| Bebuso site 1 | 0/1 (0) | 1/26 (3.85) | 2/28 (7.14) | 0/3 (0) | 0/7 (0) | 3/65 (4.62) |
| Bebuso site 2 | 0/2 (0) | 1/15 (6.67) | 0/9 (0) | 1/2 (50) | 2/28 (7.14) | |
| Dukusen | 0/4 (0) | 0/49 (0) | 1/39 (2.56) | 1/8 (12.5) | 0/10 (0) | 2/110 (1.82) |
| Mageda | 0/5 (0) | 0/5 (0) | 1/5 (20) | 0/1 (0) | 1/16 (6.25) | |
| Nshyieso site 1 | 0/3 (0) | 0/2 (0) | 0/1 (0) | 0/6 (0) | ||
| Nshyieso site 2 | 0/1 (0) | 3/38 (7.89) | 3/39 (7.69) | 0/6 (0) | 1/9 (11.11) | 7/93 (7.53) |
| Pataban | 0/5 (0) | 0/5 (0) | 1/5 (20) | 0/1 (0) | 1/16 (6.25) | |
| Serebouso | 2/4 (50) | 2/40 (5) | 0/44 (0) | 0/8 (0) | 0/10 (0) | 4/106 (3.77) |
| total | 2/13 (15.38) | 10/223 (4.48) | 6/205 (2.93) | 4/45 (8.89) | 3/53 (5.66) | 25/539 (4.64) |
Some of the IS2404 positive cultures harbored more than one genus of FLA (Acanthamoeba [n = 13 (2.4%)], Vahlkampfiidae [n = 20 (3.7%)] and Naegleria [n = 7 (1.3%)]). The amoebae had similar ITS sequences to those of Acanthamoeba sp., A. lenticulata, A. castellanii, Naegleria sp. strain WTP29, N. lovaniensis, N. philippinensis and V. avara, V. inornata, Acanthamoeba sp. T11 genotype and Acanthamoeba spp. T4 genotype as reported by Gryseels et al. [4]. Three of the positive cultures could not be identified with the primers used (Table S1).
Identification of intracellular mycobacteria
We could not cultivate M. ulcerans from the FLA, however, other mycobacteria were cultured from intracellular origins in 162 (30.06%) specimens; 109 (67.28%) among these originated from specimens from which we also isolated FLA. All isolates were confirmed by DSE for AFB and partial sequencing of the 16S rRNA gene. There was no growth of bacteria after the supernatants containing the killed extracellular mycobacteria were cultured for a week. One hundred and thirty one isolates showed >99% sequence similarity match with the available database in GenBank (NCBI and RIDOM 16S rDNA) comprising a total of 23 mycobacterial species (Table S3). Eight of the remaining sequence data were too short to be identified and 23 (14.20%) isolates had mixed growth, which made identification impossible. The most frequently isolated species were M. arupense (39.69%), M. fortuitum (7.63%) and M. lentiflavum (4.58%).
After screening the FLA cultures for the mycobacterial 16S rRNA gene, 159 (42.97%) of the 370 were positive but mycobacteria were not identified to the species level.
Evolution of study parameters during the sampling period
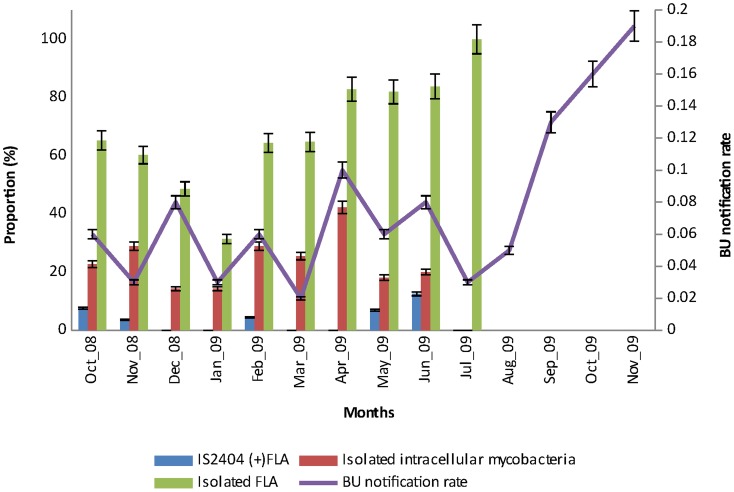
The detection of intracellular mycobacteria peaked in April 2009 followed by a peak in the detection of IS2404 positive FLA in June 2009 and the isolation of FLA in July 2009. The highest number of BU cases was however reported four months later in November 2009 after FLA isolation peaked (Figure 2).
Figure 2. Proportions of FLA isolated from specimens, IS2404 detected in FLA cultures, mycobacteria isolated from an intracellular source over the sampling period (October 2008–July 2009) and BU notification rate until four months after the sampling period.
Error bars on the left Y-axis represent 95% confidence interval (CI) of the proportions of isolated FLA (68.3±1.66), IS2404 detected in FLA cultures (3.5±0.37) and intracellular mycobacteria (21.49±0.95) sampled monthly from environmental specimens (n = 539) for the specified period. Error bars on the right Y-axis represent the 95% CI of BU notification rate of the 5 endemic communities (0.08±0.04) with a population size of 6,296.
Time accounted for a 13.80% variance in the BU notification rate (new BU cases per month) using a hierarchical multiple regression model (F (4, 534) = 26.00, p<0.001). The three other parameters, intracellular mycobacteria, detection of IS2404 target and detection of mycobacterial DNA in FLA, together explained an additional 2.5% of the variance in BU notification rate, after controlling for time, R squared change = 0.025, F change (3, 534) = 5.251, p<0.001. In the final model, only two parameters were statistically significant, with time recording a higher beta value (beta = 0.312, p<0.001) than detection of mycobacterial DNA in FLA (beta = 0.152, p<0.001). The isolation frequency of FLA varied significantly through time ((χ2 (1, N = 539) = 28.479, p<0.001) as well. There was a positive correlation of BU notification rate with detection of mycobacterial DNA in FLA (r = 0.27, n = 539, p<0.0005) but not with detection of the IS2404 target in FLA (r = 0.07, n = 539, p = 0.127).
Using a direct logistic regression model, time accounted for between 5.1% (Cox and Snell R square) and 7.2% (Nagelkerke R squared) of the variance in FLA isolation but could not predict the variances in the detection of IS2404 in FLA ((χ2 (1, N = 539) = 2.034, p = 0.154) and isolation of intracellular mycobacteria ((χ2 (1, N = 539) = 0.132, p = 0.717).
M. ulcerans persists in amoebae for up to 28 days
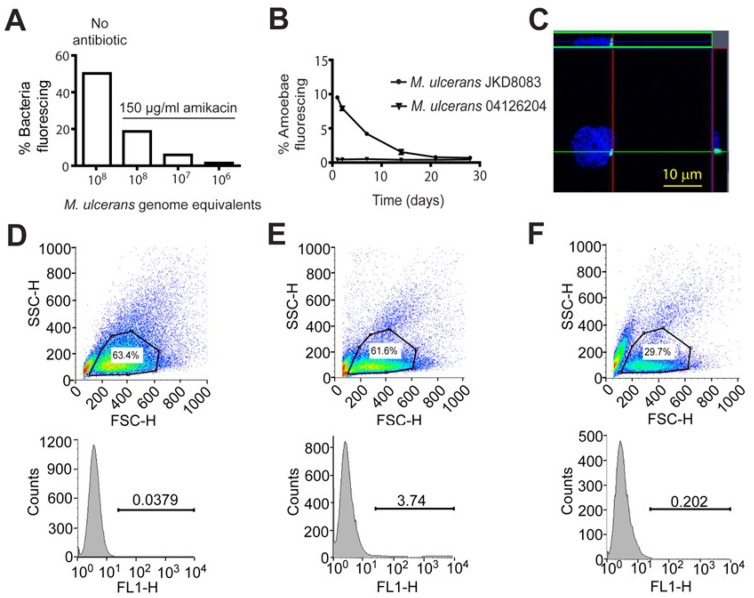
M. ulcerans infections of A. castellanii were performed over a four week period and quantified using flow cytometry. Methods involving the removal of extracellular bacteria using amikacin have been reported previously [4], [30] and were independently tested here (Figure 3A). M. ulcerans JKD8083 bacteria alone (106–108) were treated with amikacin for 7 days to test the effect of the antibiotic on extracellular bacteria. Treatment with 150 µg/ml amikacin for 7 days left 18.7% of 108 extracellular bacteria (5.92% of 107, 1.53% of 106) fluorescing above background (Figure 3A).
Figure 3. (A) Percentage of fluorescing bacteria present following growth at room temperature for 7 days in AC buffer supplemented with 150 µg/ml amikacin.
(B) Summary of flow cytometry data of A. castelanii infected with fluorescing (JKD8083) and non-fluorescing (04126204) M. ulcerans for 28 days. The mean percentage and SD of 3 biological repeats of fluorescent trophozoites at a MOI of 1 are shown. (C) Confocal microscopy demonstrating the intracellular location of M. ulcerans 3 hours post-infection in a DAPI stained trophozoite. Scale bar indicates 10 µm. (D–F) Representative FACS plots indicating forward scatter (x-axis) and side scatter (y-axis) following 7 days of co-incubation of amoebae alone (D), M. ulcerans JKD8083 (E) and M. ulcerans 04126204 (F). Lower panels show the gated trophozoites and FL1 fluorescence vs counts. Numbers refer to percentage of gated trophozoites fluorescing.
Using flow cytometry, 50,000 events were counted at each time point. Amoebae were experimentally infected with M. ulcerans by placing them in co-culture at a multiplicity of infection of 1 for 30 min, and killing remaining extracellular mycobacteria with amikacin. At day 0, 9.51% of the amoebae were infected with M. ulcerans, but this percentage gradually decreased until at day 28 (end of experiment) only 0.7% of amoebae were infected with M. ulcerans (Figure 3B). Figure 3 (D–F) shows gated trophozoites following 7 days co-incubation of amoebae alone (D), M. ulcerans JKD8083 (E) and M. ulcerans 04126204 (F). Amoebae infected with non-fluorescing bacteria however, fluoresced at levels less than 0.05% over the 28-day time course (Figure 3B), therefore we neglected this background fluorescence.
An intracellular location of M. ulcerans within A. castellanii
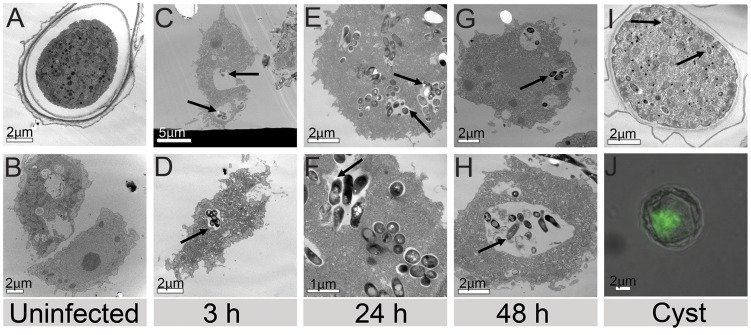
Localisation of the bacteria with respect to the amoebae was determined by both fluorescent confocal microscopy and electron microscopy (Figure 3C; Figure 4). Confocal optical sections demonstrate a mycobacterium within A. castellanii three hours post infection (Figure 3C). Video S1. shows fluorescing bacteria in A. castelanii at 24 h post infection. Examination of 1 mL aliquots of M. ulcerans-infected trophozoites and cysts by transmission electron microscopy demonstrated an intravacuolar location for M. ulcerans at 3, 24 and 48 hours post infection (Figure 4, Panels C–I). Subsequent microscopy on an extended time series reveals the presence of intracellular bacteria within cysts at 22 days post infection (Figure 4I–J).
Figure 4. Transmission electron microscopy showing uninfected (A) cysts and (B) trophozoites as well as the intravacuolar location of M. ulcerans (arrows) within infected, intact trophozoites (C, D) 3 h, (E, F) 24 h, (G, H) 48 h and (I) cysts 3 weeks post infection.
(J) is a composite image showing a cyst 22 days post infection by fluorescence microscopy.
Discussion
It has been suggested that M. ulcerans persists with difficulty in the environment as a free-living organism due to its natural fragility [32] and may be maintained in a commensal or parasitic relationship with hosts that protect the bacilli against potentially unfavorable environments. This hypothesis is supported by the observation that M. ulcerans, unlike its close environmental relatives, has degraded genome, with many pseudogenes, such as a mutation in the crt locus. This locus harbors genes responsible for the production of light-inducible carotenoids that affects its ability to withstand exposure to direct sunlight and hence diminishes its capacity to live freely [17].
A previous study by our group detected the IS2404 target twice as often in FLA cultures as in environmental specimens [4]. Extending this study, we investigated the role of FLA and other phagocytic microorganisms as a reservoir of M. ulcerans in the aquatic environment for 10 months and tested the survival of M. ulcerans within A. castellanii in vitro.
Acanthamoeba sp. and Vahlkampfiidae sp. were isolated more frequently than Naegleria from the sampled specimens. FLA were isolated more frequently from aerosols and detritus than from biofilms. Acanthamoeba has been implicated in a number of diseases including granulomatous amoebic encephalitis [33], a cerebral abscess [34] and chronic keratitis [35]. We also isolated potentially pathogenic V. avara, N. canariensis, N. philippinensis and A. lenticulata.
In this study, we detected the M. ulcerans IS2404 target in 4.64% of FLA cultures, significantly more often in Acanthamoeba sp. and Vahlkampfiidae sp. as has been reported by Gryseels et al. [4]. Our inability to detect the targets IS2606 and KR-B in IS2404 positive FLA was not surprising since most of the CT values of the IS2404 target recorded were indicative of low mycobacterial DNA concentrations as observed in other studies carried out in the same sampling sites [4], [36].
The importance of protozoa harboring human-pathogenic bacteria has recently been given much attention, especially in the case of fragile bacteria whose environmental phase would be difficult without the protection of a protozoan host [37]. Moreover, phagocytic protozoans such as FLA strongly resemble vertebrate macrophages; and it has been shown that infection success and internal proliferation is enhanced when bacteria such as Legionella and M. avium had previously resided inside protozoans [15], [38]. A number of intracellular Mycobacterium sp. were isolated from unknown hosts in the specimens, which previously have been shown to live/survive intracellularly in amoebae: M. simiae, M. fortuitum, M. septicum, M. peregrinum, M. terrae, M. gordonae, M. intracellulare and M. lentiflavum [16], [39]. The most frequently isolated species were: M. arupense, M. fortuitum and M. lentiflavum are potentially pathogenic species. These species were isolated from the environment [40]–[42] and M. arupense was isolated from wild African rodents [43]. Intracellular mycobacteria were more frequently isolated from specimens from which we also isolated FLA that may indicate their role as a reservoir for these mycobacteria. Thomas et al. [37] also reported a significant association between the presence of amoebae and the presence of mycobacteria. FLA have the additional advantage that they can form cysts, which allow them to persist through harsh periods and be dispersed via the air. It has been suggested that some infections can be acquired by inhaling aerosols containing FLA cells filled with bacteria [44], for example in the case of Legionella [45]. The detection of the IS2404 target in two of the thirteen aerosolized FLA suggests that they may act as vehicles for these mycobacteria. The aerosol transmission hypothesis of M. ulcerans was first postulated by Hayman [46], but received little attention, due to the unlikelihood of M. ulcerans being airborne as a free-living organism. The possibility that IS2404 positive mycobacteria including M. ulcerans are carried by aerosolized protozoan cysts changes this perspective. More research is, however, needed to explore this transmission route further, and in a subsequent study we investigated the presence of M. ulcerans on the skin of healthy inhabitants in the same endemic communities (manuscript in preparation).
BU notification rates varied significantly through time with the highest number of cases recorded in November 2009. BU prevalence has increased during the last quarter of the year in this locality as well as in some endemic regions of Ghana (data not shown); in this case there was no community awareness during this period. BU notification rates correlated positively with detection frequency of mycobacterial DNA in FLA cultures but not with detection of IS2404 in FLA cultures, suggesting that detection of IS2404 in FLA cannot predict concurrent BU incidence. However, the time series of this data set was not long enough to test for a potential lagging phase between peaks of IS2404 detection in FLA and BU incidence.
Similar to our previous study [4], over the time course of experimental infection of A. castellanii with M. ulcerans, M. ulcerans is present within amoebae for up to 28 days albeit at low levels. In addition, both electron microscopy and standard fluorescence microscopy revealed the presence of intracellular bacteria within cysts at 22 days post infection (Figure 4I–J). This is not unexpected due to the previously demonstrated presence and survival of a variety of environmental mycobacteria in cysts [39].
The persistence of strong GFP fluorescence of M. ulcerans within A. castellanii throughout the experiment indicated that the mycolactone polyketide synthases genes are abundantly expressed intracellularly, as GFP gene expression is under the control of the mlsA1 promoter [26]. These data also suggest that mycolactone may be produced by the bacteria within the vacuole. While this study was not designed to test the effect of M. ulcerans on A. castellanii, our observations of fluorescing M. ulcerans persisting through 28 days within intact A. castellanii suggest that A. castellanii is not adversely affected by mycolactone or the presence of the bacteria as was also shown for A. polyphaga [4].
The ability of M. marinum to persist within amoebae is widely documented [39], [47], [48]. Following the initial time points a decrease in M. ulcerans-infected amoebae as reported previously for M. ulcerans, M. shottsii and M. pseudoshottsii [4], [10] was seen which suggests that M. ulcerans does not replicate within amoebae and is not as well adapted as M. marinum to resist initial amoebic digestion, but is perhaps able to persist once within the vacuolar compartment by preventing lysosomal maturation of the vacuole by as yet undetermined mechanisms.
This study showed the occurrence of the IS2404 marker in FLA, especially in the genera Acanthamoeba and Vahlkampfiidae. After co-culturing amoebae and M. ulcerans the pathogen persisted at low levels suggesting that it probably only transiently occupies FLA and that it is unlikely that protozoa are a long-term reservoir for this pathogen. While the data we present here confirm that FLA can host mycobacteria that harbor the IS2404 marker (including M. ulcerans), the lack of predictive power of detection of IS2404 positive FLA in predicting BU notifications suggests FLA are not directly involved in transmission of M. ulcerans to humans. We suggest future work should focus on reservoirs that act as M. ulcerans amplifiers that link protozoans with humans.
Supporting Information
Real-time PCR CT values of IS2404 target in FLA cultures.
(DOCX)
Identification of FLA per sample and sampling site.
(DOCX)
Identification of intracellular mycobacteria per sample and sampling site.
(DOCX)
Fluorescing bacteria in A. castelanii at 24 h post infection.
(ZIP)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Flemish Interuniversity Council – University Development Cooperation (VLIR-UOS) and by the Stop Buruli Initiative funded by the UBS Optimus Foundation (Zurich, Switzerland). SG was an FWO PhD fellow (1.1.671.10.N.00) during part of this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Portaels F, Silva MT, Meyers WM (2009) Buruli ulcer. Clin Dermatol 27: 291–305. [DOI] [PubMed] [Google Scholar]
- 2. Marion E, Deshayes C, Chauty A, Cassisa V, Tchibozo S, et al. (2011) Detection of Mycobacterium ulcerans DNA in water bugs collected outside the aquatic environment in Benin. Med Trop (Mars) 71: 169–172. [PubMed] [Google Scholar]
- 3. Williamson HR, Benbow ME, Campbell LP, Johnson CR, Sopoh G, et al. (2012) Detection of mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl Trop Dis 6: e1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gryseels S, Amissah D, Durnez L, Vandelannoote K, Leirs H, et al. (2012) Amoebae as Potential Environmental Hosts for Mycobacterium ulcerans and Other Mycobacteria, but Doubtful Actors in Buruli Ulcer Epidemiology. PLoS Negl Trop Dis 6: e1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merritt RW, Walker ED, Small PLC, Wallace JR, Johnson PDR, et al. (2010) Ecology and transmission of buruli ulcer disease: a systematic review. PLoS Negl Trop Dis 4: e911 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3001905&tool=pmcentrez&rendertype=abstract. Accessed 4 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fyfe JAM, Lavender CJ, Handasyde KA, Legione AR, OBrien CR, et al. (2010) A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis 4: e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson PDR, Azuolas J, Lavender CJ, Wishart E, Stinear TP, et al. (2007) Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis 13: 1653–1660 10.3201/eid1311.061369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowbotham TJ (1980) Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol 33: 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landers P, Kerr KG, Rowbotham TJ, Tipper JL, Keig PM, et al. (2000) Survival and growth of Burkholderia cepacia within the free-living amoeba Acanthamoeba polyphaga. Eur J Clin Microbiol Infect Dis 19: 121–123 10.1007/s100960050442 [DOI] [PubMed] [Google Scholar]
- 10. Gupta T, Fine-Coulson K, Karls R, Gauthier D, Quinn F (2013) Internalization of Mycobacterium shottsii and Mycobacterium pseudoshottsii by Acanthamoeba polyphaga. Can J Microbiol 59: 570–576 Available: http://www.ncbi.nlm.nih.gov/pubmed/23899000. [DOI] [PubMed] [Google Scholar]
- 11. Mba Medie F, Ben Salah I, Henrissat B, Raoult D, Drancourt M (2011) Mycobacterium tuberculosis complex mycobacteria as amoeba-resistant organisms. PLoS One 6: e20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas V, McDonnell G (2007) Relationship between mycobacteria and amoebae: ecological and epidemiological concerns. Lett Appl Microbiol 45: 349–357 Available: http://www.ncbi.nlm.nih.gov/pubmed/17897376. Accessed 4 May 2014. [DOI] [PubMed] [Google Scholar]
- 13. Brown MRW, Barker J (1999) Unexplored reservoirs of pathogenic bacteria: Protozoa and biofilms. Trends Microbiol 7: 46–50. [DOI] [PubMed] [Google Scholar]
- 14. Steinert M, Birkness K, White E, Fields B, Quinn F (1998) Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol 64: 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cirillo JD, Falkow S, Tompkins LS, Bermudez LE (1997) Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun 65: 3759–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishna-Prasad BN, Gupta SK (1978) Preliminary report on engulfment and retention of mycobacteria by trophozoites of axenically grown Acanthamoeba castellanii Douglas. Curr Sci 47: 245–247. [Google Scholar]
- 17. Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, et al. (2007) Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17: 192–200 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1781351&tool=pmcentrez&rendertype=abstract. Accessed 4 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greub G, Raoult D (2004) Microorganisms resistant to free-living amoebae. Clin Microbiol Rev 17: 413–433 10.1128/CMR.17.2.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eddyani M, De Jonckheere JF, Durnez L, Suykerbuyk P, Leirs H, et al. (2008) Occurrence of free-living amoebae in communities of low and high endemicity for Buruli ulcer in southern Benin. Appl Environ Microbiol 74: 6547–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page F (1976) An illustrated key to freshwater and soil amoebae. Ambleside, England: Titus Wilson & Son, Ltd. [Google Scholar]
- 21.Ash LR, Orihel T (1987) Parasites: a guide to laboratory procedures and identification. Ed. Chicago: American Society of Clinical Pathologists. [Google Scholar]
- 22. Durnez L, Stragier P, Roebben K, Ablordey A, Leirs H, et al. (2009) A comparison of DNA extraction procedures for the detection of Mycobacterium ulcerans, the causative agent of Buruli ulcer, in clinical and environmental specimens. J Microbiol Methods 76: 152–158 10.1016/j.mimet.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 23. Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, et al. (1990) Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fyfe JAM, Lavender CJ, Johnson PDR, Globan M, Sievers A, et al. (2007) Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl Environ Microbiol 73: 4733–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Portaels F, De Muynck A, Sylla MP (1988) Selective isolation of mycobacteria from soil: a statistical analysis approach. J Gen Microbiol 134: 849–855 10.1099/00221287-134-3-849 [DOI] [PubMed] [Google Scholar]
- 26. Kirschner P, Springer B, Vogel U, Meier A, Wrede A, et al. (1993) Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol 31: 2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Böddinghaus B, Rogall T, Flohr T, Blöcker H, Böttger EC (1990) Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol 28: 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobias NJ, Seemann T, Pidot SJ, Porter JL, Marsollier L, et al. (2009) Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies of Mycobacterium ulcerans and buruli ulcer. PLoS Negl Trop Dis 3: e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moffat JF, Tompkins LS (1992) A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun 60: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bermudez LE, Young LS (1994) Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun 62: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abd H, Johansson T, Golovliov I, Sandström G, Forsman M (2003) Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl Environ Microbiol 69: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Portaels F, Chemlal K, Elsen P, Johnson PD, Hayman JA, et al. (2001) Mycobacterium ulcerans in wild animals. Rev Sci Tech 20: 252–264 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11288515. [DOI] [PubMed] [Google Scholar]
- 33. Visvesvara GS, Mirra SS, Brandt FH, Moss DM, Mathews HM, et al. (1983) Isolation of two strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J Clin Microbiol 18: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harwood CR, Rich GE, McAleer R, Cherian G (1988) Isolation of Acanthamoeba from a cerebral abscess. Med J Aust 148: 47–49. [DOI] [PubMed] [Google Scholar]
- 35. Auran JD, Starr MB, Jakobiec FA (1987) Acanthamoeba Keratitis. Cornea 6: 2–26 Available: http://journals.lww.com/corneajrnl/Fulltext/1987/06010/Acanthamoeba_Keratitis.2.aspx. [PubMed] [Google Scholar]
- 36. Vandelannoote K, Durnez L, Amissah D, Gryseels S, Dodoo A, et al. (2010) Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS Microbiol Lett 304: 191–194 10.1111/j.1574-6968.2010.01902.x [DOI] [PubMed] [Google Scholar]
- 37. Thomas V, Herrera-Rimann K, Blanc DS, Greub G (2006) Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72: 2428–2438 10.1128/AEM.72.4.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cirillo JD, Falkow S, Tompkins LS (1994) Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun 62: 3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adékambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M (2006) Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl Environ Microbiol 72: 5974–5981 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1563627&tool=pmcentrez&rendertype=abstract. Accessed 4 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu R, Yu Z, Zhang H, Yang M, Shi B, et al. (2012) Diversity of bacteria and mycobacteria in biofilms of two urban drinking water distribution systems. Can J Microbiol 58: 261–270 10.1139/w11-129 [DOI] [PubMed] [Google Scholar]
- 41. Thomson R, Tolson C, Carter R, Coulter C, Huygens F, et al. (2013) Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol 51: 3006–3011 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3754680&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castillo-Rodal AI, Mazari-Hiriart M, Lloret-Sánchez LT, Sachman-Ruiz B, Vinuesa P, et al. (2012) Potentially pathogenic nontuberculous mycobacteria found in aquatic systems. Analysis from a reclaimed water and water distribution system in Mexico City. Eur J Clin Microbiol Infect Dis 31: 683–694 Available: http://www.ncbi.nlm.nih.gov/pubmed/21805195. [DOI] [PubMed] [Google Scholar]
- 43. Durnez L, Eddyani M, Mgode GF, Katakweba A, Katholi CR, et al. (2008) First detection of mycobacteria in African rodents and insectivores, using stratified pool screening. Appl Environ Microbiol 74: 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angenent LT, Kelley ST, St Amand A, Pace NR, Hernandez MT (2005) Molecular identification of potential pathogens in water and air of a hospital therapy pool. Proc Natl Acad Sci U S A 102: 4860–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abu Kwaik Y, Gao LY, Stone BJ, Venkataraman C, Harb OS (1998) Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol 64: 3127–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayman J (1991) Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol 20: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 47. Kennedy GM, Morisaki JH, DiGiuseppe Champion PA (2012) Conserved Mechanisms of Mycobacterium marinum Pathogenesis within the Environmental Amoeba Acanthamoeba castellanii. Appl Environ Microbiol 78: 2049–2052 10.1128/AEM.06965-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Solomon JM, Leung GS, Isberg RR (2003) Intracellular replication of Mycobacterium marinum within Dictyostelium discoideum: efficient replication in the absence of host coronin. Infect Immun 71: 3578–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time PCR CT values of IS2404 target in FLA cultures.
(DOCX)
Identification of FLA per sample and sampling site.
(DOCX)
Identification of intracellular mycobacteria per sample and sampling site.
(DOCX)
Fluorescing bacteria in A. castelanii at 24 h post infection.
(ZIP)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.