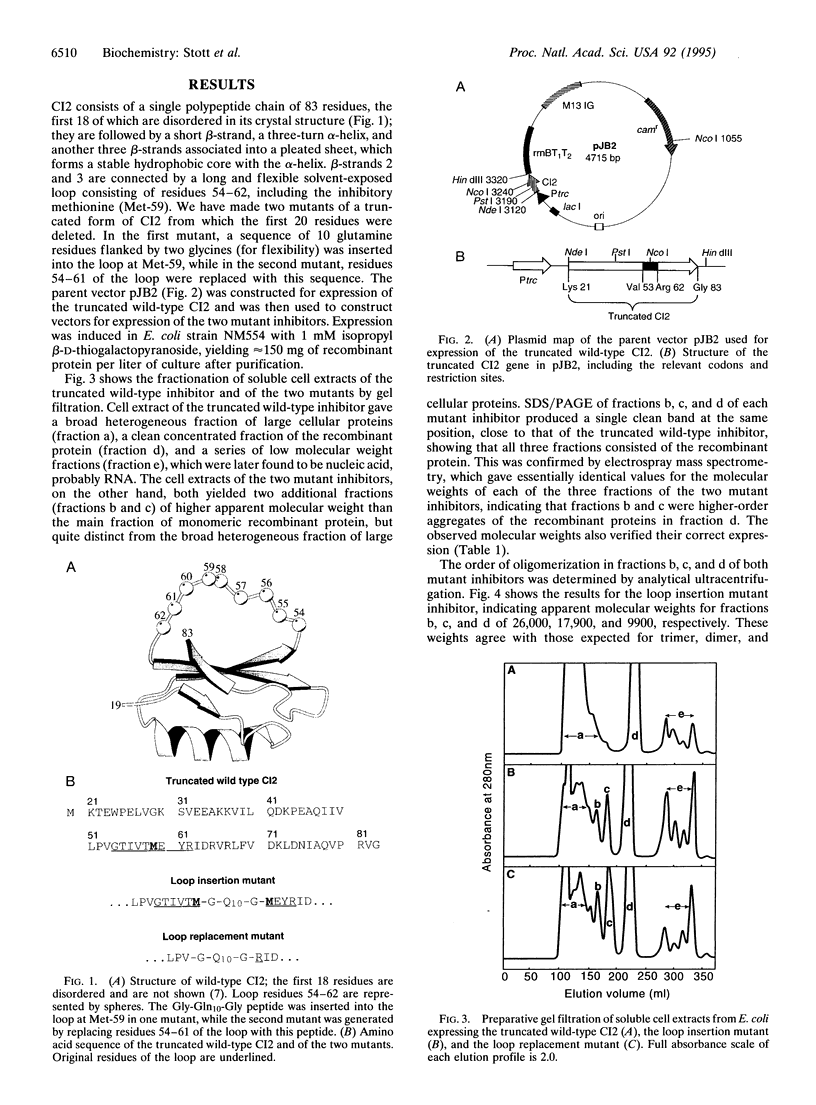
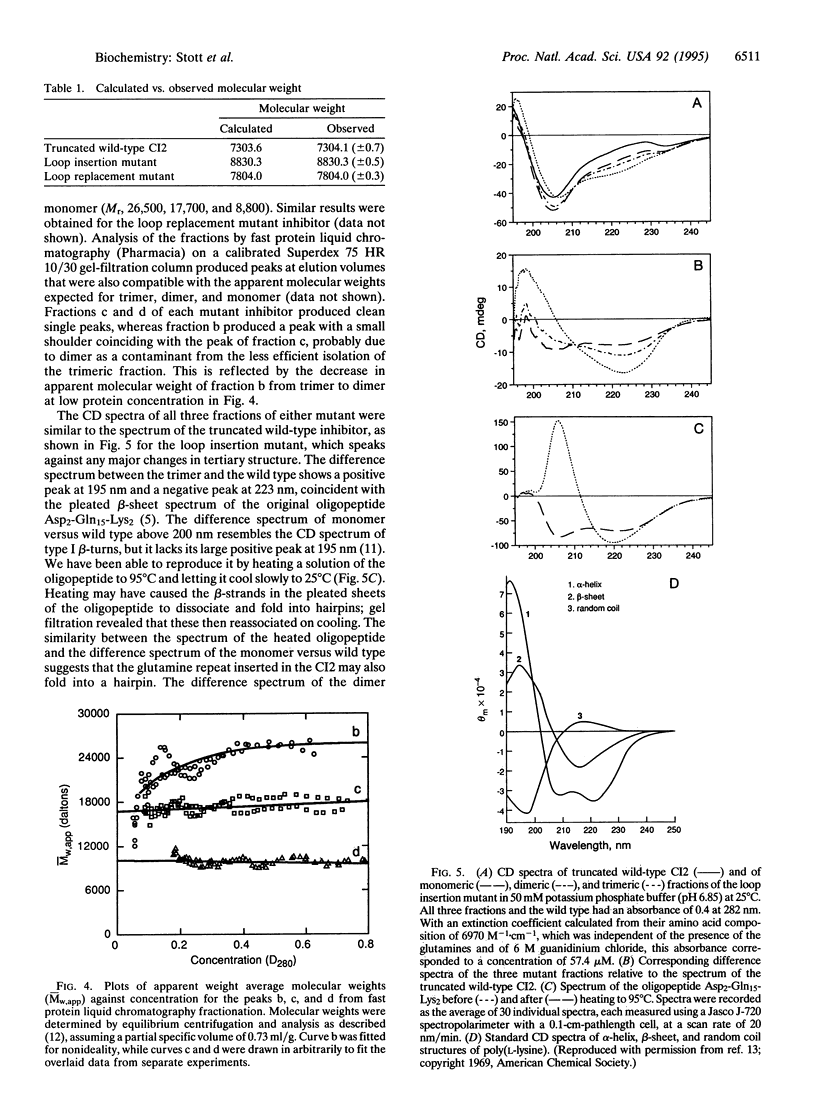
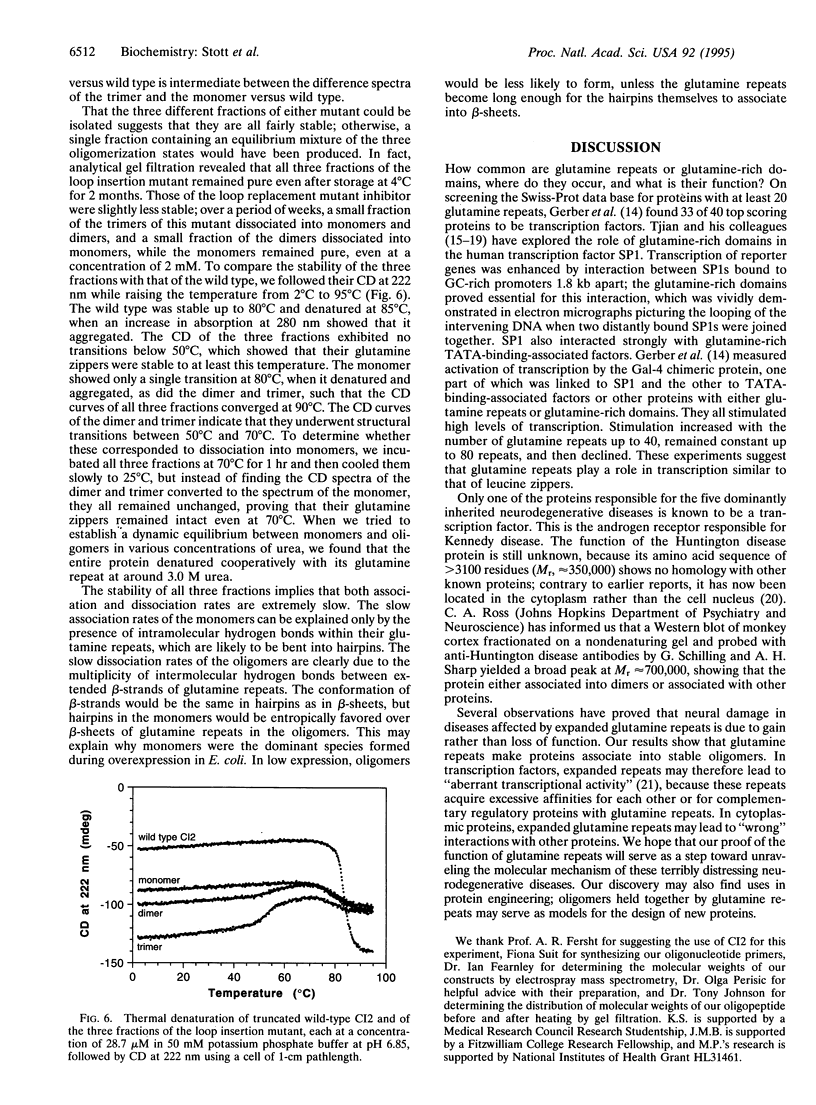
Abstract
Many transcription factors and some other proteins contain glutamine repeats; their abnormal expansion has been linked to several dominantly inherited neuro-degenerative diseases. Having found that poly(L-glutamine) alone forms beta-strands held together by hydrogen bonds between their amide groups, we surmised that glutamine repeats may form polar zippers, an unusual motif for protein-protein interactions. To test this hypothesis, we have engineered a Gly-Gln10-Gly peptide into the inhibitory loop of truncated chymotrypsin inhibitor 2 (CI2), a small protein from barley seeds, by both insertion and replacement. Gel filtration resolved both mutant inhibitors into at least three fractions, which analytical ultracentrifugation identified as monomers, dimers, and trimers of the recombinant protein; the truncated wild-type CI2 formed only monomers. CD difference spectra of the dimers and trimers versus wild type indicated that their glutamine repeats formed beta-pleated sheets, while those of the monomers versus wild type were more suggestive of type I beta-turns. The CD spectra of all three fractions remained unchanged even after incubation at 70 degrees C; neither the dimers nor the trimers dissociated at this temperature. We argue that the stability of all three fractions is due to the multiplicity of hydrogen bonds between extended strands of glutamine repeats in the oligomers or within a beta-hairpin formed by the single glutamine repeat of each monomer. Pathological effects may arise when expanded glutamine repeats cause proteins to acquire excessively high affinities for each other or for other proteins with glutamine repeats.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993 Jan 29;72(2):247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Jackson S. E., Moracci M., elMasry N., Johnson C. M., Fersht A. R. Effect of cavity-creating mutations in the hydrophobic core of chymotrypsin inhibitor 2. Biochemistry. 1993 Oct 26;32(42):11259–11269. doi: 10.1021/bi00093a001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Molecular genetics of neurological diseases. Science. 1993 Oct 29;262(5134):674–676. doi: 10.1126/science.8235586. [DOI] [PubMed] [Google Scholar]
- McPhalen C. A., Svendsen I., Jonassen I., James M. N. Crystal and molecular structure of chymotrypsin inhibitor 2 from barley seeds in complex with subtilisin Novo. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7242–7246. doi: 10.1073/pnas.82.21.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre A. N., Trifiro M. A., Kaufman M., Kazemi-Esfarjani P., Figlewicz D., Rouleau G., Pinsky L. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1993 Oct;5(2):184–188. doi: 10.1038/ng1093-184. [DOI] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Perczel A., Hollósi M., Sándor P., Fasman G. D. The evaluation of type I and type II beta-turn mixtures. Circular dichroism, NMR and molecular dynamics studies. Int J Pept Protein Res. 1993 Mar;41(3):223–236. doi: 10.1111/j.1399-3011.1993.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Johnson T., Suzuki M., Finch J. T. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Staden R., Moens L., De Baere I. Polar zippers. Curr Biol. 1993 May 1;3(5):249–253. doi: 10.1016/0960-9822(93)90174-m. [DOI] [PubMed] [Google Scholar]
- Perutz M. Polar zippers: their role in human disease. Protein Sci. 1994 Oct;3(10):1629–1637. doi: 10.1002/pro.5560031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., McInnis M. G., Margolis R. L., Li S. H. Genes with triplet repeats: candidate mediators of neuropsychiatric disorders. Trends Neurosci. 1993 Jul;16(7):254–260. doi: 10.1016/0166-2236(93)90175-l. [DOI] [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Tennent G. A., Butler P. J., Hutton T., Woolfitt A. R., Harvey D. J., Rademacher T. W., Pepys M. B. Molecular characterization of Limulus polyphemus C-reactive protein. I. Subunit composition. Eur J Biochem. 1993 May 15;214(1):91–97. doi: 10.1111/j.1432-1033.1993.tb17900.x. [DOI] [PubMed] [Google Scholar]




