Abstract
OBJECTIVE
To compare the efficacy of intermittent androgen deprivation therapy (IADT) vs continuous androgen deprivation therapy (CADT) for the treatment of advanced prostate cancer; we performed a meta-analysis of randomized controlled trials (RCTs), assessing the risks of disease progression, all-cause, and disease-specific mortality.
MATERIALS AND METHODS
We conducted a systematic search of several bibliographic systems to identify all RCTs of IADT in men with newly diagnosed metastatic or biochemical only prostate cancer. We abstracted outcome data, study characteristics, and participant demographics. We performed heterogeneity tests and calculated the summarized risk differences (RD) and risk ratios at 95% confidence intervals (CI), using inverse variance methods in random-effects approaches.
RESULTS
We identified 8 RCTs (N = 4664) comparing mortality between IADT and CADT. For all men combined, we observed small but nonsignificant differences in all-cause mortality (RD = 0.02, 95% CI = −0.02, 0.06), disease-specific mortality (RD = 0.04, 95% CI = −0.01, 0.08), and disease progression (RD = −0.03, 95% CI = −0.09, 0.04). Among the prespecified subgroup with histologically confirmed, newly diagnosed metastatic disease, we found no difference in overall survival (RD = 0.00, 95% CI = −0.09, 0.09).
CONCLUSION
We found no difference in overall survival, but a small increased risk in disease-specific survival for men treated with IADT relative to CADT was observed. IADT could be considered as an alternative to CADT because of better quality of life outcome. Patients should be informed of the possible risks and benefits of both therapies. More research confirming the benefits of IADT vs CADT is needed to inform treatment decisions.
Androgen deprivation therapy (ADT) is commonly used to treat advanced prostate cancer (PCa)1,2 in men who are newly diagnosed with metastatic disease or have progressed after treatment for localized disease. ADT consists of permanent surgical castration or periodic medical blockade, using gonadotropin-releasing hormone (GnRH) agonists or antagonists, which are occasionally coupled with anti-androgens. Medically administrated ADT accounts for over 95% of ADT in the United States and can be delivered continuously or intermittently. Although medical ADT is prescribed to treat over 80% of men diagnosed with advanced PCa in the US,3 there is no consensus regarding intermittent vs continuous administration.
Although continuous ADT (CADT) has been the standard in care, intermittent ADT (IADT) has been proposed since the 1990s as an alternative option because of promising preclinical findings that IADT can delay disease progression to androgen-independent PCa by reviving the apoptotic potential of prostate tumor cells.4,5 Additionally, IADT is appealing because of potential advantages such as improved sexual function during off-treatment periods, decreased risk of ADT-related adverse events, and reduced direct medical care costs.1,6 IADT has been recently recommended as a first-line hormonal therapy for advanced PCa by the European Association of Urology7 and the United Kingdom National Institute for Health and Clinical Excellence.8 However, the effect of IADT on long-term survival, compared with CADT, remains uncertain. This might be a main contributor for the lack of explicit endorsement for IADT as the preferred treatment in the US and Canadian guidelines.
It remains inconclusive if IADT results in worse survival outcomes when compared with CADT. Most published randomized controlled trial (RCTs) failed to show differences in survival between IADT and CADT,9–14 yet a recent large trial of 1535 men reported that IADT is associated with worse survival outcomes than CADT in metastatic PCa.15 Additionally, several studies found that IADT has an elevated disease-specific mortality compared with CADT,9,10,15,16 whereas other studies have reported otherwise.11,14 Therefore, we conducted this meta-analysis study combining currently available RCTs data to compare the efficacy of IADT relative to CADT with respect to all-cause and disease-specific mortality.
MATERIALS AND METHODS
We conducted a thorough search of PubMed, EMBASE, Central, and Web of Science databases to find full text clinical trials and conference abstracts published through September 10, 2012. Key words included (“prostate cancer” or “prostate neoplasms”) and (“intermittent hormonal deprivation” or “intermittent androgen deprivation” or “intermittent androgen suppression” or “intermittent hormonal therapy”). As an additional search method, we identified conference proceedings and examined published articles references that met search criteria. We included phase III RCTs, where men had clinically evident metastatic disease or rising prostate-specific antigen (PSA) after radiation or surgery, because ADT is indicated as a primary or salvage therapy in each respective situation. We excluded studies that did not compare IADT with CADT, and omitted reviews, editorials, guidelines, or phase-I and phase-II trials. For studies published as conference abstracts, we attempted to contact authors of the abstracts to obtain current and full results. We also contacted corresponding authors of all articles that did not include data from variables pertinent to this analysis. We made at least 3 follow-up attempts for queries sent.
Two independent reviewers performed data abstraction of each study’s characteristics. Characteristics included patients’ diagnosis (metastatic, recurrent, mixed), age, ADT formulation (GnRH-only, GnRH with anti-androgens), IADT criteria (based on PSA level, testosterone level, or calendar time), and length of follow-up. Outcome data pertaining to both treatment groups included the numbers of participants, all-cause death, and disease-specific death. The authors also abstracted data on the proportion of adverse events and patient-reported quality of life (QoL).
We tested for heterogeneity of the published study findings using the Cochran’s Q statistic and I2 statistics. We used the Q statistics to determine true heterogeneity, defined by a P-value less than .1. We used the I2 statistic to indicate the percentage of variance explained by variation between studies17 and to quantify the extent of heterogeneity, with 25% as the threshold for low heterogeneity, 50% for moderate heterogeneity, and 75% for high heterogeneity. We performed and reported random-effect modeling, using the inverse variance method to control for heterogeneity between studies. The summarized estimates on risk differences (RD) and risk ratios (RR) were considered statistically significant if their 95% confidence intervals (CI) did not include zero and unity, respectively.
Because most RCTs concerned with advanced PCa did not separately report mortality rates for patients with metastasis vs recurrent disease after treatment for localized PCa at enrollment, our main analyses included both types of patients from all trials. However, we also conducted a subgroup analysis among men in 4 studies, which only enrolled men with histologically confirmed metastasis. All analyses were performed using the METABIN function in the package META in R for Windows version 2.14.2.
RESULTS
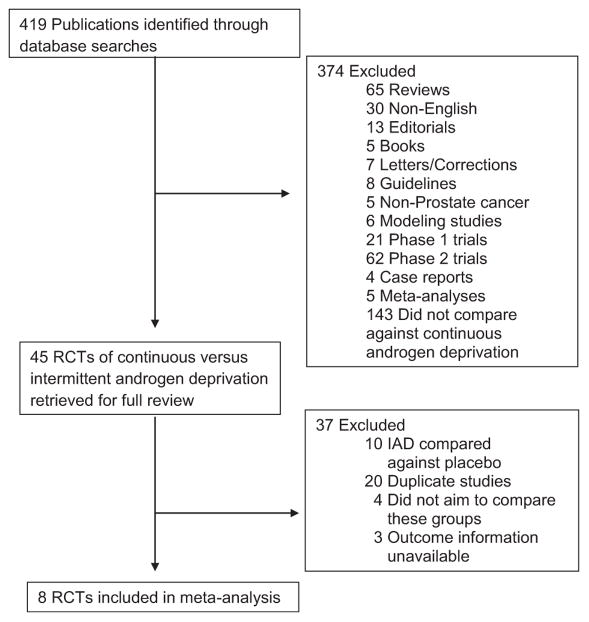
Initially, we identified 419 publications potentially relevant to our analysis. On further review, we identified 12 phase-III RCTs for extensive review. The search process is described in Figure 1. We excluded trials that did not assess mortality outcomes18,19 or provide count data on outcomes of interest after attempted contacts.20,21 We included 4664 men with advanced PCa from 8 trials in this meta-analysis. Table 1 summarizes the study characteristics of these trials.
Figure 1.
Flow chart of study selection.
Table 1.
Characteristics of 8 randomized controlled trials of intermittent vs continuous androgen deprivation therapy in metastatic or recurrent prostate cancer patients
| Authors | Patients’ Diagnosis | Total Sample Size | Median Age | ADT Regimen | IADT Definition | Median Follow-up (y) |
|---|---|---|---|---|---|---|
| de Leval et al (2002) | Mixed | 68 | 72 | GnRH + antiandrogen | PSA level | 2.4 |
| Irani et al (2008) | Mixed | 129 | 73 | GnRH + antiandrogen | 6-month block | 3.6 |
| Calais da Silva et al (2009) | Mixed | 626 | 73 | GnRH + antiandrogen | PSA level | 4.3 |
| Langenhuijsen et al (2011) | Metastatic | 193 | 68 | GnRH + antiandrogen | PSA level | 2.6 |
| Salonen et al (2012) | Mixed | 554 | 72 | GnRH only | PSA level | 5.4 |
| Mottet et al (2012) | Metastatic | 173 | 69 | GnRH + antiandrogen | PSA level | 3.7 |
| Hussain et al (2012)* | Metastatic | 1535 | – | GnRH + antiandrogen | PSA level | 9.2 |
| Crook et al (2012) | Recurrent | 1386 | 74 | GnRH only | PSA level | 6.9 |
ADT, androgen deprivation therapy; GnRH, gonadotropin-releasing hormone; IADT, intermittent androgen deprivation therapy; PSA, prostate-specific antigen.
Published in an abstract form.
Seven of 8 trials with 4586 men reported all-cause mortality as an end point. The average median follow-up in these 8 studies is 4.8 years. The estimates of all-cause mortality were homogenous across published trials (heterogeneity test, P-value = .13 and .15; and I2 statistics = 39% and 37% for RD and RR, respectively). Under the random-effect modeling, we observed a 2% increased mortality in men receiving IADT compared with those receiving CADT. This elevated risk had a 95% CI that included 0 on the RD scale or 1 on RR scale (P >.05). (Fig. 2A1: summarized RD = 0.02, 95% CI = −0.02, 0.06; RR = 1.03, 95% CI = 0.96, 1.11). When we restricted the subgroup analysis to 4 trials with men having histologically confirmed metastatic PCa (n = 2168), we did not find a significant difference in all-cause mortality (Fig. 2A2: RD = 0.00, 95% CI = −0.09, 0.09, RR = 1.00, 95% CI = 0.87, 1.17).
Figure 2.
Risk difference and risk ratio for comparative efficacy (all-cause and disease-specific mortality, disease progression) and risk of hot flushes between intermittent and continuous androgen deprivation therapy (IADT vs CADT) in 4664 men with advanced prostate cancer enrolled in 8 clinical trials. Studies are listed in an ascending order of median follow-up duration.
Six clinical trials with 4292 men reported PCa-specific death. The PCa-specific deaths were heterogenous across studies (heterogeneity test, P-value = .04 and .05; and I2 statistics = 57% and 56% for RD and RR, respectively). The summarized estimates of 6 published RCTs found a small but not statistically significant increase of disease-specific mortality in men receiving IADT vs CADT (Fig. 2B: summarized RD = 0.04, 95% CI = −0.01, 0.08; RR = 1.15, 95% CI = 0.97, 1.36).
Five trials including 2803 men with advanced PCa reported progression events. There was no difference in risk of progression by treatment group (Fig. 2C summarized RD = −0.03, 95% CI = −0.09, 0.04; RR = 0.97, 95% CI = 0.97, 95% CI = 0.86, 1.10).
Few studies provided count data on the occurrence of adverse event cases. In 3 trials of 954 men with advanced PCa, the IADT group had a 9% lower risk of hot flashes compared with the CADT group (Fig. 2D summarized RD = −0.09, 95% CI = −0.15, −0.03; RR = 0.82, 95% CI = 0.67, 1.01). Because we were unable to obtain original data after repeated attempts to contact authors, we do not provide quantitative effects in terms of adverse events or QoL. Therefore, we summarized study findings with respect to these outcomes in Table 2. Most studies concluded that IADT significantly reduced treatment-related side effects, such as hot flashes and headaches. Most clinical trials used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 instrument to assess QoL associated with IADT compared with CADT, but few studies reported the raw scores in IADT and CADT groups. Several studies concluded that IADT had a better QoL outcome in hot flashes and sexual function,9–11,19,20 although some studies found no clinically significant difference.12,16
Table 2.
Published trials about risk of adverse events and comparative quality of life between intermittent and continuous androgen deprivation therapy (ADT vs CADT)
| Author* | Adverse Events Assessed | Findings |
|---|---|---|
| de Leval et al (2002) | Information unavailable | Two of 33 CADT-treated patients and 1 of 35 IADT-treated patient experienced severe gastrointestinal tract-related adverse events. No unexpected events were recorded. |
| Calais da Silva et al (2009) | Sexual function, hot flushes, gynecomastia, headache, skin complaints, and so forth | Side effects were more pronounced among CADT patients, including hot flushes (20% vs 30%, P <.01), gynecomastia (12% vs 20%, P = .02), and skin complaints (3% vs 7%, P = .03) but not headache (7% vs 12%, P = .06). |
| Miller et al (2007)* | Toxicity | No differences in the incidence of patients with adverse events or in any other safety parameter between groups. |
| Langenhuijsen et al (2011) | Hot flushes, visual disturbances, nausea, constipation, dyspnea, erectile dysfunction, depression, liver enzyme increase, anemia, gynecomastia, alcohol intolerance | Most patients experienced 1 or more concurrent adverse side effects during treatment (95% and 91% in CADT and IADT, respectively). CADT group had more side effects including hot flushes (59% vs 50%), nausea (20% vs 11%), constipation (17% vs 7%), dyspnea (12% vs 6%), and depression (11% vs 6%). |
| Mottet et al (2012) | Headache, hot flushes, vital sign (heart rate, blood pressure and weight). | IADT had significantly fewer treatment-related adverse events than CADT (84% vs 94%, P = .042). Compared with CADT, IADT group had significantly lower incidence of headache (47% vs 32%) and hot flushes (64% vs 60%). |
| Hussain et al (2012)* | Information unavailable | Grade 3 or 4 adverse events occurred in 30% and 33% of IADT-treated and CADT-treated patients, respectively. |
| Author* | QoL Measurement | Findings |
|---|---|---|
| de Leval et al (2002) | Not specified | Most patients reported ADT-related side effects, such as hot flashes, loss of libido and erectile dysfunction, which were resolved in most IADT-treated patients during off-treatment period. Overall, IADT-treated group had a better QoL outcome compared with CADT-treated group. |
| Calais da Silva et al (2009) | QLQ-C30 | This study concluded IADT and CADT do not have a clinically meaningful difference with respect to QoL. CADT-treated group showed better scores for the emotional domain (P = .01) and less severe nausea, vomiting, and insomnia, compared with IADT-treated group. Men treated with intermittent therapy reported better sexual function during off-treatment periods and at 15-month follow-up (28% vs 10% in IADT and CADT, respectively). |
| Miller et al (2007)* | Not specified | Patient self-reported overall health and sexual activity was favorable for IADT compared with CADT. Eighty eight percent IADT experienced more than 50% of the number of treatment days as treatment-free days. Off-treatment periods are >40% and attribute to improved QoL. |
| Verhagen et al (2008)* | QLQ-C30 | Physical and emotional function significantly better in the IADT (P <.05). No observed difference between IADT and CADT for role and social function. Cognitive function significantly reduced in IADT 87% to baseline, 69% (P <.05). |
| Irani et al (2008) | QLQ-C30 | No significant differences in QoL score between CADT and IADT groups. Possibly because of insufficient recovery of testosterone level from a fixed 6-month suspension in the IADT group. |
| Langenhuijsen et al (2011) | QLQ-C30 | No clinically significant differences in QoL score between CADT and IADT groups. |
| Mottet et al (2012) | QLQ-C30 | Moderate differences observed in QoL and no significant difference in functional and symptom scales. |
| Crook et al (2012) | QLQ-C30 | IADT-treated group showed a better QoL score for hot flashes (P <.001), libido (P <.001), and urinary symptom (P = .006). Both groups were not found to be significantly different with respect to functional domains (physical, role, and global health). |
CADT, continuous androgen deprivation therapy; QLQ-C30, quality of life questionnaire-core 30; QoL, quality of life.
Published in an abstract form.
COMMENTS
After combining results from 8 RCTs, we observed a small but nonsignificant trend of increased mortality in men treated with IADT than CADT. We did not find statistically significant differences in disease progression delay between IADT and CADT. Additionally, it appeared that men receiving IADT experienced significant reductions in hot flashes compared with those receiving CADT. Our findings suggested that IADT should still be considered as an alternative therapeutic approach to CADT but with caveat and cautions.
The present study focused on the relative efficacy of IADT because its uncertainty might be one of the paramount factors hindering the adoption of IADT in the US clinical practice guidelines. The slight increase in all-cause mortality in IADT recipients might partly be because of a higher percentage of PCa deaths experienced by this group: we observed a 4% higher disease-specific mortality in the IADT group in this meta-analysis. We also observed a small but nonsignificant reduction in risk of disease progression for IADT vs CADT-treated men, which might be because of a combination of biologic reasons (eg resistance to ADT) or possibly because of inadequate power. These results indicated that IADT might slightly decrease survival when compared with CADT, although the differences might not be of clinical significance.
Two recently published large noninferiority trials reached different conclusions regarding survival. A recently published trial of 1386 men with rising PSA levels after radiotherapy reported a similar survival time between men receiving IADT vs those receiving CADT (8.8 vs 9.1 years for IADT and CADT, respectively, hazard ratio for death = 1.02, 95% CI = 0.86, 1.21; 7-year PCa mortality: 18% vs 15% for IADT and CADT, respectively, P = .24).10 This trial is consistent with our conclusion. However, in another recent RCT of 1535 men with metastatic PCa, IADT had a slightly shorter overall survival (5.1 vs 5.8 years) and a 9% increase in the risk of all-cause mortality (RR = 1.09. 95% CI = 0.95–1.24) when compared with CADT.15 Thus, the population of inference is not the same as in the first trial. Additionally, this trial set 20% as the noninferiority margin and based on that, reported that IADT cannot be considered equivalent to CADT and thus concluded that CADT should remain the standard treatment for metastatic PCa patients.
Findings from these 2 important trials suggest that the comparative efficacy of IADT and CADT might be dependent on the indication for treatment. Whether the treatment was initiated at documented metastases15 or during biochemical failure only10 (with no evidence of metastatic disease) might have the potential to influence outcomes. However, most of the earlier published trials comparing CADT with IADT, enrolled advanced PCa participants, including those with newly diagnosed metastasis and those with biochemical failure. Because these studies did not separate results by indications for treatment, we were unable to separate the 2 groups. Therefore, we reported our summarized effect estimates for men with advanced PCa. Additionally, we conducted a subgroup analysis in 4 trials of 2169 men with histologically confirmed metastasis (Fig. 2A2) and observed no difference in all-cause mortality between those who received IADT and CADT. Future studies that calculate the risks and benefits of IADT vs CADT should consider stratifying enrollment by indication for treatment (documented metastases vs none) and also measuring and reporting other variables such as PSA doubling time. The stratification would better reflect participants’ disease status at enrollment and help optimize ADT to different stages of advanced PCa.
Independent of its effect on survival, IADT might be advantageous relative to CADT in reducing ADT-related adverse effects and improving patients’ QoL, particularly during off-treatment periods. Limited studies have failed to show differences in generic QoL domains12,13; however, few studies have reported the number of ADT-related symptoms, such as hot flashes, gynocomastia, and impaired sexual function.9,10,18–20 Owing to the reversibility of testosterone levels after ADT suspension or termination coupled with the fact that IADT uses fewer cycles of ADT treatments than conventional CADT, it is plausible that IADT might be associated with fewer ADT-related symptoms. Based on data provided in 3 of 8 trials, we found IADT significantly reduced hot flashes by 9% compared with CADT.
IADT might also have a greater advantage over CADT in terms of serious adverse events among patients aged 75 or older or with comorbidities. Several observational studies have shown that the use of ADT increases the risk of cardiac dysfunction, diabetes, and major bone fractures because of the alteration of the endocrine system.22–26 These results have led the Food and Drug Administration to revise drug labels to note the potential harm of long-term ADT. A large trial with 1386 men receiving salvage ADT after radiation treatment did not find any difference in the risk of myocardial events or bone fractures.10 This null finding might be partly explained by low statistical power given the infrequency of these events. Over 60% of patients with advanced PCa are aged 75 or older, an age group with a high prevalence of cardiovascular risk factors and comorbidities. Therefore, IADT might have a more favorable risk-benefit ratio in this subgroup relative to CADT. Larger studies that include clinical and patient-reported outcomes would permit analysis of variations in risk-benefit ratios across clinically relevant subgroups to better inform clinical practice.
There are 2 major limitations of our study. First, most trials were conducted in patients with advanced PCa and did not distinguish between patients who received treatment for histologically confirmed metastases (primary therapy) or for disease relapse with rising PSA level (salvage therapy). Second, our results might have limited generalizability to patients in general practice owing to patient selection that often occurs in enrollment of randomized clinical trials. Studies using larger, more generalizable population-based cohorts are needed to complement results from published RCTs by enabling more subgroup analysis of more typical patients treated in general clinical practice.
CONCLUSION
The possible inferior survival associated with IADT has likely limited the widespread adoption of IADT in the US clinical practice. Our results suggest that IADT might result in a modest increase in disease-specific death but significant reduction in hot flashes events compared with CADT. Our findings support IADT as a reasonable option particularly for men who wish to avoid hot flashes despite the small increase in the risk of poorer survival. Our data might assist physicians and patients in making more informed clinical decisions regarding the type of ADT to be used for the treatment of advanced PCa.
Acknowledgments
The authors thanked to Drs. Verhagen, Salonen, Crook, Irani, Klotz, and Miller for providing information regarding their studies. This study was funded in part by grants R01CA142934 and P30CA051008 from the National Cancer Institute.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Ismail M, Ferroni M, Gomella LG. Androgen suppression strategies for prostate cancer: is there an ideal approach? Curr Urol Rep. 2011;12:188–196. doi: 10.1007/s11934-011-0178-0. [DOI] [PubMed] [Google Scholar]
- 2.Youssef E, Tekyi-Mensah S, Hart K, et al. Intermittent androgen deprivation for patients with recurrent/metastatic prostate cancer. Am J Clin Oncol. 2003;26:e119–e123. doi: 10.1097/01.coc.0000091351.09243.15. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2009;29:647–653. doi: 10.1016/j.urolonc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruchovsky N, Rennie PS, Coldman AJ, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–2282. [PubMed] [Google Scholar]
- 5.Sato N, Gleave ME, Bruchovsky N, et al. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumor model. J Steroid Biochem Mol Biol. 1996;58:139–146. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 6.Shore ND, Crawford ED. Intermittent androgen deprivation therapy: redefining the standard of care? Rev Urol. 2010;12:1–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.UK National Institute for Health and Clinical Excellence, in Prostate Cancer. Diagnosis and Treatment. Cardiff, UK: National Collaborating Centre for Cancer; 2008. [Available online at http://www.nice.org.uk/nicemedia/pdf/CG58FullGuideline.pdf; cited October 2012] [Google Scholar]
- 9.Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–171. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 12.Langenhuijsen JF, Badhauser D, Schaaf B, et al. Continuous vs. intermittent androgen deprivation therapy for metastatic prostate cancer [Epub ahead of print] Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Mottet N, Van Damme J, Loulidi S, et al. Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial [Epub ahead of print] BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11120.x. [DOI] [PubMed] [Google Scholar]
- 14.Salonen AJ, Taari K, Ala-Opas M, et al. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol. 2012;187:2074–2081. doi: 10.1016/j.juro.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 15.Hussain M, Tangen CM, Higano CS, et al. Intermittent (IAD) versus continuous androgen deprivation (CAD) in hormone sensitive metastatic prostate cancer (HSM1PC) patients (pts): results of S9346 (INT-0162), an international phase III trail. 2012 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2012;30(suppl 18) (June 20 Supplement_Abstract 4) [Google Scholar]
- 16.Irani J, Celhay O, Hubert J, et al. Continuous versus six months a year maximal androgen blockade in the management of prostate cancer: a randomised study. Eur Urol. 2008;54:382–391. doi: 10.1016/j.eururo.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Calais da Silva FE, Goncalves F, Santos A, et al. Evaluation of quality of life, side effects and duration of therapy in a phase 3 study of intermittent monotherapy versus continuous combined androgen deprivation. Eur Urol. 2008;7:205. (abstract 540) [Google Scholar]
- 19.Verhagen PCMS, Wissenburg LD, Wildhagen MF, et al. Quality of life effects of intermittent and continuous hormonal therapy by cyproterone acetate (CPA) for metastatic prostate cancer. Eur Urol. 2008;7:206. [Google Scholar]
- 20.Miller K, Steiner U, Lingnau A. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer. 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2007;25(suppl 18) (June 20_Supplement_Abstract 5015) [Google Scholar]
- 21.Tunn UW, Canepa G, Hillger H, et al. Intermittent androgen deprivation in patients with PSA-Relapse after radical prostatectomy – final results of a European randomized prospective phase-III clinical trial AUO study AP 06/95, EC 507. J Urol. 2007;177:201. [Google Scholar]
- 22.Alibhai SM, Duong-Hua M, Sutradar R, et al. Impact of androgen deprivation therapy (ADT) on bone, cardiovascular, and endocrine outcomes: a propensity-matched analysis of 20,000 patients. 2008 ASCO Annual Meeting Proceedings (post-meeting edition) J Clin Oncol. 2008;26(suppl 15) (May 20_Supplement_Abstract 5012) [Google Scholar]
- 23.Keating NL, O’Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 26.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]