Abstract
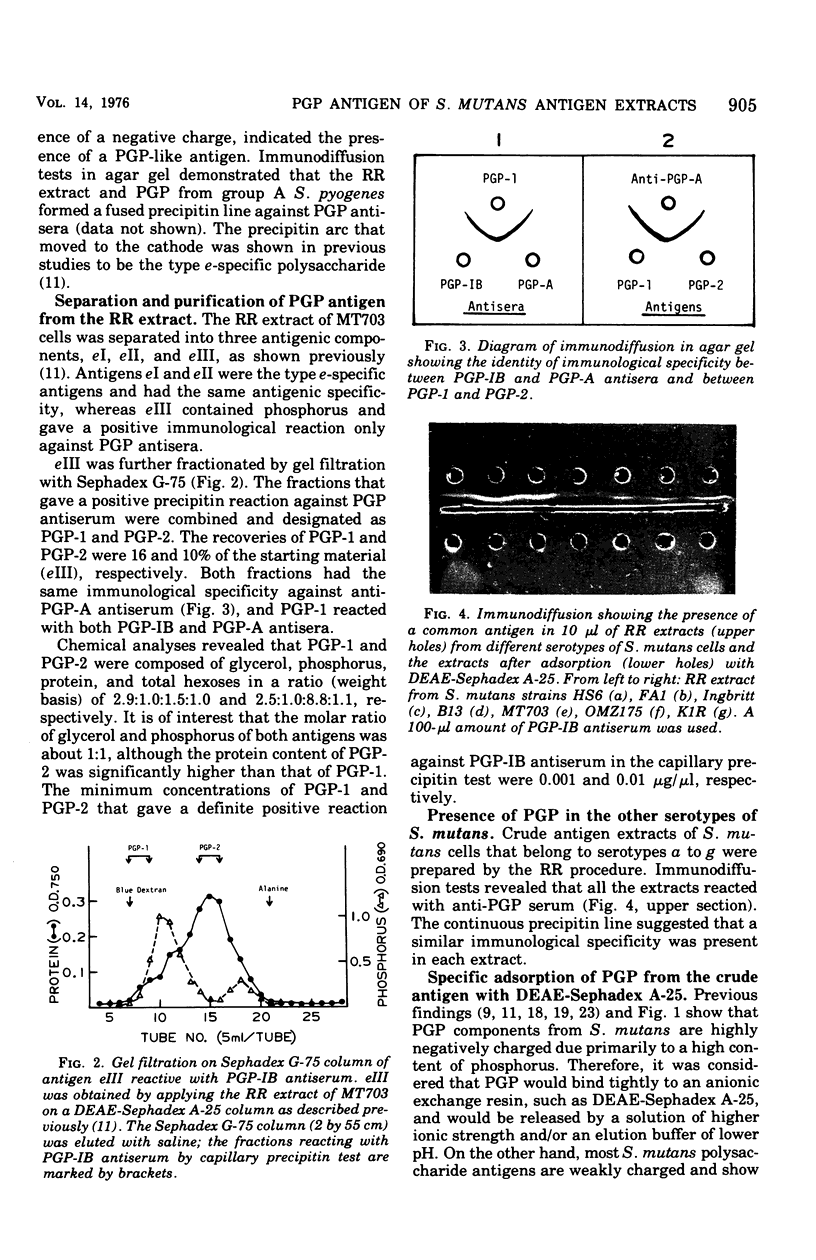
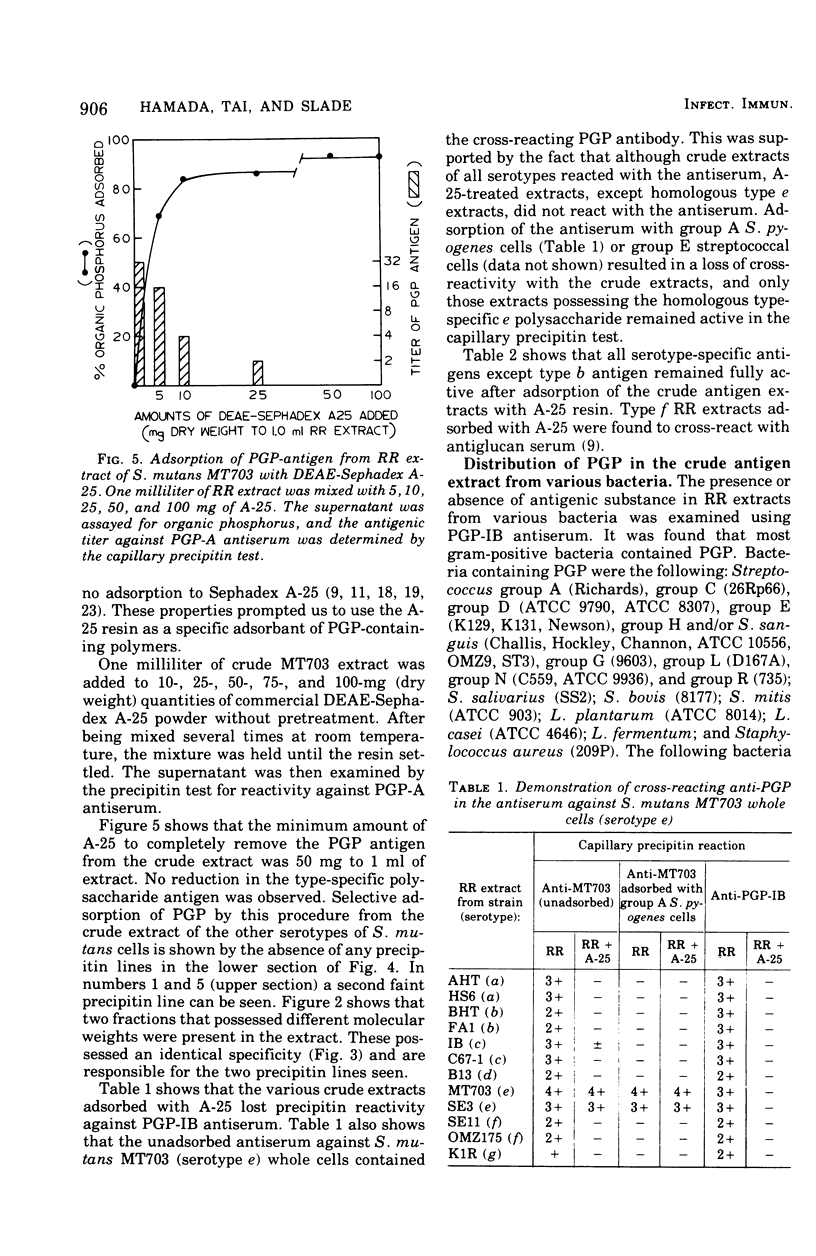
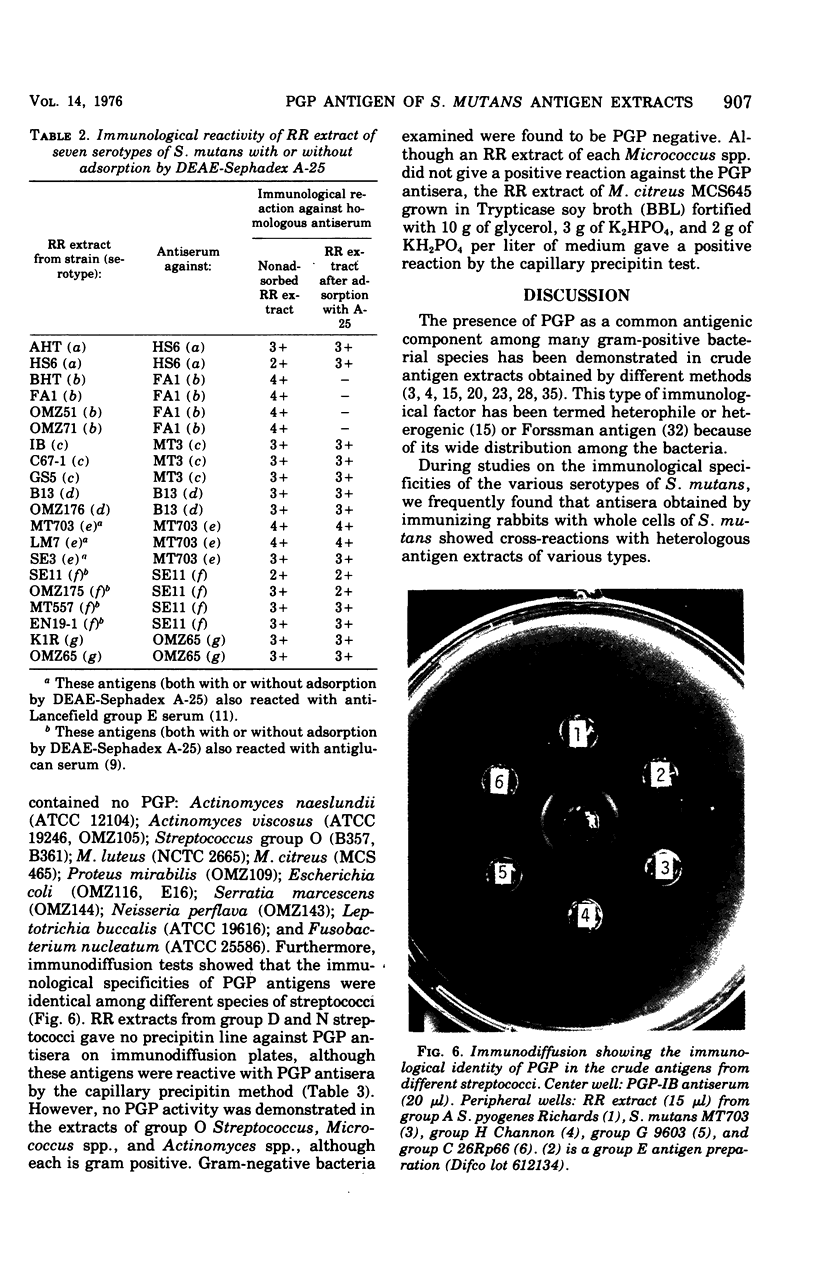
Hot saline extracts of Streptococcus mutans have been shown to contain antigenic substances which occasionally react nonspecifically with some antisera against whole cells of various serological groups and types of streptococci. Chromatography of the extract of S. mutans strain MT703 (serotype e) on a diethylaminoethyl-Sephadex A-25 column gave two principal antigens. One antigen was eluted without adsorption to the resin and was identified as the serotype-specific polysaccharide. The other antigen, which contained a large quantity of phosphorus, was absorbed to and released from the resin by gradient elution. It was reactive against the antisera specific for polyglycerophosphate (PGP) from group A Streptococcus pyogenes and/or S. mutans strain Ingbritt (type c). The PGP antigen was further purified by gel filtration with Sephadex G-75. Two peaks, PGP-1, and PGP-2, were obtained. Each possessed the same antigenic specificity to anti-PGP serum as shown by immunodiffusion. Chemical analyses revealed that the molar ratio of phosphorus to glycerol in both was about 1:1, although the protein content between the two was significantly different. PGP antigen was found to be widely distributed in hot saline extracts from various gram-positive bacteria, with a few exceptions. However, all gram-negative bacteria examined were free of PGP. The PGP in the hot saline extracts of various gram-positive bacteria possessed an essentially identical antigenic specificity. The addition of diethylaminoethyl-Sephadex A-25 resin to hot saline extracts successfully removed the cross-reacting PGP antigen. After adsorption of the extract from S. mutans, the supernatant contained only type-specific polysaccharide antigen, except type b, in which both type b-specific polysaccharide and PGP antigens were absorbed with the resin. This simple procedure should be useful for the removal of the PGP-type teichoic acid from antigen extracts of bacteria that contain uncharged polysaccharides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Baddiley J., Heckels J. E. Molecular arrangement of teichoic acid in the cell wall of Staphylococcus lactis. Nat New Biol. 1973 Jan 3;241(105):29–31. doi: 10.1038/newbio241029a0. [DOI] [PubMed] [Google Scholar]
- Chiu T. H., Emdur L. I., Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974 May;118(2):471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpenning F. W., Cooper H. R., Rosen S. Cross-reactions of Streptococcus mutans due to cell wall teichoic acid. Infect Immun. 1975 Sep;12(3):586–591. doi: 10.1128/iai.12.3.586-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley J., Duckworth M., Baddiley J. The occurrence of lipoteichoic acids in the membranes of gram-positive bacteria. J Gen Microbiol. 1972 Dec;73(3):587–591. doi: 10.1099/00221287-73-3-587. [DOI] [PubMed] [Google Scholar]
- Decker G. P., Chorpenning F. W., Frederick G. T. Naturally-occurring antibodies to bacillary teichoic acids. J Immunol. 1972 Jan;108(1):214–222. [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Hamada S., Gill K., Slade H. D. Chemical and immunological properties of the type f polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):203–211. doi: 10.1128/iai.14.1.203-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Ooshima T., Sobue S., Kotani S. Epidemiological survey of Streptococcus mutans among Japanese children. Identification and serological typing of the isolated strains. Jpn J Microbiol. 1976 Feb;20(1):33–44. doi: 10.1111/j.1348-0421.1976.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Adherence of serotype e Streptococcus mutans and the inhibitory effect of Lancefield group E and S mutans type e antiserum. J Dent Res. 1976 Apr;55(Spec No):C65–C74. doi: 10.1177/002203457605500328011. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Purification and immunochemical characterization of type e polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):68–76. doi: 10.1128/iai.14.1.68-76.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann E., Lüderitz O., Knox K., Weinfeld N. Structural requirements for bone resorption by endotoxin and lipoteichoic acid. J Dent Res. 1975 Jun;54(SPEC):B94–B99. doi: 10.1177/00220345750540023401. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunter E. Gewinnung des präzipitierenden gruppenspezifischen Streptokokken-Polysaccharids durch Erhitzen von Streptokokken im Autoklaven. Zentralbl Bakteriol Orig. 1965 Jul;197(1):72–78. [PubMed] [Google Scholar]
- Linzer R., Gill K., Slade H. D. Chemical composition of Streptococcus mutans type c antigen: comparison to type a, b, and d antigens. J Dent Res. 1976 Jan;55:A109–A115. doi: 10.1177/002203457605500103011. [DOI] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Purification and characterization of Streptococcus mutans group d cell wall polysaccharide antigen. Infect Immun. 1974 Aug;10(2):361–368. doi: 10.1128/iai.10.2.361-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTY M. The occurrence of polyglycerophosphate as an antigenic component of various gram-positive bacterial species. J Exp Med. 1959 Apr 1;109(4):361–378. doi: 10.1084/jem.109.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Extraction, purification, and chemical and immunological properties of the Streptococcus mutans group "a" polysaccharide cell wall antigen. Infect Immun. 1973 Aug;8(2):190–198. doi: 10.1128/iai.8.2.190-198.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. C., Penny B. B. A comparison by gel diffusion of the Lancefield and Rantz extraction techniques used in grouping haemolytic streptococci. Med Lab Technol. 1974 Jan;31(1):43–49. [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Sharpe M. E., Brock J. H., Knox K. W., Wicken A. J. Glycerol teichoic acid as a common antigenic factor in lactobacilli and some other gram-positive organisms. J Gen Microbiol. 1973 Jan;74(1):119–126. doi: 10.1099/00221287-74-1-119. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driel D., Wicken A. J., Dickson M. R., Knox K. W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973 Jun;43(5):483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Characterization of group N streptococcus lipoteichoic acid. Infect Immun. 1975 May;11(5):973–981. doi: 10.1128/iai.11.5.973-981.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]





