Abstract
Background
p16INK4a is a tumor suppressor protein which is induced in cells upon the interaction of high-risk HPV E7 with the retinoblastoma protein by a positive feedback loop, but cannot exert its suppressing effect. Previous reports suggested that p16INK4a immunostaining allows precise identification of even small CIN or cervical cancer lesions in biopsies. The prognostic value of overexpressed p16INK4a in cervical cancer has been evaluated for several years while the results remain controversial. We performed a systematic review and meta-analysis of studies assessing the clinical and prognostic significance of overexpression of p16INK4a in cervical cancer.
Methods
Identification and review of publications assessing clinical or prognostic significance of p16INK4a overexpression in cervical cancer until March 1, 2014. A meta-analysis was performed to clarify the association between p16INK4a overexpression and clinical outcomes.
Results
A total of 15 publications met the criteria and comprised 1633 cases. Analysis of these data showed that p16INK4a overexpression was not significantly associated with tumor TNM staging (I+II vs. III+IV) (OR = 0.75, 95% confidence interval [CI]: 0.35–1.63, P = 0.47), the tumor grade (G1+ G2 vs. G3) (OR = 0.78, 95% CI: 0.39–1.57, P = 0.49), the tumor size (<4 vs. ≥4 cm) (OR = 1.10, 95% CI: 0.45–2.69, P = 0.83), or vascular invasion (OR = 1.20, 95% CI: 0.69–2.08, P = 0.52). However, in the identified studies, overexpression of p16INK4a was highly correlated with no lymph node metastasis (OR = 0.51, 95% CI: 0.28–0.95, P = 0.04), increased overall survival (relative risk [RR]: 0.42, 95% CI: 0.24–0.72, P = 0.002) and increased disease free survival (RR: 0.60, 95% CI: 0.44–0.82, P = 0.001).
Conclusions
This meta-analysis shows overexpression of p16INK4a in cervical cancer is connected with increased overall and disease free survival and thus marks a better prognosis.
Introduction
Cervical cancer is the third most common malignancy in women worldwide and a leading cause of cancer-related death in women in developing countries [1]. The relationship between the development of cervical cancer and persistent infection with high-risk human papilloma virus (HR-HPV) is well established [2]. A plethora of research on the development of objective biomarkers allowing to distinguish the transformation from productive HPV infections to carcinoma and to predict disease severity has been performed. The cellular tumor suppressor protein cyclin-dependent kinase inhibitor 2A (p16INK4a) has been identified as a biomarker for transforming HPV infections [3]. It decelerates the cell cycle by inactivating the cyclin-dependent kinases (CDK4/CDK6) involved in the phosphorylation of the retinoblastoma protein (pRb) [4]. This process is leading to senescence in normal cells. In the presence of the HR-HPV oncogene E7, p16INK4a transcription is induced by the histone demethylase KDM6B [5]. HPV E7 expression causes an acute dependence on KDM6B expression for cervical cancer cell survival. Thus, the p16INK4A tumor suppressor is a critical KDM6B downstream transcriptional target and its expression is critical for cell survival [6].
Over the past decade, several studies have evaluated the prognostic value of p16INK4a protein expression in cervical cancer with conflicting results. Some concluded that p16INK4a expression had no influence on survival [7] while others reported that p16INK4a expression was predictive of improved survival outcome for cervical carcinoma [8], [9]. In order to evaluate this question, we conducted a systematic review and meta-analysis to determine the association between the overexpression of p16INK4a and common clinical and pathologic features of cervical cancer.
Materials and Methods
Search strategy
A comprehensive literature search of the electronic databases PubMed (www.pubmed.com), EMBASE (www.embase.com) and Wanfang (www.wanfangdata.com.cn) was performed up to March 1, 2014. The following search terms and their combinations were used: “cervical cancer,” “cervical carcinoma,” “carcinoma of cervix”, “CDKN2A”, “p16” and “p16INK4a”. The citation lists associated with all the studies retrieved in the search were used to identify other potentially relevant publications. Review articles were also scanned to find additional eligible studies but none could be identified from the reference lists. The title and abstract of each study identified in the search was scanned to exclude any clearly irrelevant publications. The remaining articles were browsed to determine whether they contained information on the topic of interest.
Selection criteria
Diagnosis of cervical cancer was proven by histopathological methods. Studies of p16INK4a expression based on cervical cancer tissue (after either surgical excision or biopsy sampling) were included. Studies based on serum or any other kinds of specimen were excluded. All studies on the correlation of p16INK4a overexpression with clinicopathological markers and the association of p16INK4a overexpression on overall survival (OS) or disease-free survival (DFS) of cervical cancer patients were included. For inclusion into the analysis, there was no limitation on the minimum number of patients of every single study. When there were multiple articles by the same group based on similar patients and using same detection methods, only the largest or the most recent article was included.
Data extraction
Information was carefully extracted from all eligible publications independently by two of the authors according to the inclusion criteria listed above. Disagreement was resolved by a consensus discussion between the two authors. Data tables were composed to extract all relevant data from texts, tables, and figures of each included study, including author, publication year, country of patient’s origin, tumor stage, number of patients, research technique used, and cutoff value of overexpression of p16INK4a. In case the prognosis was only plotted as Kaplan-Meier curve in some articles, the software GetData Graph Digitizer 2.24 (free software downloaded at http://getdata- graph-digitizer.com) was applied to digitize and extract the data.
Statistical analysis
The cut-off for p16INK4a –positivity according to stained cells is given in table 1 for every study included. ORs with 95% confidence intervals (CI) were used to evaluate the association between p16INK4a overexpression and clinicopathological factors, including tumor TMN staging, tumor grade, tumor size, vascular invasion and lymph node status. To stratify for the analysis, the following data of p16INK4a overexpression and clinicopathological factors were combined into single categories with comparable clinicopathologic relevance: tumor TMN staging (I+II vs. III+IV); tumor grade (G1+G2 vs. G3); tumor size (<4 vs. ≥4 cm); vascular invasion or not; lymph node negative or positive.
Table 1. Main characteristics and results of the eligible studies.
| Study | Patient’s country | Year | Tumor stage(UICC) | Technique | Percentage of p16INK4a positive cells | Number of patients | Cut off (IHC) |
| Li et al. | China | 2000 | ND | IHC | 54% | 72 | ND |
| Alfsen et al. | Norway | 2003 | I-II | IHC | 73% | 138 | >50% |
| Van de Putte et al. | Norway | 2003 | ND | IHC | 43% | 212 | >50% |
| Han et al. | China | 2004 | I–IV | IHC | 42% | 43 | >50% |
| Masoudi et al. | Canada | 2006 | ND | IHC | 83% | 130 | ND |
| Huang et al. | China | 2007 | I–IV | IHC | 65% | 89 | >1% |
| Cao et al. | China | 2008 | I-III | IHC | 24% | 41 | >25% |
| Bodner et al. | Austria | 2011 | I–IV | IHC | 56% | 39 | >1% |
| Huang et al. | Taiwan | 2011 | I–IV | IHC | 74% | 145 | staining scores>2 |
| Schwarz et al. | USA | 2011 | I–IV | IHC | 94% | 126 | >1% |
| Mu et al. | China | 2012 | I-III | IHC | 63% | 275 | ND |
| Jiang et al. | China | 2012 | I-II | IHC | 79% | 76 | >5% |
| Weng et al. | China | 2012 | I–IV | IHC | 61% | 62 | >10% |
| Son et al. | Korea | 2012 | ND | IHC | 74% | 35 | >5% |
| Liu et al. | China | 2012 | I–IV | IHC | 83% | 150 | >5% |
IHC; immunohistochemistry; ND: not documented.
The RR with 95% CI was used for assessing the association of p16INK4a and the survival outcome combined over studies. An observed OR or RR <1 implied a better prognosis for the group with p16INK4a overexpression and would be considered to be statistically significant if the 95% CI did not exceed 1. The existence of heterogeneity between studies was evaluated using the Dersimonian and Laird’s Q test [10]. I 2 was used to quantify heterogeneity and an I 2 value >50% was considered to represent substantial heterogeneity between studies. Relative to fixed-effects models, random-effects models were used and were more appropriate for the current study, because of the heterogeneity visible from the forest plots which often cannot be revealed by the Q test given its low power. The influence of individual studies on the summary effect estimate was displayed using the sensitivity analysis. In addition, funnel plots and the Egger’s test were used to estimate the possible publication bias [11]. Cochrane Review Manager, version 5.2 (Cochrane Library, Oxford, UK) was used to calculate the ORs or RRs and their variations from each investigation.
Results
Description of studies
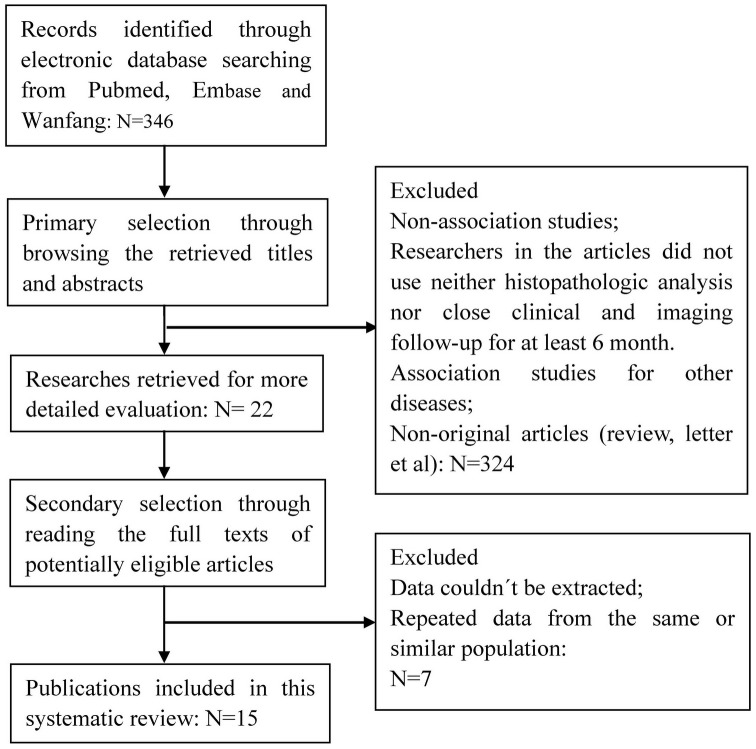
A total of 15 publications met the criteria for this analysis [7]–[9], [12]–[23] (Fig. 1). The total number of patients was 1633, ranging from 35 to 275 patients per study. Main characteristics of the eligible studies are summarized in Table 1 including the cut-off definition for p16 INK4a positivity. Thirteen articles dealt with clinicopathological factors. Eight studies determined overall survival (OS) or disease free survival (DFS). Seven studies only reported the association between p16INK4a overexpression and clinicopathological factors without OS or DFS analysis. There was only one method used to evaluate p16INK4a expression in cervical cancer specimens i.e. immunohistochemistry (IHC).
Figure 1. Literature search strategy and selection of articles.
A total of 346 articles were selected for the meta-analysis by browsing the databases PubMed, Embase and Wanfang, of which 324 were excluded after reviewing the title and abstract, seven articles were excluded after reviewing the full publications, the reasons for exclusion were: (a) Non-association studies (b) researchers in the article did not use neither histopathologic analysis nor close clinical and imaging follow-up for at least 6 months, (c) association studies for other diseases (d), non-original articles, (e) data couldn’t be extracted or (f) repeated data from the same or similar population. Finally, a total of 15 studies with 1633 patients, who fulfilled all of the inclusion criteria, were considered for the analysis.
Correlation of p16INK4a expression with clinicopathological parameters
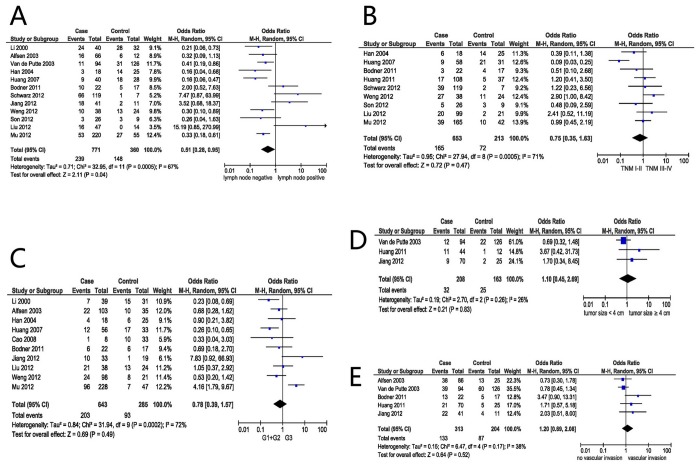
The association between p16INK4a and several clinicopathological parameters is illustrated in Figure 2. Overexpression of p16INK4a was highly correlated with no lymph node metastasis (OR = 0.51, 95% CI: 0.28–0.95, P = 0.04) (Fig. 2A). However, overexpression of p16INK4a was not significantly associated with tumor TNM staging (I+II vs. III+IV) (OR = 0.75, 95% CI: 0.35–1.63, P = 0.47) (Fig. 2B), the tumor grade (G1+ G2 vs. G3) (OR = 0.78, 95% CI: 0.39–1.57, P = 0.49)(Fig. 2C), the tumor size (<4 vs. ≥4 cm) (OR = 1.10, 95% CI: 0.45–2.69, P = 0.83) (Fig. 2D), or vascular invasion (OR = 1.20, 95% CI: 0.69–2.08, P = 0.52) (Fig. 2E).
Figure 2. Forest plot depiction of p16INK4a expression and OR for clinical pathologic features.
Clinicopathological parameters investigated are lymph node status (A), TMN classification (B), tumor grade (C), size of tumor (D), vascular invasion (E). OR with corresponding confidence intervals are shown.
p16INK4a expression and 5-year survival outcome
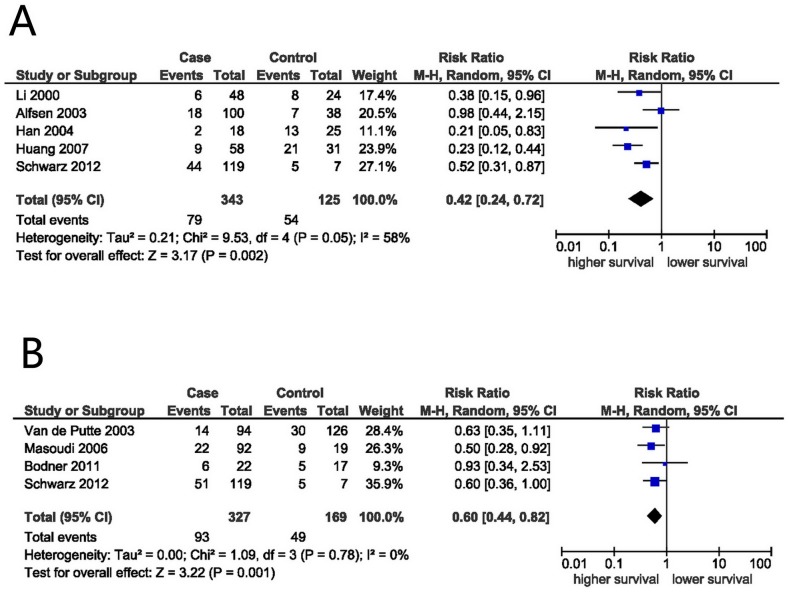
Using the methods described above, the OS and/or DFS of 849 patients in 8 studies were analyzed. The main results of this meta-analysis are shown in Figure 2. Five-year OS rate was extracted from 5 studies. The meta-analysis of the 5 studies for the prognostic value of p16INK4a overexpression showed that p16INK4a overexpression was associated with a favorable OS. This was obtained from the DerSimonian–Laird random-effects model with a value of 0.42 (95% CI: 0.24–0.72, P = 0.002) (Fig. 3), although there was heterogeneity between studies (I2 = 58%, Ph = 0.05). In fact, 4 out of 5 studies have also concluded p16INK4a expression as a favorable prognostic factor in cervical cancer patients (Fig. 3A).
Figure 3. Analysis of p16INK4a expression and survival of cervical cancer patients.
Forest plot of RR for OS (A) and DFS (B) among included studies. Combined RR was calculated by a random mode.
p16INK4a expression and DFS in cervical cancer
Meta-analysis of 4 applicable studies showed that p16INK4a expression was associated with favorable DFS (RR: 0.60, 95% CI: 0.44–0.82, P = 0.001; Fig. 3B). No heterogeneity was observed between these studies (I2 = 0%, Ph = 0.78).
Sensitivity analysis
In order to test for a bias introduced by the low numbers of available eligible publications we performed a sensitivity analysis. For this a single study involved in the meta-analysis was omitted for each round of analysis to investigate the influence of the individual data set of the particular study to the pooled ORs. We found that the corresponding pooled ORs were not essentially altered by substraction of any study (data not shown), indicating that our results were statistically robust.
Publication Bias
Begg’s funnel and Egger’s test were performed to assess the publication bias in this meta-analysis. The shape of the Funnel plots did not reveal obvious evidence of asymmetry. Egger’s test showed no significant publication bias for tumor grade (P = 0.18), TMN classification (P = 0.835), tumor size (P = 0.851), lymph node status (P = 0.051), vascular invasion (P = 0.142), DFS (P = 0.602) and OS (P = 0.624) (Table 2).
Table 2. Egger’s test of funnel plot asymmetry.
| clinicopathological parameters | t value | df | P value |
| tumor grade | 0.59 | 11 | 0.18 |
| TMN classification | 0.28 | 10 | 0.835 |
| tumor size | 0.11 | 5 | 0.851 |
| lymph node metastasis | 1.73 | 16 | 0.051 |
| vascular invasion | 3.68 | 4 | 0.142 |
| disease free survival | 0.67 | 2 | 0.602 |
| overall survival | 0.29 | 4 | 0.624 |
df: deflection.
Discussion
The p16INK4a protein is a cyclin-dependent kinase inhibitor that regulates the G1/S cell cycle checkpoint by inactivating cyclin D1-CDK4/6 complex activity and thereby enhancing pRb activity and suppressing cell growth [24]. It was shown recently that HPV-transformed cervical cancer cells are dependent on its expression and knock down will lead to reduced proliferation [6]. Many IHC-studies have demonstrated that p16INK4a protein is highly overexpressed in dysplastic epithelial cells of the uterine cervix and that it is associated with HR-HPV infection. At the same time its expression is basal in normal epithelium and benign lesions due to few spontaneous senescent cells [25]. Furthermore, overexpression of p16INK4a appears to correlate with the degree of cervical neoplasia, which may improve the histological diagnosis and hence the management of cervical lesions [26]. Tsoumpou et al [27] performed a meta-analysis of 61 published studies on the correlation of the p16INK4a immunostaining to the degree of cytological or histological abnormality. They reported that the proportion of cervical smears overexpressing p16INK4a increased with the severity of cytological abnormality. In histology only 2% of normal biopsies and 38% of CIN1 showed diffuse staining for p16INK4a compared to 68% of CIN2 and 82% of CIN3 [27]. Thus, p16INK4a is regarded a surrogate biomarker for cervical intraepithelial neoplasia.
Recently, the clinical significance of p16INK4a overexpression in cervical cancer has been reported by many investigators. However, the results of these reports are still conflicting. To address the predictive value of p16INK4a overexpression in cervical cancer, we performed a meta-analysis of the published studies to obtain a more precise estimation of the above stated association. This meta-analysis summarizes all currently available and relevant data on the impact of p16INK4a overexpression on the prognosis of cervical cancer including 1633 cases. Altogether our results using the pooled RR of OS indicate that a low p16INK4a expression indicates a poorer prognosis for patients diagnosed with cervical cancer than p16INK4a overexpression. Our findings were consistent with the theory that HR-HPV is a triggering factor in the development of cervical cancer, but would concomitantly induce a p16INK4a -mediated protection mechanism [28]. The reason is not clear at present but presumably overexpression of p16INK4a can be recognized by the immune system as an antigen of naturally low expressed protein. This p16INK4a protein can eventually initiate an antitumor response in cervical cancer patients [29].
However, the lack of standardized cut-off points for positive expression could have led to underestimation of the true prognostic significance of p16INK4a overexpression. No clear guidelines are available regarding their use in routine practice [30]. Tsoumpou et al [27] reviewed 40 publications where 35 used Klaes’ scoring system. The percentage of p16INK4a positivity of high grade squamous intraepithelial lesion varied between 44% and 92%. Thus, a predictive evaluation of the scoring systems to reach a consensus on the threshold of positivity is needed.
Strong p16INK4a expression is a proven useful surrogate marker for tumors with transcriptionally active HR-HPV, which is known to be associated with less genetically altered and less complex tumors that respond better to therapy and have improved outcomes. The positivity for p16INK4a has been proposed as a prognostic marker for a more favorable outcome in head and neck squamous cell carcinoma and in lung cancer [31], [32]. Some authors have suggested that p16INK4a plays a major role not only in suppression of cell division but also in suppression of lymphangiogenesis and lymphatic metastasis [30].
Our analysis is supported by the clinical observations of Riou et al [33] who reported that HPV-negative cervical cancer patients had a significantly higher risk of overall relapse and a higher risk of distant metastatic tumor than HPV-positive patients. Tumors that lack HR-HPV positivity may have a larger number of mutations in genes coding for cell cycle regulating proteins to be transformed, and thus may be more therapy resistant [34]. This is corroborated by the fact that HR-HPV positive carcinoma generally is more susceptible to radiochemotherapy than HPV-negative counterparts [35].
This meta-analysis has certain limitations that should be considered when interpreting the results. First, our results are based on unadjusted estimates. A more precise analysis could be conducted using the original individual data sets that, however, are not available to us. Nevertheless, such an approach would allow for adjustment by other co-variates including age, ethnicity, family history, environmental factors, treatment and lifestyle [36]. Second, heterogeneity is a potential problem when interpreting the results of meta-analyses. We minimized the likelihood of this problem by performing a careful search for published studies using explicit criteria for study inclusion, precise data extraction, and strict data analysis. However, significant heterogeneity between studies existed in some comparisons. The presence of heterogeneity results from differences in many factors, including the age distribution, lifestyle factors and the standard of the IHC technique used particularly concerning the positivity threshold. Third, only published full text studies were included in this meta-analysis. Non-significant or negative findings may not be published or only published as abstract at conferences and could therefore not be evaluated. We included data of 1633 patients currently available in this meta-analysis that should lay the foundation to perform a larger and prospective study.
In conclusion, despite the limitations of this meta-analysis, our study suggests that p16INK4A overexpression is significantly associated with better prognosis in terms of longer DFS and OS in patients with cervical cancer. Hopefully this analysis will stimulate further research with rigid criteria and large study populations to resolve any remaining controversy of the role of p16INK4A expression for the prognosis of patients with cervical cancer.
Supporting Information
PRISMA checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The data of this meta-analysis are freely and publicly available in literature repositories which are listed in the references.
Funding Statement
The authors have no support or funding to report.
References
- 1. Arbyn M, Castellsagué X, De Sanjose S, Bruni L, Saraiya M, et al. (2011) Worldwide burden of cervical cancer in 2008. Annals of oncology 22: 2675–2686. [DOI] [PubMed] [Google Scholar]
- 2. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology 189: 12–19. [DOI] [PubMed] [Google Scholar]
- 3. Roelens J, Reuschenbach M, von Knebel Doeberitz M, Wentzensen N, Bergeron C, et al. (2012) p16INK4a immunocytochemistry versus human papillomavirus testing for triage of women with minor cytologic abnormalities. Cancer cytopathology 120: 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wentzensen N, von Knebel Doeberitz M (2007) Biomarkers in cervical cancer screening. Disease markers 23: 315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLaughlin-Drubin ME, Crum CP, Münger K (2011) Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proceedings of the National Academy of Sciences 108: 2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLaughlin-Drubin ME, Park D, Munger K (2013) Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proceedings of the National Academy of Sciences 110: 16175–16180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alfsen GC, Reed W, Sandstad B, Kristensen GB, Abeler VM (2003) The prognostic impact of cyclin dependent kinase inhibitors p21WAF1, p27Kip1, and p16INK4/MTS1 in adenocarcinomas of the uterine cervix. Cancer 98: 1880–1889. [DOI] [PubMed] [Google Scholar]
- 8. Schwarz JK, Lewis Jr JS, Pfeifer J, Huettner P, Grigsby P (2012) Prognostic significance of p16 expression in advanced cervical cancer treated with definitive radiotherapy. International Journal of Radiation Oncology* Biology* Physics 84: 153–157. [DOI] [PubMed] [Google Scholar]
- 9. Anschau F, Schmitt VM, Lambert APF, Gonçalves MAG, Machado DC (2009) Transition of cervical carcinoma in situ to invasive cancer: Role of p16INK4a expression in progression and in recurrence. Experimental and molecular pathology 86: 46–50. [DOI] [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 11. Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X (2000) The prognostic value of P16 expression in cervical cancer. Central China Medical Journal 24: 85–86. [Google Scholar]
- 13. de Putte GV, Holm R, Lie AK, Tropé CG, Kristensen GB (2003) Expression of p27, p21, and p16 protein in early squamous cervical cancer and its relation to prognosis. Gynecologic oncology 89: 140–147. [DOI] [PubMed] [Google Scholar]
- 14. Dai SZ, Li XR, Wang N, Luo B (2004) The prognostic impact of P16 and HPV16/18E7 in cervical cancer. Medical Journal of Qilu 2004 19: 283–86. [Google Scholar]
- 15. Han CR, Li ZQ, Yu JB (2004) The expression and clincal significance of P16 and P15 in cervical carcinoma. Journal of Mudanjiang medical college 25: 1–5. [Google Scholar]
- 16. Masoudi H, Van Niekerk D, Gilks C, Cheang M, Bilek K, et al. (2006) Loss of p16 INK4 expression in invasive squamous cell carcinoma of the uterine cervix is an adverse prognostic marker. Histopathology 49: 542–545. [DOI] [PubMed] [Google Scholar]
- 17. Huang L-W, Lee C-C (2012) P16INK4A overexpression predicts lymph node metastasis in cervical carcinomas. Journal of clinical pathology 65: 117–121. [DOI] [PubMed] [Google Scholar]
- 18. Bodner K, Laubichler P, Kimberger O, Czerwenka K, Zeillinger R, et al. (2011) Expression of p16 protein and epidermal growth factor receptor in patients with adenocarcinoma of the uterine cervix: an immunohistochemical analysis. Archives of gynecology and obstetrics 283: 611–616. [DOI] [PubMed] [Google Scholar]
- 19. Jiang T, Li LY, Pan M, Zeng SY, Qiao ZQ, Wu WY (2005) The prognostic impact of P16 in cervical cancer. The practical journal of cancer 20: 581–83. [Google Scholar]
- 20. Weng M-y, Li L, Feng S-y, Hong S-j (2012) Expression of Bmi-1, P16, and CD44v6 in Uterine Cervical Carcinoma and Its Clinical Significance. Cancer Biology & Medicine 9: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Son S-M, Noh K-I, Lee H-C, Park Y-J, Jeong E-H, et al. (2012) Evaluation of p16INk4a, pRb, p53 and Ki-67 expression in cervical squamous neoplasia. cell cycle 5: 6. [Google Scholar]
- 22. Liu W, Wang YK, Yang HY, Sun SZ (2012) The prognostic impact of NDRG1 and P16 in cervical cancer. Medical Journal of Qilu 27(4): 309–310. [Google Scholar]
- 23. Huang Y, Tang Y, Yang H. Expression of p16INK4a in cervical cancer (2007) Hainan medicine. 18(7): 44–45. [Google Scholar]
- 24. Koh J, Enders GH, Dynlacht BD (1995) Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature 375: 506–10. [DOI] [PubMed] [Google Scholar]
- 25. Wang J-L, Zheng B-Y, Li X-D, Nokelainen K, Ångström T, et al. (2004) p16INK4A and p14ARF expression pattern by immunohistochemistry in human papillomavirus-related cervical neoplasia. Modern pathology 18: 629–637. [DOI] [PubMed] [Google Scholar]
- 26. Horn L-C, Reichert A, Oster A, Arndal SF, Trunk MJ, et al. (2008) Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. The American journal of surgical pathology 32: 502–512. [DOI] [PubMed] [Google Scholar]
- 27. Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, et al. (2009) p16INK4a immunostaining in cytological and histological specimens from the uterine cervix: A systematic review and meta-analysis. Cancer treatment reviews 35: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nature Reviews Cancer 2: 342–350. [DOI] [PubMed] [Google Scholar]
- 29.Miriam Reuschenbach (2014) Targeting p16 INK4a by therapeutic vaccination in patients with HPV-associated cancers – results of a phase I study.in Onkologie-Symposium in Lübeck.
- 30. Walts AE, Bose S (2009) p16, Ki-67, and BD ProEx C immunostaining: a practical approach for diagnosis of cervical intraepithelial neoplasia. Human pathology 40: 957–964. [DOI] [PubMed] [Google Scholar]
- 31. Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. Journal of Clinical Oncology 24: 5630–5636. [DOI] [PubMed] [Google Scholar]
- 32. Tong J, Sun X, Cheng H, Zhao D, Ma J, et al. (2011) Expression of p16 in non-small cell lung cancer and its prognostic significance: a meta-analysis of published literatures. Lung Cancer 74: 155–163. [DOI] [PubMed] [Google Scholar]
- 33. Riou G, Bourhis J, Favre M, Orth G, Jeannel D, et al. (1990) Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. The Lancet 335: 1171–1174. [DOI] [PubMed] [Google Scholar]
- 34. Crook T, Vousden K (1992) Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. The EMBO journal 11: 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, et al. (2008) Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute 100: 261–269. [DOI] [PubMed] [Google Scholar]
- 36. Koopman L, van der Heijden GJ, Hoes AW, Grobbee DE, Rovers MM (2008) Empirical comparison of subgroup effects in conventional and individual patient data meta-analyses. International journal of technology assessment in health care 24: 358–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The data of this meta-analysis are freely and publicly available in literature repositories which are listed in the references.