Abstract
Diabetic retinopathy (DR) impairs vision of patients with type 1 and type 2 diabetes, associated with vascular dysfunction and occlusion, retinal edema, hemorrhage, and inappropriate growth of new blood vessels. The recent success of biologic treatments targeting vascular endothelial growth factor (VEGF) demonstrates that treating the vascular aspects in the later stages of the disease can preserve vision in many patients. It would also be highly desirable to prevent the onset of the disease or arrest its progression at a stage preceding the appearance of overt microvascular pathologies. The progression of DR is not necessarily linear but may follow a series of steps that evolve over the course of multiple years. Abundant data suggest that diabetes affects the entire neurovascular unit of the retina, with an early loss of neurovascular coupling, gradual neurodegeneration, gliosis, and neuroinflammation before observable vascular pathologies. In this article, we consider the pathology of diabetic retinopathy from the point of view that diabetes causes measurable dysfunctions in the complex integral network of cell types that produce and maintain human vision.
Keywords: diabetic retinopathy, neurodegeneration, neurovascular unit, maladaption, metabolism
Clinical features of diabetic retinopathy
Diabetic retinopathy (DR) afflicts approximately 93 million people worldwide, and 28 million of these have vision-threatening DR.1–3 These numbers are expected to increase as the prevalence of type 2 diabetes continues to climb.4 The diagnosis and treatment of DR are primarily focused on vascular abnormalities that appear at later stages of the disease. DR is staged into several levels of severity, including mild, moderate, and severe nonproliferative DR (NPDR), followed by proliferative DR (PDR), depending on the extent of vascular lesions. As DR severity increases, vascular abnormalities, including plasma leakage, dilation, microaneurysms, and hemorrhages, occur at increasing frequency, with the growth of abnormal capillaries (angiogenesis) defining PDR. This vascular focus is largely due to the fact that retinal vasculature abnormalities are unambiguously identified by visual inspection, and advanced vascular abnormalities correlate with a disruption of vision. Diabetic macular edema (DME) results when fluid accumulation increases retinal thickness and causes light-distorting fluid-filled cysts within retinal tissue, as well as serous detachments separating the neural retina from the underlying pigmented epithelium. Retinal edema is examined noninvasively by optical coherence tomography (OCT) imaging of the retina. OCT can precisely measure retinal layer thickness while detecting intraretinal cysts and serous retinal detachments. Fluorescein angiography clearly defines microaneurysms as hyperfluorescent dots, often associated with additional diffuse fluorescence within the retinal tissue, indicating dye leaking from the vasculature. If unchecked, this focal vascular leakage leads to precipitation of vision-obstructing opaque deposits of plasma lipoproteins (hard exudates). Vitreous cavity hemorrhages, caused by penetration of abnormal and bleeding capillaries into the vitreous gel, occur with the progression to PDR. These vessels fail to form a tight blood–retinal barrier (BRB) and thus leak and contribute to edema. Untreated PDR leads to fibrovascular tissue formation and resulting epiretinal membranes require surgical removal by vitrectomy, as they adhere to the vitreous and cause traction retinal detachment.
The ability of vascular endothelial growth factor (VEGF) to promote both vascular permeability and angiogenesis made it a likely contributor to the vascular dysfunctions observed in severe DR. Fluid balance within retinal tissue is controlled by the balance of transport across the inner vascular BRB and fluid resorption across the retinal pigmented epithelium.5 Like the blood–brain barrier, the BRB is composed of tight junctions between endothelial cells that stringently control the flux of molecules between plasma and neural tissue. Breakdown of this normally tight barrier is thought to be a major factor in the pathogenesis of DME,6 although compromise of normal water removal mechanisms via aquaporin proteins could also contribute to the intraretinal accumulation of fluid.7–9 VEGF causes disassembly of endothelial cell junctions and acts as a potent endothelial cell mitogen, so inappropriate accumulation of VEGF in the diabetic retinas was hypothesized to promote both edema and angiogenesis. In 1994, Aiello et al.10 found markedly increased VEGF protein levels in the vitreous fluid of patients with DR. Their initial report and numerous subsequent studies demonstrated that the concentration of VEGF in vitreous fluid of DME and PDR patients can be increased up to 10 times that of normal levels.10–20 Furthermore, VEGF levels are significantly higher in vitreous fluid from patients with active PDR compared to the vitreous fluid from those with inactive or quiescent PDR.17, 18, 21, 22 These discoveries led to the concept that blocking VEGF action in the retina would improve DME and stall the progression of PDR. This hypothesis was clinically tested by an off-label application of humanized antibody against VEGF165 (bevacizumab, Genentech) that had been developed for cancer therapy, and several small trials have suggested efficacy toward edema and improved vision in approximately 25% of patients.23 In these studies, patients received a limited number of bevacizumab injections (from 1 to 9), often on an as-needed basis. Ranibizumab (Genentech) is an Fc antibody fragment designed specifically for intraocular use and it is now FDA-approved for DME treatment on the basis of randomized trials.24, 25 A VEGF receptor fusion protein (aflibercept; Regeneron) also improved visual acuity greater than 15 letters in up to 46% of patients after 1 year of treatment every 4 weeks.26 Also, ranibizumab reduced the risk of NPDR severity progression in eyes treated for DME by approximately 67% over a 2-year period of monthly injections.27 This last finding, if confirmed, suggests that VEGF overexpression may be directly related to the progression of established DR in a significant portion of patients.
Although success has not been total, these anti-VEGF treatments represent a major advance in DR therapy. Intravitreal injections are invasive but well-tolerated by patients, and the risks associated with repeated intravitreal injections are surprisingly low. For example, endophthalmitis rates are less than 1/2000, and to date, no major long-term ocular or systemic risks of anti-VEGF treatments have been identified.28 The success of anti-VEGF treatments, and indications that additional factors may be involved in the control of retinal vascular permeability and angiogenesis in DME and PDR, have encouraged research on alternative therapeutic targets (reviewed in Ref. 29). Additional pro-angiogenic factors are increased in vitreous fluid from PDR patients, including angiopoietin-2 (Ang-2),17, 18, 30 cysteine-rich 61 (CYR61),31, 32 erythropoietin (EPO),16, 18, 33 osteopontin (OPN),34, 35 platelet-derived growth factor (PDGF),36–39 and stromal cell–derived factor (SDF-1, CXCL12).40, 41 Concentrations of several anti-angiogenic factors are also decreased in the vitreous fluid of PDR patients and/or increased following laser photocoagulation therapy. These include angiostatin (AS),42 endostatin (ES),43 pigment epithelium–derived growth factor (PEDF),16, 30, 34 and tissue kallikrein (TK).44 In addition, after treatment of PDR with laser surgery or intravitreal bevacizumab, a transition from angiogenesis to fibrosis can occur when vitreous levels of VEGF decrease and levels of connective tissue growth factor (CTGF, CCN2) increase.45
The hope is that targeting these alternative permeability-inducing or pro-angiogenic factors will help patients for whom anti-VEGF treatments are not effective. However, the greater goal is to address the early pathophysiologic changes that lead to vision loss so that patients with diabetes can maintain good vision without the need for invasive or destructive procedures.46 This objective requires finding means to halt the progression of DR pathology before the accumulation of appreciable vascular defects, thus avoiding the detrimental effects of edema, microaneurysms, hemorrhages, and inappropriate angiogenesis.
Insufficient vascular coupling of retinal metabolic demand suggests that retinal neurovascular unit dysfunction is an early sign of DR
The challenge of maintaining good vision in an expanding population of patients with diabetes requires a new understanding of the pathophysiology of DR. It is not entirely clear why some organs (e.g., retina, peripheral nerves, and kidney) are relatively susceptible to diabetic complications. DR and diabetic nephropathy have been considered microvascular complications of diabetes, thus suggesting that the commonality is their dependence on microvascular functions. However, this apparent link does not explain the susceptibility of peripheral nerves or the recently appreciated cerebral complications of diabetes.47, 48 In addition, while retinal and kidney microvessels share some parallels, they are fundamentally different; for example, kidney vessels do not possess a tight blood–tissue barrier. Thus, an important question to consider is what may link retina, brain, and peripheral nerves to make these tissues relatively susceptible to diabetic complications. Lesions within the neurosensory retina are now understood to play an important role in DR. There are clear indications that retinal function is disturbed shortly after the onset of diabetes, and that neurodegeneration is an ongoing component of DR pathology.
We propose that the retinal response to diabetes involves a successful adaptation to altered systemic conditions, followed by eventual loss of neurovascular unit function in response to progressive metabolic disruption, resulting in subtle preclinical findings, followed by eventual advanced vision-threatening retinopathy (Fig. 1 and Table 1). Retinal function depends on the synergy of several neuronal subtypes, including photoreceptors, horizontal and bipolar cells, amacrine and ganglion cells, along with their supporting glia (astrocytes and Müller cells), as well as inner vascular (endothelial cells and pericytes) and outer blood–retinal (choroidal vessels and pigmented epithelium) barriers that mediate the supply of nutrients and control the transfer of water and ions into and out of the tissue.49 This complex integral network of cell types and structures allow humans to see across a wide range of light intensity (10 orders of magnitude), and to discern nearly infinite degrees of colors. This functional network appears to adapt fairly well to the systemic metabolic alterations caused by diabetes, for patients can maintain vision and exhibit no apparent clinical pathology for 5 to 10 years after the onset of diabetes. However, the complexity and functional demands of the retina may make it susceptible to eventual loss of tissue homeostasis in the presence of diabetes.
Figure 1.
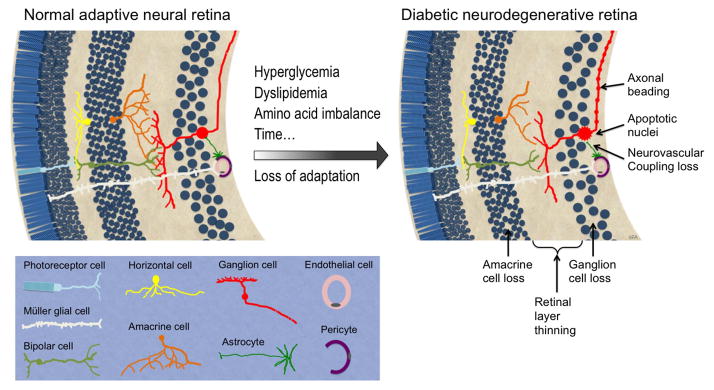
DR changes to the neural retina. Over time, neurons in the inner retinal layers lose adaptation to systemic metabolic alterations caused by diabetes and succumb to cell stress, as evidenced by reduced axonal and dendritic process branching, axonal beading, apoptotic cell death, accumulative cell loss, and retinal layer thinning.
Table 1.
Evolution of diabetic retinopathy and failure of adaptive mechanisms
| Preclinical retinopathy | NPDR | PDR | ||||
|---|---|---|---|---|---|---|
| Symptoms | References | References | References | |||
| None | None, blurred vision, or glare | None, floaters, or decreased vision | 153, 154, 155, 156 157, 158, 159, 160 | |||
| Clinical signs | Normal appearing retina | Retinal vasodilation, microaneurysms, hemorrhages, cotton-wool spots, venous beading | Partial vitreous gel separation from retina; retinal and/or iris neovascularization; epiretinal membranes | |||
| Functional events | Decreased ERG oscillatory amplitudes and increased latency | 76–78 | Normal or decreased visual acuity | Visual acuity usually decreased | ||
| Decreased visual field sensitivity | 161 | Visual field defects | 153, 161, 162 | Progressive visual field defects | ||
| Decreased flicker responses | 63–65, 67 | Increased blood flow | 163 | Reduced dark adaptation | ||
| Decreased blue- yellow color sensation | 164, 165 | Fluorescein angiography: vascular leakage and occlusion | 166 | Fluorescein leakage from neovascularization | ||
| Reduced vasoconstriction in response to oxygen | ||||||
| Cellular alterations | Synaptic loss | 167 | ||||
| Neuronal apoptosis | 80, 95, 100, 168, 169 | Cytoid bodies and nerve fiber layer swelling | 82, 105–107, 170, 171 | Retinal and/or iris neovascularization | ||
| Nerve fiber loss and retinal thinning | 172–174 | Neuronal loss and degeneration; lipid- and fluid-containing cysts | 107, 175 | Progressive neuronal and axonal degeneration | ||
| Glial cell dysfunction | 81, 176, 177 | Gliosis of Müller cells and reduced astrocytes | 176 | |||
| Reduced endothelial cell tight junctions | 178 | Early fibrotic reactions with increased TGF-β | 179, 180 | Epiretinal membranes | ||
| Vascular occlusion and intraretinal shunt vessels | 181 | |||||
| Microglial cell proliferation | 182 | |||||
| Increased cytokine and pro-angiogenic proteins | Immune cells in epiretinal membranes | |||||
| Metabolic alterations | Reduced insulin receptor/Akt activity | 89, 90 | ||||
| Reduced retinal protein synthesis | 183 | |||||
| Altered retinal lipid composition | 151, 184, 185 | |||||
| Defective crystallin expression | 113, 186 | |||||
| Reduced retinal glutamate/glutamine metabolism | 119, 187, 188 | |||||
| ER stress | 93 | |||||
| Oxidative stress | 91, 92 | |||||
| Impaired K(ATP) current and purinergic toxicity | 69, 72, 73 | |||||
| Reduced synaptic protein expression | 167 | |||||
| Altered retinal amino acid profiles | 189 | |||||
An important aspect of neuronal tissue equilibrium is balancing local blood supply and metabolic demand. It has been known for many years that cerebral blood flow responds to brain metabolism, and in the 1980s and 1990s it was realized that vascular tone is mechanistically coupled to neuronal metabolism; it was then hypothesized that this neurovascular coupling may involve signaling through astrocytes.50–53 Such a link was demonstrated in 2003 by Zonta et al.54 using brain slices. Subsequently, Iadecola55 was first to popularize the term neurovascular unit to describe the concept that neurons, astrocytes, smooth muscle cells (or pericytes), and endothelial cells form a functional unit that controls cerebral blood flow in response to metabolic demand. The term has proved useful in understanding the links between neural degeneration and vascular dysfunctions that occur from stroke, Parkinson disease, and other neurodegenerations.56 Matea and Newman57 applied the neurovascular unit concept to describe the functional and structural interactions between neurons, glial cells, and vascular cells in the inner retina. The outer retina photoreceptors and Müller cells receive nutrients and dispose of waste products via the choroidal blood supply through the pigmented epithelium. Thus, ironically, the oxygen-rich outer retina is devoid of vessels, whereas the oxygen-poor inner retina has a well-defined, though relatively sparse, vascular supply. In both the inner retina and brain, neurovascular coupling regulates blood flow to meet the oxygen and nutrient demands created by metabolic and electrical activities, while the blood–tissue barriers control the flux of water and ions, protect against the influx of plasma proteins, and regulate inflammation.
Thus, the neurovascular unit allows integration of metabolic needs and vascular tone by integrating multiple molecular signals in context to maintain normal visual function throughout a range of physiologic conditions. The functions of the neurovascular unit in brain and retina are demonstrated by a normal adaptive response of retinal blood vessels to match metabolic demand and to minimize excessive or insufficient blood and nutrient delivery, termed autoregulation.58 In humans, retinal vascular diameter and blood flow respond dynamically to changing physiologic conditions, including blood pressure, blood gas concentration, and visual stimulation. For example, retinal function is protected from wide variations in systemic arterial pressures; retinal blood flow remains constant over a range of perfusion pressures up to an increase of 36% over baseline.59 Other features of autoregulation are revealed by vasoconstriction in response to breathing 100% oxygen (hyperoxia) and vasodilation resulting from exposure to hypercapnia (increased pCO2).60 Hyperoxia reduces the volume of blood flow needed to provide the retina with appropriate oxygen influx, whereas hypercapnia increases the requirement for blood flow. These physiologic responses occur within seconds to minutes and diminish rapidly when the stimulus is removed.
The cellular coupling that links the neurovascular unit includes light-induced vasodilation and vasoconstriction of retinal arterioles. Flickering light stimulation of the retina increases metabolic demand in the inner retina, which is accompanied by vasodilation of arterioles.60–62 Metea and Newman57 found that these responses result from direct glial–vascular signaling without neuronal involvement, and are mediated by integrated responses to arachidonic acid intermediates, nitric oxide and K+. Specifically, stimulating or inhibiting nitric oxide synthase determines if signals initiated by 5-6-epoxyeicosatrienoic acid (5-6-EET) and 20-hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE) lead to vasodilation or vasoconstriction in response to light. These same arachidonic acid derivatives also mediate light-induced vasomotor responses and are associated with increased glial cell [Ca2+]. Metea and Newman57 concluded that glial-evoked vasomotor responses are due to direct glial-to-vessel signaling without neuronal intermediates. Also, light- and glial-evoked vasomotor responses are mediated by the same arachidonic acid metabolites, and light-evoked vasodilation and vasoconstriction depend on neuron-to-glia signaling. Thus, retinal vascular responses depend on multiple local and systemic factors and may vary in different retinal regions.
Deficient neurovascular coupling, as evidenced by altered vasoconstriction and flicker light responses, is an early sign of retinal disequilibrium caused by diabetes. Multiple studies have examined the effects of diabetes on the reactivity of retinal vessels. Before the appearance of clinically evident retinal vascular lesions or edema, patients with pre-diabetes or overt type 2 diabetes exhibit greater than 50% reductions of vasoconstriction in response to hyperoxia and vasodilation in reaction to flicker stimulus compared to healthy controls.63, 64 Similar changes occur in type 1 diabetes.65 The impairment does not appear to result from a defective response to nitric oxide release, as vasodilation stimulated by oral nitroglycerin is normal in the diabetic subjects.66 Pemp et al. conclude that, “neither the reduced vasodilator response to flicker stimulation nor abnormal retinal autoregulation, as observed previously, is the consequence of a generally reduced vascular reactivity of retinal vessels in this disease.”66 Additional work by the Schmetterer laboratory shows that patients with well-controlled type 1 diabetes (mean HBA1c < 7.5%) for less than 10 years with clinically normal retinas and normal pattern electroretinographic responses exhibit increased basal diameters of retinal arterial and reduced flicker-induced vasodilation in both retinal arteries and veins compared to non-diabetic controls.67 This result suggests the mechanisms that regulate flicker responses may be distinct from those that mediate electrical responses of the neurosensory retina. The increased vascular diameter is an early manifestation of disturbed autoregulation that begins shortly after the onset of diabetes.64 These early events suggest the possibility that the retina changes its adaptive reflexes in response to mild hyperglycemia, or another metabolic perturbation of diabetes. Dorner et al. found that short-term hyperglycemia reduces the vasodilation response to flicker by 55% in healthy subjects.68 Thus, diabetes appears to alter the ability of the retina to autoregulate, but the specific mechanism remains uncertain.
The mechanism by which diabetes affects the neurovascular coupling response to flickering light is not clear. Vasoreactivity also depends on focal electrical responses along the course of retinal arteriole/capillary segments.69 Diabetes accelerates the axial decay of voltage along microvessels by fivefold compared to normal.70 This defect results from spermine-induced inhibition of voltage-gated calcium channels in arterioles71 and of K(ATP) channels in capillaries, thus eliminating the normal topographical heterogeneity of functional K(ATP) channels required for normal vasoresponses.72 Impeded axial voltage transmission would be expected to hinder the control of blood flow by impeding upstream responses to downstream signals. Vessels from diabetic rat retinas are also susceptible to death due to purinergic toxicity via pore formation following activation of P2X(7) purinoceptors by extracellular NAD+.73 Together, these changes limit the ability of retinal vessels to autoregulate and increase neuronal vulnerability to death from metabolic insults. They reveal important regional responses of retinal vessels to ionic and small molecule signals that regulate the neurovascular unit under physiologic conditions.
Defects detected by electroretinography suggest that deficiencies in neuroglial function are early features of DR
Neuroglial function of the retina is assessed by electroretinography (ERG), the ocular equivalent of electroencephalography. The ERG has been used to investigate the ocular effects of diabetes for at least five decades, with the first studies finding delays in the response rate (increased implicit time) in patients with severe nonproliferative and proliferative retinopathy.74 The studies were interpreted to indicate that Müller cells are damaged in advanced diabetic retinopathy. More recent studies revealed defects in the ERG responses of patients with newly diagnosed type 1 and type 2 diabetes who have no clinically evident retinopathy.75–77 Multifocal ERG revealed focal areas of electrical depression and increased latency (implicit time) in the peripheral retina that predicted the development of retinal vascular lesions in specific retinal zones.78 Lecleire-Collet et al.79 observed that alterations in the amplitude and implicit time pattern of ERGs in patients with diabetes correlate with deficits in flicker light–induced vasodilation. These changes were interpreted to indicate altered function of Müller, ganglion, amacrine, and bipolar cells, all of which are adversely affected by experimental diabetes.80–83 The ERG is a highly sensitive index of retinal function but is difficult to apply in routine clinical practice because of the length of testing (> 1 h for dark-adapted testing) and need for corneal contact lenses.
Together, these findings of depressed vascular coupling and altered neuroglial electrical responses that occur before the onset of clinically evident vascular lesions suggest that diabetes causes considerable alterations to retinal function before the onset of typical clinical signs of DR. These neurosensory retina alterations might be early adaptations to diabetes or may simply be negative consequences of adaptive mechanisms necessary to maintain function of the retina in the diabetic state. These findings do not necessarily indicate the blood vessels are normal during this period. Indeed, mice with deficient PDGF receptor β expression in brain pericytes exhibit blood–brain barrier breakdown at 1 month of age, while behavioral function and brain cellular structure remain intact until 6 months of age and neuroinflammation at 16 months of age.84 Therefore, primary blood–brain barrier defects do not cause immediate brain damage in animals that are otherwise healthy, implying that the central nervous system has intrinsic cytoprotective mechanisms, as suggested by Iadecola.85 Degenerative brain diseases exhibit concomitant defects in neuroglial and vascular function (reviewed in Ref. 86), but the sequence of neurovascular unit disintegration in DR remains uncertain at this time. Thus, normal appearing retinal blood vessels in diabetic patients may not reveal subtle defects that could indicate early retinopathy.
Maladaptive changes observed in animal models of diabetes and human specimens
The transition from physiologic adaptation to disease occurs slowly over the course of years, and indication of maladaptive changes may lie below the resolution of clinical evaluation; therefore, studies in animal models are particularly important. Functional changes such as impaired ERG and flicker responses, similar to those in humans, occur in rats and mice within several months of diabetes onset.87, 88 Concurrent biochemical alterations characteristic of diabetes (e.g., reduced insulin receptor/Akt activity,89, 90 oxidative stress,91, 92 and endoplasmic reticulum stress93) occur within 1 to 6 months after the onset of diabetes and in the context of histologically normal retinas. These alterations may have direct effects on retinal neurovascular unit function by causing cellular dysfunction, perturbation in neurotransmitter production, astrogliosis, and neuroinflammation. In diabetic rats, retinal cell death and astrogliosis were reversed both by reduction of hyperglycemia via treatment with the sodium-linked glucose transporter inhibitor, phloridzin (without added insulin), and by injecting very small doses of insulin in the subconjunctival space to restore retinal insulin receptor signaling (without affecting systemic glycemia).94 Both manipulations restored retinal Akt activity. Akt, also known as protein kinase B (PKB), is a key positive regulator of cell metabolism, growth, and survival.95 Akt is highly responsive to insulin receptor binding leading to PI3K activation, which occurs in nervous tissues as well as classically insulin-responsive tissues (liver, muscle, and adipose).96 Thus, the corrective effects of local insulin delivery were expected, while the effects of glycemic normalization were somewhat surprising. We interpret these results to indicate that both central features of type 1 diabetes, insulin deficiency and associated hyperglycemia, can contribute directly or indirectly to cellular dysregulation in the rodent retina. The findings do not exclude contributions from dyslipidemia or altered amino acid metabolism, which are also part of the diabetic milieu.
If the retina appears normal and has normal visual function as assessed by standard methods (visual acuity and fields), clinicians generally deem that retinopathy is not present. Nevertheless, studies in animal models reveal multiple cellular alterations, suggesting that diabetes causes subtle but progressive dysfunction and degeneration of the neural retina. Notably, multiple studies have shown that uncontrolled insulin-deficient diabetes increases the basal rate of neuronal cell death by several-fold, beginning within 1–3 months after the onset of hyperglycemia and in postmortem retinas of humans with less than 5 years of diabetes.80, 97–102 Diabetic rodent models do not generally exhibit advanced stages of DR pathologies observed in humans, such as DME and PDR, but they eventually exhibit breakdown of the BRB and vascular cell death.103 The rapid onset of neuronal apoptosis was not initially expected because the earliest vascular cell death in diabetic rats requires at least 6 months to appear.104 It should be noted that several groups have failed to detect increased neuronal apoptosis in diabetic rats or mice when they examined retinal cross sections.88, 105–107 Indeed, Barber et al.80 also found no quantitative increase in TUNEL-positive cells when using cross sections, even though occasional sections revealed TUNEL-positive cells. However, when retinal flat mounts are examined and sufficient animals are studied to provide appropriate statistical power, the sampling error of sections is circumvented and the infrequent, but increased rate of cell death is apparent.80 Fort et al.94 also reported the ability of a nucleosome ELISA to consistently detect modest increases in retinal cell death and responses to treatments in diabetic rodents. Therefore, we submit that the accelerated retinal cell death in diabetes is a conserved event in all species; however, appropriate group numbers and methods must be used if its occurrence is to be accurately detected.
Moreover, diabetes affects both neurites (axons and dendrites) and cell bodies of retinal neurons, as evidenced by neuritic swellings108, 109 (Figs. 2–4), TUNEL-positive nuclei, and caspase-3 activation82 within a few months of diabetes onset. These changes in well-defined animal models reveal ganglion cell body swelling and axonal fragmentation, and closely mimic those in postmortem human retinas from patients with diabetes.110 Both retrograde and anterograde axonal transport is impeded in the optic nerve of diabetic rats.111, 112 Retinal ganglion cell axonopathy, including reactive gliosis of axonal astrocytes, occurs within weeks of diabetes, before loss of retinal ganglion cells.112 This effect on retinal ganglion cells differs from that in glaucoma, in which axonal degeneration is thought to be the primary defect.113, 114
Figure 2.
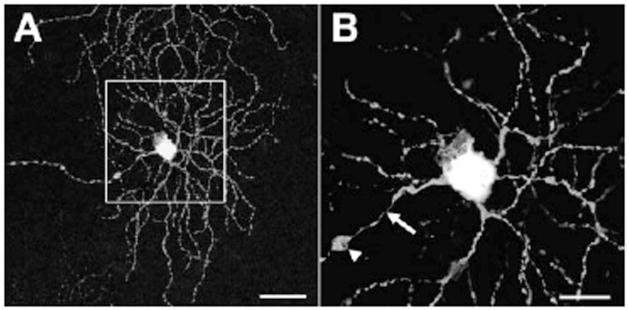
Axonal swellings of Thy1-YFP–positive retinal ganglion cells in a mouse model of diabetic retinopathy. An example of axonal swellings on retinal ganglion cells of Thy1-YFP transgenic mice crossed with Ins2Akita diabetic mice after 3 months of diabetes. (A) shows an entire dendritic arbor of a retinal ganglion cell in a diabetic retina; (B) shows an enlarged image of the boxed region in A, with axon swelling (arrowhead) at approximately 60 μm from the soma and preceded by a prominent thinning of the axon (arrow). Scale bars: 50 μm in A and 20 μmin B. Taken from Gastinger et al.,109 with permission.
Figure 4.
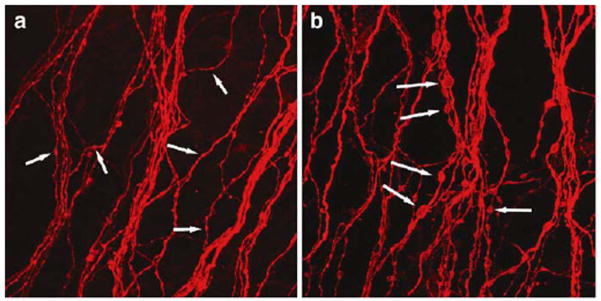
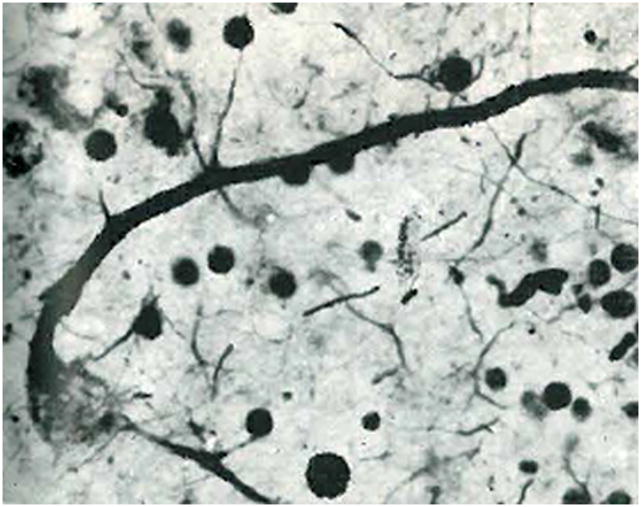
Enlarged axonal beading of parasol cells in human diabetic retinopathy. (a) Axons within a control retina exhibiting low-caliber beading consistent with transportation beads (arrows). (b) Axons within a diabetic retina showing abnormally large and irregular beads along the axons (arrows). Taken from Meyer-Rüsenberg et al.,157 with permission.
The presence of ongoing cell death in animals and humans under moderate metabolic control80 suggests several potential explanations. One possibility is that hyperglycemia or insulin resistance is sufficient to injure neurons. Indeed, mild diabetic retinopathy occurs in approximately 8% of people with pre-diabetes,115 much as diabetic peripheral neuropathy occurs in up to 25% of people with pre-diabetes,116 and multiple cases of diabetic retinopathy have been reported in patients with normal or minimally altered glucose tolerance (reviewed in Ref. 117). These established clinical observations suggest that hyperglycemia alone may not be a necessary or sufficient cause of DR.
Another explanation for early and ongoing cell death is that intrinsic adaptive mechanisms fail to serve their function, as illustrated with two examples. First, alpha crystallins belong to the small heat shock protein family of molecular chaperones and regulate apoptosis by inhibiting the pro-apoptotic Bcl2 family member Bax. In diabetic rats, the expression of these chaperones increases while their solubility decreases, leading to disruption of their interaction with Bax.118 Second, autophagy, a lysosome-based process to recycle organelles and long-lived proteins, normally protects neurons from death, but we have found that the expression and cleavage of retinal autophagy proteins are altered in clinical and experimental diabetes (unpublished data). Iadecola and Anrather85 have summarized a host of intracranial and systemic endogenous protections against stroke, including enhanced growth factor production, bone marrow–derived progenitor cells, and blood pressure control. At present the understanding of protective mechanisms in the retina are less well-understood, but the potential for therapeutic benefits of endogenous neuroprotection could be substantial. Additional work is needed to determine how diabetes causes cellular stress and how stress response mechanisms are altered in retinal neurons, and when neurovascular coupling is lost beyond the ability to prevent vision loss.
Proposed mechanisms of DR related to impaired Akt/mTOR signaling
Clearly, the diabetes epidemic mandates new strategies to prevent retinal cell death and preserve vision,119, 120 and understanding the mechanisms of neural cell death in DR is essential because neuronal integrity is central to vision.121 The mechanisms of retinal neurodegeneration are complex and likely multifactorial. At a systemic level, insulin-deficient diabetes is fundamentally a consequence of deficient insulin receptor action in sensitive tissues, notably liver, skeletal muscle, and adipose tissue, with attendant unopposed excess action of glucagon, leading to a catabolic state with breakdown of energy stores, impaired substrate oxidation, and accumulation of lipids, glucose, and some amino acids within the blood. In contrast to the acutely insulin-sensitive tissues (liver, skeletal muscle, and adipose), the retina differs in that insulin receptor kinase activity does not fluctuate with feeding and fasting.122 In addition, even though insulin at high nanomolar concentrations can stimulate retinal insulin receptors, insulin-like growth factors 1 and 2, rather than insulin, are probably the primary endogenous ligands for retinal insulin receptors (unpublished data). Nonetheless, the retina uses largely conserved insulin receptor signaling pathways, notably PI-3-kinase, Akt isoforms 1 and 3, and p70 S6 kinase, but not p42/44 MAP kinase, to mediate cell survival.89, 94, 123, 124 Analysis of enzyme activities by immunoprecipitation and quantification of substrate phosphorylation reveals that the activity of the retinal insulin receptor, PI-3 kinase, Akt pathway is approximately double that of gastrocnemius muscle in normal rats.125 This relatively high enzyme activity also parallels a higher specific rate of protein synthesis in retina compared to muscle (unpublished data). This may be due to the extremely high metabolic and synthetic rates of photoreceptors. Rajala et al. have detailed how photons activate the insulin receptor of photoreceptors to mediate cell survival via hexokinase126, 127 and phototransduction by activating photoreceptor cyclic nucleotide gated channels.128, 129 Accordingly, deletion of the insulin receptor or Akt2 removes neuroprotective inputs to photoreceptors, making them highly susceptible to light-induced stress.130, 131
The mechanism of diminished Akt activity observed in retinas of diabetic rats does not coincide with loss of phosphorylation associated with growth factor signaling.89 We recently completed an exhaustive mass spectrometric analysis of all Akt1 protein phosphorylation changes occurring in diabetic rat retinas in which Akt1-specific activity is significantly down-regulated. Although approximately 4% of Akt1 protein from the diabetic retinas exhibited a novel dual phosphorylation of S124 and S129 in the hinge region, no changes in activating phosphorylations were identified (unpublished data). Thus, loss of activating phosphorylation does not seem to be the mechanism of decreased Akt1 activity in the diabetic retina. It is also probable that diabetes-induced protein oxidative and nitrosive alterations impair Akt in the neural retina. Such modifications would explain diminished Akt activity without loss of activating phosphorylations. Increased protein nitration in the neural retina of diabetic humans and rats has been documented. For example, nitrotyrosine immunoreactivity was found throughout the inner neural retina, including the nerve fiber, ganglion cell, inner plexiform, and inner nuclear layers of retinas from diabetic humans and rats.132, 133 Work by El-Remessy and colleagues demonstrated how nitration of the anti-apoptotic TrkA receptor plays a key role in the loss of NGF signaling and neurodegeneration in the diabetic retina134 and how nitration of PI3-K p85 subunit inhibits VEGF survival signaling in retinal endothelial cells.135 High fat diet–induced nitration of insulin signaling proteins and insulin resistance were reversed by catalytic removal of peroxynitrite, the reaction product of superoxide and nitric oxide, which causes nitration of tyrosine residues.136, 137 Although nitration of tyrosines in Akt are likely in DR, two cysteines, Cys296138 and Cys224,139 have also been identified as nitrosylation sites of Akt1. This Akt nitrosylation mechanism accounts for loss of Akt activity in aging muscle and brain ischemia, where activating phosphorylations of Akt were not decreased but nitrosylation of Akt was increased.140, 141 Akt nitrosylation is thought to contribute to insulin resistance in diabetic muscle.142 In high fat diet–induced pre-diabetes and diabetes, muscle insulin resistance coincided with nitrosylation of the insulin receptor beta, insulin receptor substrate 1 (IRS-1), and Akt.143
We hypothesize that defects in mTOR signaling may also underlie the neurodegenerative aspects of DR, as it does brain degenerations,144, 145 because it coordinates protein synthesis and autophagy in response to growth factors and amino acids.146, 147 mTOR is associated with two relatively independent complexes, mTORC1 and mTORC2, which contain regulatory-associated protein of mTOR (raptor) and rapamycin-insensitive companion of mTOR (rictor) proteins, respectively. Raptor-associated mTORC1 promotes protein synthesis in muscle and liver by phosphorylating initiating factor 4E-binding protein (4E-BP1) and S6 kinase (S6K1), and is inhibited by both torin and rapamycin.148 In contrast, rictor-associated mTORC2 promotes cellular viability and cytoskeleton organization by phosphorylating Akt Ser473 and atypical protein kinase C, and is inhibited by torin but not rapamycin.148 Conditional deletion of rictor in postnatal mice and flies impairs synaptic efficacy via reduced actin polymerization149, 150 and causes cerebellar Purkinje neurons to be severely stunted.151 Both raptor and rictor regulate dendrite arborization in hippocampal neurons in response to insulin and IGF-1.152 Therefore, it is reasonable to postulate that disruption of neuronal mTOR function may contribute to defects in axons and dendrites that are essential for vision. mTOR also suppresses autophagy.153 Depending on the context, autophagy can protect neurons or promote neuronal cell death,154, 155 but increasing evidence suggests that autophagy dysfunction can contribute to neurodegeneration by allowing the accumulation of mutant or defective proteins.145 We recently showed that hyperglycemia decreases mTOR activity in diabetic rat retinas and R28 cells by lowering phosphatidic acid content, and replenishment of phosphatidic acid inhibits retinal cell death induced by IL-1β.156 These findings reinforce the importance of further understanding how diabetes impairs Akt and alters mTOR signaling in the retina.
Conclusion
Numerous studies over the past five decades conclusively show that neurosensory retinal defects evolve with or before the onset of the earliest vascular lesions that define DR pathology. This information may allow early detection of DR using sensitive techniques that measure retinal sensory neuropathy. However, several questions remain, such as: (1) the nature of the insults that lead to retinal damage; (2) how and when the retina loses adaptation to ongoing diabetes resulting in vision loss; (3) how clinical tests can be optimized to detect early retinal neuropathic changes in clinical practice; and (4) how such a diagnosis can be employed to prevent appreciable vision loss in persons with diabetes.
Figure 3.
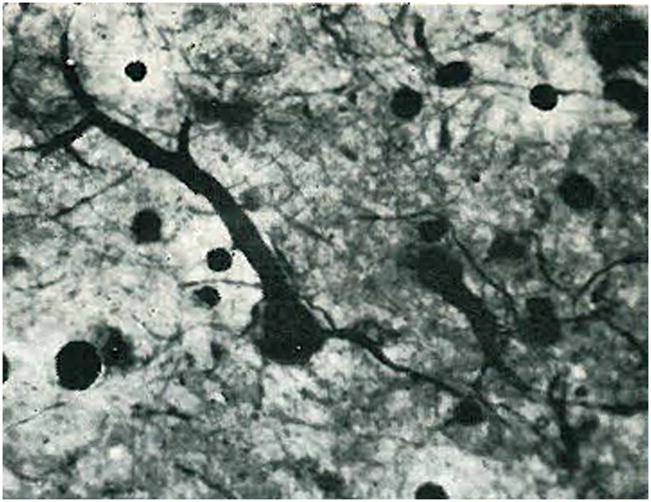
Early evidence of degenerating ganglion cells in human diabetic retinopathy. Ganglion cell bodies and neurites are fragmented and the dendrites are swollen. Hortega stain of frozen sections. Reprinted from Wolter,110 with permission.
Figure 5.
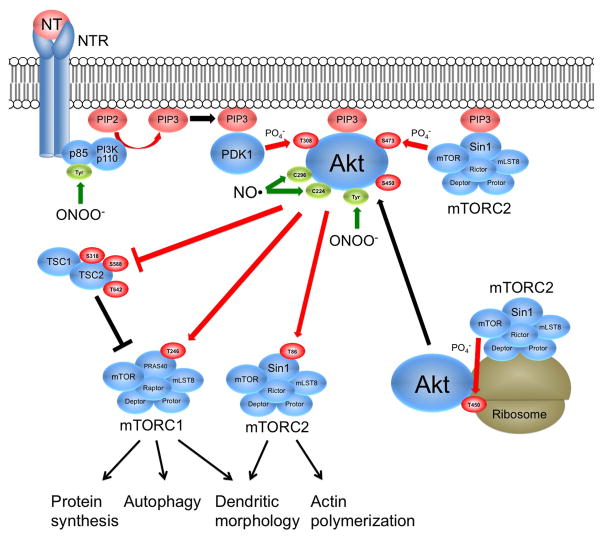
Summary of known Akt and mTOR regulation pathways. The mTOR kinase within rictor-containing mTORC2 complex located at ribosomes catalyzes the co-translational phosphorylation of Akt Thr450, thus stabilizing the newly formed Akt protein. Neurotrophins (NT) (e.g., NGF, IGFs, BDNF) bind their respective receptors (NTR), thus activating phosphoinositide 3-kinase (PI3K), resulting in formation of phosphoinositide(3,4,5)-trisphosphate (PIP3). PIP3 recruits phosphoinositide-dependent kinase 1 (PDK1), Akt, and mTORC2 complex to the membrane by association with pleckstrin homology (PH) domains. PDK1 phosphorylates Thr308 of Akt and mTOR within mTORC2 phosphorylates Akt Ser473, thus fully activating Akt. TSC2 inhibits mTORC1 through GTPase activation of the small G-protein Rheb (not shown). Akt-induced phosphorylation of TSC2 at several sites relieves inhibition of mTORC1. In addition, Akt increases the phosphorylation of PRAS40 in mTORC1 and Sin1 in mTORC2. mTORC1 primarily stimulates protein synthesis via its effects on mRNA translation and regulates autophagy, whereas mTORC2 controls dendritic morphology and actin polymerization (see text). Phosphorylations are shown in red and nitrations and nitrosylations are shown in green. Protein nitration occurs when peroxynitrite (ONOO−), formed from nitric oxide (NO) and superoxide (O2−), reacts with tyrosine residues (Tyr) forming nitrotyrosine. Nitration has been implicated in inhibition of PI3K and Akt activities. Protein nitrosylation occurs when NO reacts with the thiol group of cysteine residues. Nitrosylation of Cys298 and Cys224 have been implicated in the inhibition of Akt activity.
Acknowledgments
Supported by NIH R01EY020582 (SFA), American Diabetes Association (SFA), Juvenile Diabetes Research Foundation International (SFA), NIH R01EY20582 (SFA, TWG), NIH DP3DK094292 (TWG), Research to Prevent Blindness Physician-Scientist Award (TWG), and the Taubman Institute (TWG).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. doi:610.1136/bjophthalmol-2011-300539 Epub 302011 Dec 300531. [DOI] [PubMed] [Google Scholar]
- 2.Sivaprasad S, et al. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57:347–370. doi: 10.1016/j.survophthal.2012.01.004. Epub 2012 Apr 2028. [DOI] [PubMed] [Google Scholar]
- 3.Yau JW, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. doi:510.2337/dc2311-1909. Epub 2012 Feb 2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko F, et al. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999–2002 and 2005–2008. Jama. 2012;308:2361–2368. doi: 10.1001/jama.2012.85685. doi:2310.1001/jama.2012.85685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfensberger TJ, Gregor ZJ. Macular edema--rationale for therapy. Dev Ophthalmol. 2010;47:49–58. doi: 10.1159/000320073. Epub 000322010 Aug 000320010. [DOI] [PubMed] [Google Scholar]
- 6.Lang GE. Diabetic macular edema. Ophthalmologica. 2012;227:21–29. doi: 10.1159/000337156. Epub 2012 Apr 2024. [DOI] [PubMed] [Google Scholar]
- 7.Cui B, et al. Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp Eye Res. 2012;98:37–43. doi: 10.1016/j.exer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Goodyear MJ, Crewther SG, Junghans BM. A role for aquaporin-4 in fluid regulation in the inner retina. Vis Neurosci. 2009;26:159–165. doi: 10.1017/S0952523809090038. [DOI] [PubMed] [Google Scholar]
- 9.Hollborn M, et al. Expression of aquaporins in the retina of diabetic rats. Curr Eye Res. 2011;36:850–856. doi: 10.3109/02713683.2011.593108. [DOI] [PubMed] [Google Scholar]
- 10.Aiello LP, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 11.Abu El-Asrar AM, et al. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2011;25:25. doi: 10.1007/s00592-011-0330-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–420. doi: 10.3109/02713683.2012.661114. Epub 2012 Mar 2012. [DOI] [PubMed] [Google Scholar]
- 13.Abu El-Asrar AM, et al. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi: 10.1155/2012/697489. Epub 692012 Oct 697416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funatsu H, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi Y, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30:339–344. doi: 10.1097/IAE.0b013e3181bd2f44. [DOI] [PubMed] [Google Scholar]
- 16.Mohan N, et al. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–441. doi: 10.1016/j.jdiacomp.2012.05.005. doi:410.1016/j.jdiacomp.2012.1005.1005. Epub 2012 Jun 1012. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe D, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Loukovaara S, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2012;26:1755–3768. doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 19.Nawaz MI, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013;22:008. doi: 10.1016/j.exer.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Adamis AP, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 21.Funatsu H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8. doi: 10.1007/s00417-004-0950-7. Epub 2004 Jul 2017. [DOI] [PubMed] [Google Scholar]
- 22.Patel JI, et al. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806. doi: 10.1016/j.exer.2005.10.002. Epub 2005 Dec 2001. [DOI] [PubMed] [Google Scholar]
- 23.Zechmeister-Koss I, Huic M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: a systematic review. Br J Ophthalmol. 2012;96:167–178. doi: 10.1136/bjophthalmol-2011-300674. Epub 2011 Dec 2011. [DOI] [PubMed] [Google Scholar]
- 24.Elman MJ, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. e1035. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen QD, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Do DV, et al. One-year outcomes of the DA VINCI Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–1665. doi: 10.1016/j.ophtha.2012.02.010. doi:1610.1016/j.ophtha.2012.1602.1010. Epub 2012 Apr 1624. [DOI] [PubMed] [Google Scholar]
- 27.Ip MS, et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–1152. doi: 10.1001/archophthalmol.2012.1043. doi:1110.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 28.Elman MJ, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Park JK, Duh EJ. Novel targets against retinal angiogenesis in diabetic retinopathy. Curr Diab Rep. 2012;12:355–363. doi: 10.1007/s11892-012-0289-0. doi:310.1007/s11892-11012-10289-11890. [DOI] [PubMed] [Google Scholar]
- 30.Huber M, Wachtlin J. Vitreous levels of proteins implicated in angiogenesis are modulated in patients with retinal or choroidal neovascularization. Ophthalmologica. 2012;228:188–193. doi: 10.1159/000339952. Epub 2012 Aug 2014. [DOI] [PubMed] [Google Scholar]
- 31.You JJ, et al. Elevation of angiogenic factor Cysteine-rich 61 levels in vitreous of patients with proliferative diabetic retinopathy. Retina. 2012;32:103–111. doi: 10.1097/IAE.0b013e318219e4ad. doi:110.1097/IAE.1090b1013e318219e318214ad. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Yu W, Dong F. Cysteine-rich 61 (CYR61) is up-regulated in proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250:661–668. doi: 10.1007/s00417-011-1882-7. doi:610.1007/s00417-00011-01882-00417. Epub 02011 Dec 00413. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe D, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 34.Abu El-Asrar AM, et al. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm. 2012;2012:493043. doi: 10.1155/2012/493043. Epub 492012 Sep 493029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kase S, et al. Increased osteopontin levels in the vitreous of patients with diabetic retinopathy. Ophthalmic Res. 2007;39:143–147. doi: 10.1159/000102936. Epub 2007 May 2015. [DOI] [PubMed] [Google Scholar]
- 36.Lee WJ, et al. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 2012;96:1426–1430. doi: 10.1136/bjophthalmol-2012-301913. doi:1410.1136/bjophthalmol-2012-301913. Epub 302012 Aug 301928. [DOI] [PubMed] [Google Scholar]
- 37.Schoenberger SD, et al. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906–5911. doi: 10.1167/iovs.12-10410. doi:5910.1167/iovs.5912-10410. [DOI] [PubMed] [Google Scholar]
- 38.Praidou A, et al. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89:248–254. doi: 10.1111/j.1755-3768.2009.01661.x. doi:210.1111/j.1755-3768.2009.01661.x. [DOI] [PubMed] [Google Scholar]
- 39.Freyberger H, et al. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2000;108:106–109. doi: 10.1055/s-2000-5803. [DOI] [PubMed] [Google Scholar]
- 40.Butler JM, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooks HL, Jr, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801–1807. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- 42.Spranger J, et al. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43:1404–1407. doi: 10.1007/s001250051546. [DOI] [PubMed] [Google Scholar]
- 43.Funatsu H, et al. Outcome of vitreous surgery and the balance between vascular endothelial growth factor and endostatin. Invest Ophthalmol Vis Sci. 2003;44:1042–1047. doi: 10.1167/iovs.02-0374. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura S, et al. Tissue kallikrein inhibits retinal neovascularization via the cleavage of vascular endothelial growth factor-165. Arterioscler Thromb Vasc Biol. 2011;31:1041–1048. doi: 10.1161/ATVBAHA.111.223594. Epub 2011 Feb 1043. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper EJ, et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tombran-Tink J, Barnstable C, Gardner Te. Visual Dysfunction in Diabetes: the Science of Patient Impairment and Improvement. Springer; New York: 2012. [Google Scholar]
- 47.Moran C, et al. Brain Atrophy in Type 2 Diabetes: Regional distribution and influence on cognition. Diabetes care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Elderen SG, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 49.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 50.Dirnagl U. Metabolic aspects of neurovascular coupling. Adv Exp Med Biol. 1997;413:155–159. doi: 10.1007/978-1-4899-0056-2_17. [DOI] [PubMed] [Google Scholar]
- 51.Kuschinsky W. Neuronal-vascular coupling. A unifying hypothesis. Adv Exp Med Biol. 1997;413:167–176. [PubMed] [Google Scholar]
- 52.Harder DR, et al. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 53.Lou HC, Edvinsson L, MacKenzie ET. The concept of coupling blood flow to brain function: revision required? Ann Neurol. 1987;22:289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- 54.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 55.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 56.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. doi:110.1016/j.neuron.2008.1001.1003. [DOI] [PubMed] [Google Scholar]
- 57.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92:635–640. doi: 10.1113/expphysiol.2006.036376. Epub 2007 Apr 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. doi:310.1016/j.preteyeres.2012.1004.1004. Epub 2012 May 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Investigative ophthalmology & visual science. 1981;21:34–38. [PubMed] [Google Scholar]
- 60.Pournaras CJ, et al. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. doi:210.1016/j.preteyeres.2008.1002.1002. Epub 2008 Feb 1023. [DOI] [PubMed] [Google Scholar]
- 61.Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye (Lond) 1990;4:319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- 62.Shih YY, et al. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr Eye Res. 2013;38:292–298. doi: 10.3109/02713683.2012.756526. doi:210.3109/02713683.02712012.02756526. Epub 02712013 Jan 02713614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lott ME, et al. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol. 2012;90:e434–441. doi: 10.1111/j.1755-3768.2012.02445.x. doi:410.1111/j.1755-3768.2012.02445.x. Epub 02012 Jun 02448. [DOI] [PubMed] [Google Scholar]
- 64.Lott ME, et al. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Ophthalmol. 2013;91:e462–e469. doi: 10.1111/aos.12129. doi:410.1111/aos.12129. Epub 12013 Jun 12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garhofer G, et al. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pemp B, et al. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci. 2009;50:4029–4032. doi: 10.1167/iovs.08-3260. doi:4010.1167/iovs.4008–3260. Epub 2009 Apr 4015. [DOI] [PubMed] [Google Scholar]
- 67.Lasta M, et al. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2013;54:842–847. doi: 10.1167/iovs.12-10873. doi:810.1167/iovs.1112-10873. [DOI] [PubMed] [Google Scholar]
- 68.Dorner GT, et al. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision Res. 2003;43:1495–1500. doi: 10.1016/s0042-6989(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 69.Puro DG. Retinovascular physiology and pathophysiology: New experimental approach/new insights. Prog Retin Eye Res. 2012 doi: 10.1016/j.preteyeres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakaizumi A, Zhang T, Puro DG. The electrotonic architecture of the retinal microvasculature: Diabetes-induced alteration. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsushita K, et al. Diabetes-induced inhibition of voltage-dependent calcium channels in the retinal microvasculature: role of spermine. Investigative ophthalmology & visual science. 2010;51:5979–5990. doi: 10.1167/iovs.10-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishizaki E, Fukumoto M, Puro DG. Functional K(ATP) channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol. 2009;587:2233–2253. doi: 10.1113/jphysiol.2009.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugiyama T, et al. Enhancement of P2X(7)-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Investigative ophthalmology & visual science. 2004;45:1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- 74.Simonsen SE. ERG in Juvenile Diabetics: a prognostic study. In: Goldberg MF, Fine SL, editors. Symposium on the Treatment of Diabetic Retinopathy. US Department of Health, Education and Welfare; Arlington: 1969. pp. 681–689. [Google Scholar]
- 75.Juen S, Kieselbach GF. Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol. 1990;108:372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- 76.Di Leo MA, et al. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia. 1994;37:911–916. doi: 10.1007/BF00400947. [DOI] [PubMed] [Google Scholar]
- 77.Tyrberg M, et al. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc Ophthalmol. 2011;123:193–198. doi: 10.1007/s10633-011-9298-6. doi:110.1007/s10633-10011-19298-10636. Epub 12011 Nov 10636. [DOI] [PubMed] [Google Scholar]
- 78.Harrison WW, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52:772–777. doi: 10.1167/iovs.10-5931. doi:710.1167/iovs.1110-5931. Print 2011 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lecleire-Collet A, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52:2861–2867. doi: 10.1167/iovs.10-5960. doi:2810.1167/iovs.2810-5960. [DOI] [PubMed] [Google Scholar]
- 80.Barber AJ, et al. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. Journal of Clinical Investigation. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieth E, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 82.Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Investigative ophthalmology & visual science. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- 83.Bui BV, et al. Investigating structural and biochemical correlates of ganglion cell dysfunction in streptozotocin-induced diabetic rats. Exp Eye Res. 2009;88:1076–1083. doi: 10.1016/j.exer.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Investigative ophthalmology & visual science. 2004;45:1002–1008. doi: 10.1167/iovs.03-1080. [DOI] [PubMed] [Google Scholar]
- 88.Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia. 2010;58:1996–2004. doi: 10.1002/glia.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiter CEN, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- 90.Rajala RV, et al. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Investigative ophthalmology & visual science. 2009;50:1033–1040. doi: 10.1167/iovs.08-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Shabrawey M, et al. Role of NADPH Oxidase in Retinal Vascular Inflammation. Investigative ophthalmology & visual science. 2008 doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J, et al. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fort PE, et al. Differential roles of hyperglycemia and hypoinsulinemia in diabetes induced retinal cell death: evidence for retinal insulin resistance. PLoS One. 2011;6:e26498. doi: 10.1371/journal.pone.0026498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. doi:1510.1016/j.cellsig.2011.1505.1004. Epub 2011 May 1517. [DOI] [PubMed] [Google Scholar]
- 96.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. Epub 2006 Aug 2017. [DOI] [PubMed] [Google Scholar]
- 97.Hammes HP, Federoff HJ, rownlee MB. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- 98.Martin PM, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Investigative ophthalmology & visual science. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 99.McVicar CM, et al. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60:2995–3005. doi: 10.2337/db11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barber AJ, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investigative ophthalmology & visual science. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 101.Abu El-Asrar AM, et al. Expression of apoptosis markers in retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45:2760–2766. doi: 10.1167/iovs.03-1392. [DOI] [PubMed] [Google Scholar]
- 102.Kerrigan LA, et al. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Archives of Ophthalmology. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 103.Kern TS, Tang J, Berkowitz BA. Validation of structural and functional lesions of diabetic retinopathy in mice. Mol Vis. 2010;16:2121–2131. [PMC free article] [PubMed] [Google Scholar]
- 104.Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes-Metabolism Reviews. 1995;11:109–120. doi: 10.1002/dmr.5610110203. [DOI] [PubMed] [Google Scholar]
- 105.Howell SJ, et al. Degeneration of retinal ganglion cells in diabetic dogs and mice: relationship to glycemic control and retinal capillary degeneration. Mol Vis. 2013;19:1413–1421. [PMC free article] [PubMed] [Google Scholar]
- 106.Tang J, et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Investigative ophthalmology & visual science. 2013;54:3681–3690. doi: 10.1167/iovs.12-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaucher D, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47:612–623. doi: 10.1016/j.visres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 108.Gastinger MJ, et al. Abnormal centrifugal axons in streptozotocin-diabetic rat retinas. Investigative ophthalmology & visual science. 2001;42:2679–2685. [PMC free article] [PubMed] [Google Scholar]
- 109.Gastinger MJ, et al. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Investigative ophthalmology & visual science. 2008;49:2635–2642. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- 110.Wolter JR. Diabetic retinopathy. Am J Ophthalmol. 1961;51:1123–1139. doi: 10.1016/0002-9394(61)91802-5. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, et al. Alterations in retrograde axonal transport in optic nerve of type I and type II diabetic rats. Kobe J Med Sci. 1998;44:205–215. [PubMed] [Google Scholar]
- 112.Fernandez DC, et al. Early distal axonopathy of the visual pathway in experimental diabetes. Am J Pathol. 2012;180:303–313. doi: 10.1016/j.ajpath.2011.09.018. doi:310.1016/j.ajpath.2011.1009.1018. Epub 2011 Nov 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. The Journal of cell biology. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nickells RW, et al. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annual review of neuroscience. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- 115.Anonymous. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singleton JR, Smith AG. Neuropathy associated with prediabetes: what is new in 2007? Curr Diab Rep. 2007;7:420–424. doi: 10.1007/s11892-007-0070-y. [DOI] [PubMed] [Google Scholar]
- 117.Antonetti DA, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 118.Losiewicz MK, Fort PE. Diabetes Impairs the Neuroprotective Properties of Retinal Alpha-crystallins. Investigative ophthalmology & visual science. 2011;52:5034–5042. doi: 10.1167/iovs.10-6931. [DOI] [PubMed] [Google Scholar]
- 119.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 120.Ruta LM, et al. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:387–398. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 121.Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Current diabetes reports. 2010;10:380–384. doi: 10.1007/s11892-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 122.Reiter CE, Gardner TW. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res. 2003;22:545–562. doi: 10.1016/s1350-9462(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 123.Barber AJ, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. Epub 32001 Jul 32816. [DOI] [PubMed] [Google Scholar]
- 124.Xu Y, et al. Energy sources for glutamate neurotransmission in the retina: absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J Neurochem. 2007;101:120–131. doi: 10.1111/j.1471-4159.2006.04349.x. [DOI] [PubMed] [Google Scholar]
- 125.Reiter CE, et al. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab. 2003;285:E763–774. doi: 10.1152/ajpendo.00507.2002. Epub 2003 Jun 2010. [DOI] [PubMed] [Google Scholar]
- 126.Rajala RV, Anderson RE. Light regulation of the insulin receptor in the retina. Mol Neurobiol. 2003;28:123–138. doi: 10.1385/MN:28:2:123. [DOI] [PubMed] [Google Scholar]
- 127.Rajala A, et al. Light activation of the insulin receptor regulates mitochondrial hexokinase. A possible mechanism of retinal neuroprotection. Mitochondrion. 2013;13:566–576. doi: 10.1016/j.mito.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rajala A, et al. Insulin receptor signaling in cones. The Journal of biological chemistry. 2013;288:19503–19515. doi: 10.1074/jbc.M113.469064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta VK, Rajala A, Rajala RV. Insulin receptor regulates photoreceptor CNG channel activity. American journal of physiology. Endocrinology and metabolism. 2012;303:E1363–1372. doi: 10.1152/ajpendo.00199.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li G, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rajala A, et al. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. The Journal of biological chemistry. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ali TK, et al. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57:889–898. doi: 10.2337/db07-1669. doi:810.2337/db2307-1669. Epub 2008 Feb 2319. [DOI] [PubMed] [Google Scholar]
- 133.El-Remessy AB, et al. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali TK, et al. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657–668. doi: 10.1007/s00125-010-1935-1. doi:610.1007/s00125-00010-01935-00121. Epub 02010 Oct 00119. [DOI] [PubMed] [Google Scholar]
- 135.El-Remessy AB, et al. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–252. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- 136.Zhou J, Huang K. Peroxynitrite mediates muscle insulin resistance in mice via nitration of IRbeta/IRS-1 and Akt. Toxicol Appl Pharmacol. 2009;241:101–110. doi: 10.1016/j.taap.2009.08.005. doi:110.1016/j.taap.2009.1008.1005. Epub 2009 Aug 1012. [DOI] [PubMed] [Google Scholar]
- 137.Duplain H, et al. Stimulation of peroxynitrite catalysis improves insulin sensitivity in high fat diet-fed mice. J Physiol. 2008;586:4011–4016. doi: 10.1113/jphysiol.2008.154302. doi:4010.1113/jphysiol.2008.154302. Epub 152008 Jun 154326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu XM, Tompkins RG, Fischman AJ. Nitric oxide activates intradomain disulfide bond formation in the kinase loop of Akt1/PKBalpha after burn injury. Int J Mol Med. 2013;31:740–750. doi: 10.3892/ijmm.2013.1241. doi:710.3892/ijmm.2013.1241. Epub 2013 Jan 3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yasukawa T, et al. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. Epub 2005 Jan 7514. [DOI] [PubMed] [Google Scholar]
- 140.Wu M, et al. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS One. 2009;4:e6430. doi: 10.1371/journal.pone.0006430. doi:6410.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Numajiri N, et al. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc Natl Acad Sci U S A. 2011;108:10349–10354. doi: 10.1073/pnas.1103503108. doi:10310.11073/pnas.1103503108. Epub 1103502011 Jun 1103503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaneki M, et al. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 143.Carvalho-Filho MA, et al. Aspirin attenuates insulin resistance in muscle of diet-induced obese rats by inhibiting inducible nitric oxide synthase production and S-nitrosylation of IRbeta/IRS-1 and Akt. Diabetologia. 2009;52:2425–2434. doi: 10.1007/s00125-009-1498-1. doi:2410.1007/s00125-00009-01498-00121. [DOI] [PubMed] [Google Scholar]
- 144.Tang Z, et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: implication for Alzheimer disease. The Journal of biological chemistry. 2013;288:15556–15570. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- 146.Gulati P, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dennis MD, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287–8296. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Q, et al. Development of ATP-competitive mTOR inhibitors. Methods Mol Biol. 2012;821:447–460. doi: 10.1007/978-1-61779-430-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koike-Kumagai M, et al. The target of rapamycin complex 2 controls dendritic tiling of Drosophila sensory neurons through the Tricornered kinase signalling pathway. Embo J. 2009;28:3879–3892. doi: 10.1038/emboj.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang W, et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci. 2013;16:441–448. doi: 10.1038/nn.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carson RP, et al. Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Hum Mol Genet. 2013;22:140–152. doi: 10.1093/hmg/dds414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Urbanska M, et al. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem. 2012;287:30240–30256. doi: 10.1074/jbc.M112.374405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal. 2009;2:pe51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- 154.Nixon RA. Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends in neurosciences. 2006;29:528–535. doi: 10.1016/j.tins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 155.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21:387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fox TE, et al. Diabetes diminishes phosphatidic acid in the retina: A putative mediator for reduced mTOR signaling and increased neuronal cell death. Investigative ophthalmology & visual science. 2012 doi: 10.1167/iovs.11-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meyer-Rusenberg B, et al. Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:1009–1018. doi: 10.1007/s00417-006-0489-x. [DOI] [PubMed] [Google Scholar]
- 158.Chee CK, Flanagan DW. Visual field loss with capillary non-perfusion in preproliferative and early proliferative diabetic retinopathy. Br J Ophthalmol. 1993;77:726–730. doi: 10.1136/bjo.77.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frost-Larsen K, Larsen HW, Simonsen SE. The value of dark-adaptation as a prognostic tool in diabetic retinopathy. Metab Pediatr Ophthalmol. 1981;5:39–44. [PubMed] [Google Scholar]
- 160.Greenstein VC, et al. Effects of early diabetic retinopathy on rod system sensitivity. Optom Vis Sci. 1993;70:18–23. doi: 10.1097/00006324-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 161.Anonymous. Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:834–840. [PubMed] [Google Scholar]
- 162.Grunwald JE, et al. Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology. 1984;91:1447–1452. doi: 10.1016/s0161-6420(84)34124-0. [DOI] [PubMed] [Google Scholar]
- 163.Das A, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Investigative ophthalmology & visual science. 1999;40:809–813. [PubMed] [Google Scholar]
- 164.Guidry C, King JL, Mason JO., 3rd Fibrocontractive Muller cell phenotypes in proliferative diabetic retinopathy. Investigative ophthalmology & visual science. 2009;50:1929–1939. doi: 10.1167/iovs.08-2475. [DOI] [PubMed] [Google Scholar]
- 165.Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77:731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bengtsson B, Heijl A, Agardh E. Visual fields correlate better than visual acuity to severity of diabetic retinopathy. Diabetologia. 2005;48:2494–2500. doi: 10.1007/s00125-005-0001-x. [DOI] [PubMed] [Google Scholar]
- 167.Hellgren KJ, Bengtsson B, Agardh E. Functional and structural change in diabetic eyes. Interim results from an ongoing longitudinal prospective study. Acta ophthalmologica. 2012 doi: 10.1111/j.1755-3768.2012.02508.x. [DOI] [PubMed] [Google Scholar]
- 168.Grunwald JE, et al. Strict metabolic control and retinal blood flow in diabetes mellitus. Br J Ophthalmol. 1994;78:598–604. doi: 10.1136/bjo.78.8.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fong DS, Barton FB, Bresnick GH. Impaired color vision associated with diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 15. Am J Ophthalmol. 1999;128:612–617. doi: 10.1016/s0002-9394(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 170.Bresnick GH, et al. Association of hue discrimination loss and diabetic retinopathy. Arch Ophthalmol. 1985;103:1317–1324. doi: 10.1001/archopht.1985.01050090069034. [DOI] [PubMed] [Google Scholar]
- 171.Wessel MM, et al. Ultra-Wide-Field Angiography Improves the Detection and Classification of Diabetic Retinopathy. Retina. 2011 doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 172.van Guilder HD, et al. Diabetes downregulates presynaptic proteins and reduces basal synapsin 1 phosphorylation in rat retina. Eur J Neurosci. 2008;28:1–11. doi: 10.1111/j.1460-9568.2008.06322.x. [DOI] [PubMed] [Google Scholar]
- 173.Abu El-Asrar AM, et al. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye. 2007;21:238–245. doi: 10.1038/sj.eye.6702225. [DOI] [PubMed] [Google Scholar]
- 174.Smith SB, et al. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Investigative ophthalmology & visual science. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Donato LR. La retinite diabetica. Ann di ottal e Clinica Oculista di Roma. 1927;55:222–254. [Google Scholar]
- 176.Bloodworth JMB. Diabetic retinopathy. Diabetes. 1962;2:1–22. [PubMed] [Google Scholar]
- 177.Chihara E, et al. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology. 1993;100:1147–1151. doi: 10.1016/s0161-6420(93)31513-7. [DOI] [PubMed] [Google Scholar]
- 178.Takahashi H, et al. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142:88–94. doi: 10.1016/j.ajo.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 179.van Dijk HW, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Investigative ophthalmology & visual science. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ozdek S, et al. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye. 2002;16:761–765. doi: 10.1038/sj.eye.6700207. [DOI] [PubMed] [Google Scholar]
- 181.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 182.Gerhardinger C, et al. Expression of acute-phase response proteins in retinal muller cells in diabetes. Investigative ophthalmology & visual science. 2005;46:349–357. doi: 10.1167/iovs.04-0860. [DOI] [PubMed] [Google Scholar]
- 183.Antonetti DA, VanGuilder HD, Lin CM. Vascular Permeability in Diabetic Retinopathy. In: Duh E, editor. Diabetic Retinopathy. Humana Press; Totowa, NJ: 2008. pp. 333–352. [Google Scholar]
- 184.Kuiper EJ, et al. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol. 2004;88:1082–1087. doi: 10.1136/bjo.2003.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kuiper EJ, et al. Connective tissue growth factor is necessary for retinal capillary basal lamina thickening in diabetic mice. J Histochem Cytochem. 2008;56:785–792. doi: 10.1369/jhc.2008.950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bek T. Glial cell involvement in vascular occlusion of diabetic retinopathy. Acta Ophthalmologica Scandinavica. 1997;75:239–243. doi: 10.1111/j.1600-0420.1997.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 187.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 188.Chihara E. Impairment of protein synthesis in the retinal tissue in diabetic rabbits: secondary reduction of fast axonal transport. Journal of Neurochemistry. 1981;37:247–250. doi: 10.1111/j.1471-4159.1981.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 189.Tikhonenko M, et al. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59:219–227. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fox TE, et al. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–3580. doi: 10.2337/db06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fort PE, et al. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Mol Cell Proteomics. 2009;8:767–779. doi: 10.1074/mcp.M800326-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lieth E, et al. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Experimental Eye Research. 2000;70:723–730. doi: 10.1006/exer.2000.0840. [DOI] [PubMed] [Google Scholar]
- 193.Gowda K, Zinnanti WJ, Lanoue KF. The Influence of Diabetes on Glutamate Metabolism in Retinas. Journal of neurochemistry. 2011 doi: 10.1111/j.1471-4159.2011.07206.x. [DOI] [PubMed] [Google Scholar]
- 194.Frayser R, Buse MG. Branched chain amino acid metabolism in the retina of diabetic rats. Diabetologia. 1978;14:171–176. doi: 10.1007/BF00429777. [DOI] [PubMed] [Google Scholar]