Abstract
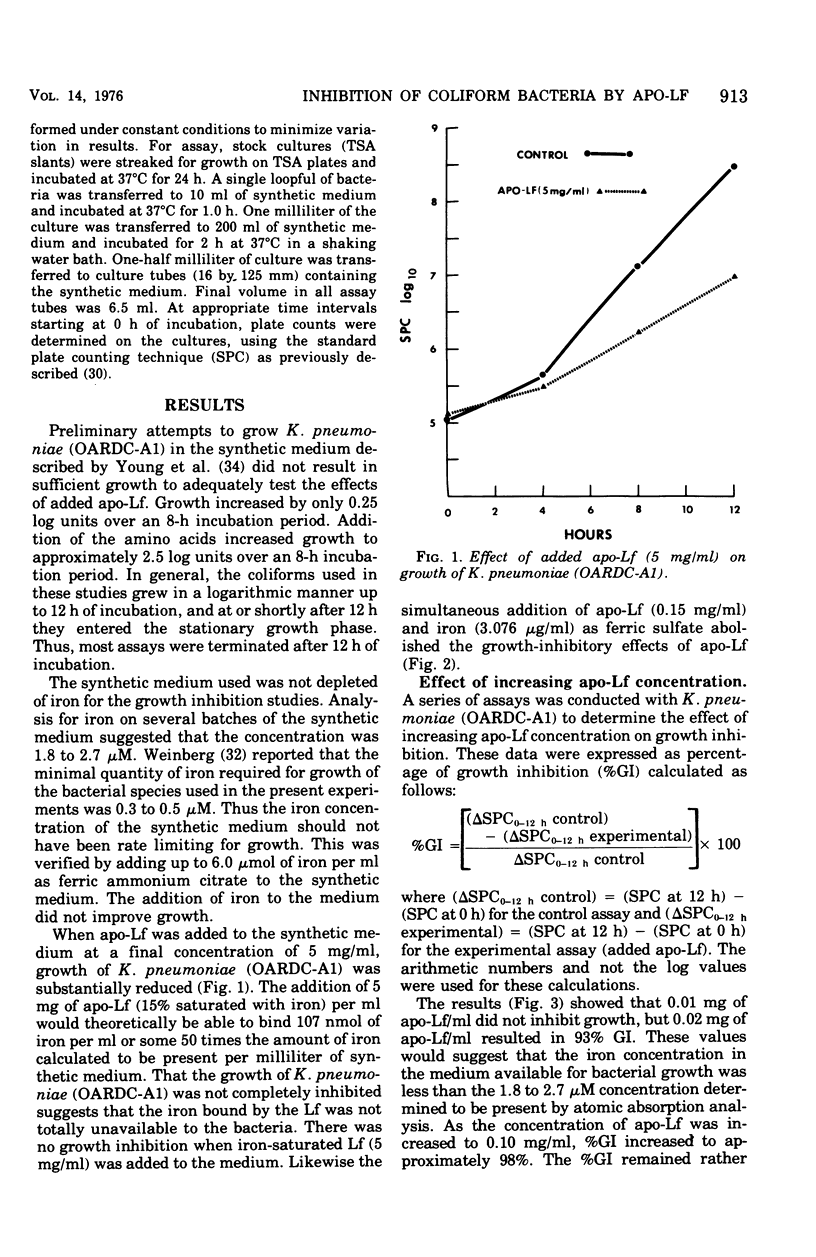
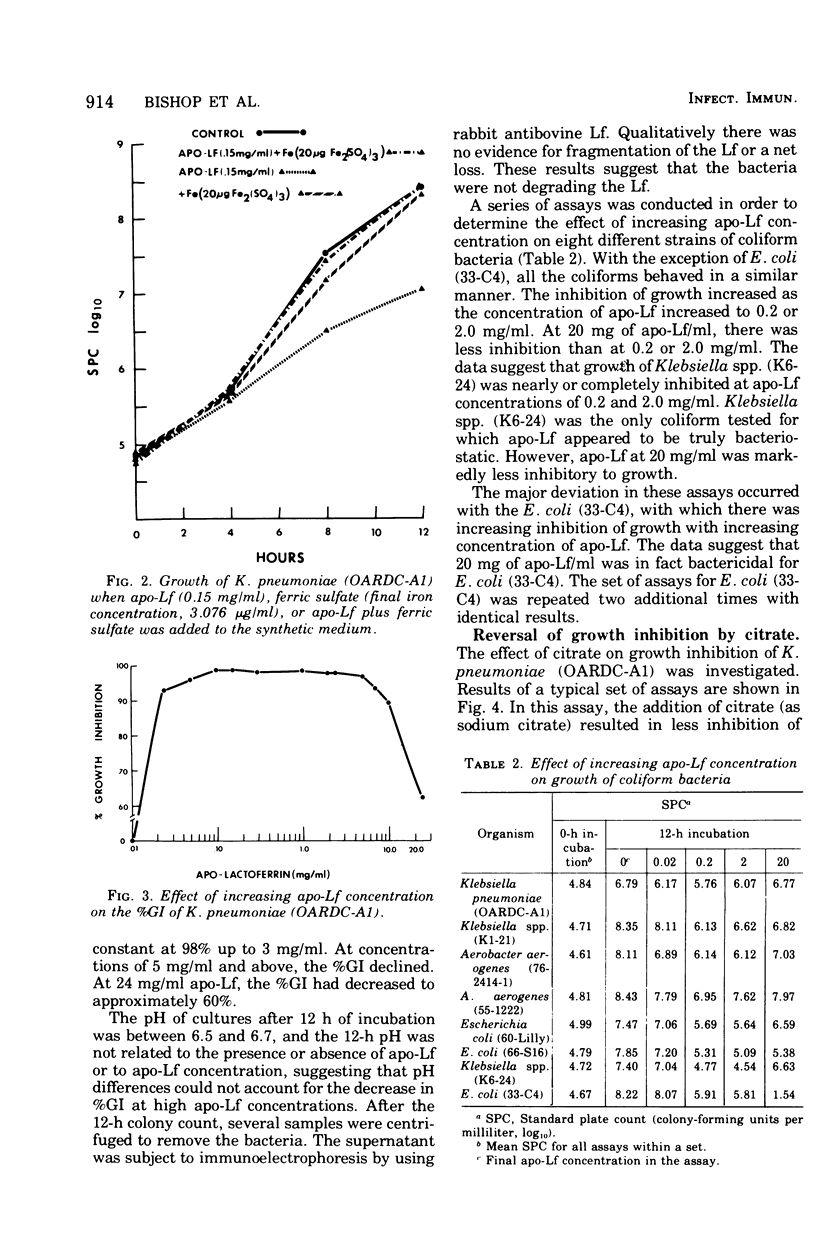
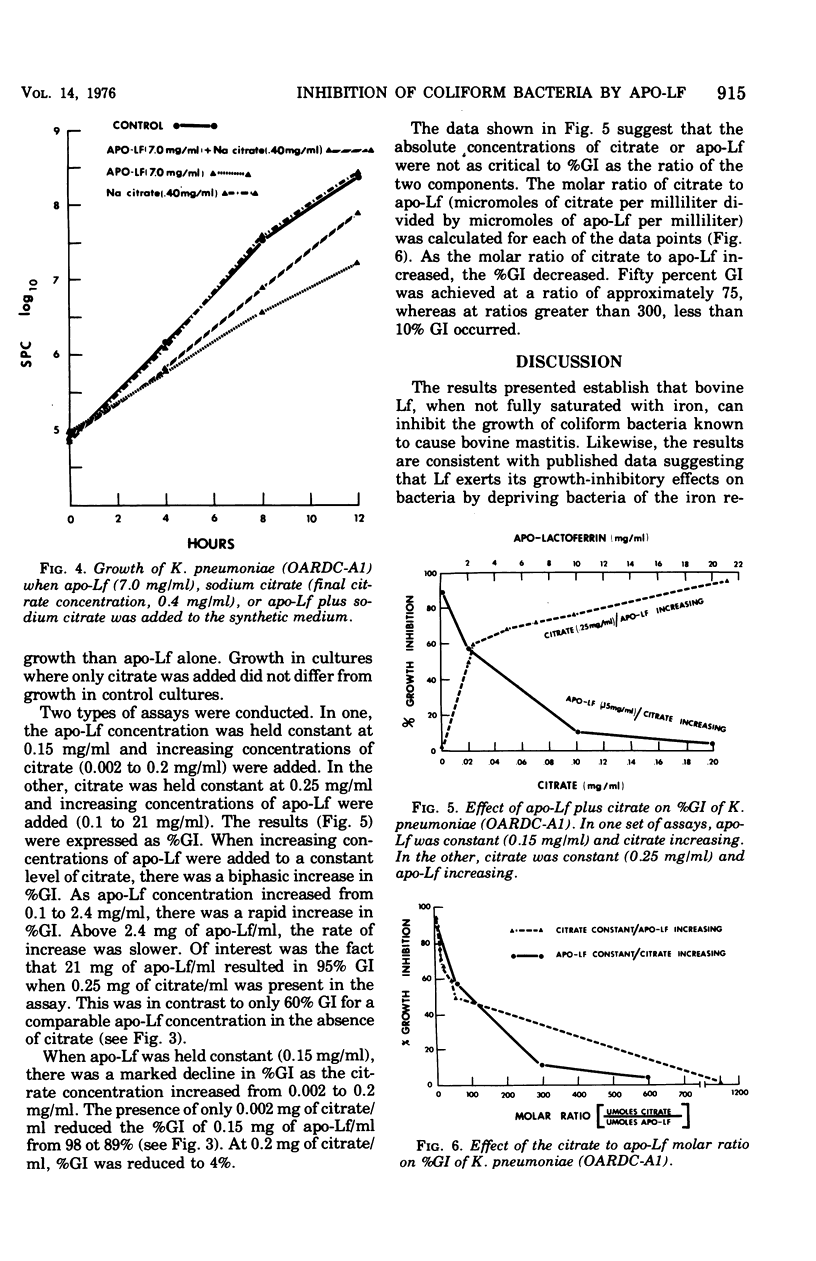
Bovine apo-lactoferrin (apo-Lf) was added to in vitro cultures of eight strains of coliform bacteria associated with bovine mastitis. As little as 0.02 mg of APO-Lf per ml resulted in marked inhibition of growth of all coliforms. Growth inhibition was lost if saturated Lf or iron plus apo-Lf was added to the synthetic medium. The inhibition of growth increased as the concentration of apo-Lf increased from 0.02 to 0.2 mg/ml for Klebsiella pneumoniae (OARDC-A1), Klebsiella spp. (K1-21), and Aerobacter aerogenes (55-12222) and 2 mg/ml for A. aerogenes (76-2414-1), Escherichia coli (60-Lilly), E. coli (66-S16), and Klebsiella spp. (K6-24). As the concentration of apo-Lf was increased above 0.2 or 2 mg/ml, there was less inhibition of growth except for E. coli (33-C4). Apo-Lf at 20 mg/ml was bactericidal for E. coli (33-C4). Results are compatible with the hypothesis that coliform bacteria respond to low-iron environments by production of iron-sequestering agents that complete effectively with apo-Lf for free iron. Addition of apo-Lf plus citrate resulted in loss of growth inhibition. The molar ratio (citrate to apo-Lf) was found to be more important than the absolute concentration of either component. A ratio of 75 resulted in 50% growth inhibition, whereas ratios of 300 and greater resulted in less than 10% growth inhibition. These results suggest that the ratio of citrate to Lf would be important in evaluating Lf as a nonspecific protective factor of bovine mammary secretions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta O., Barta V., Ingram D. G., Hubbert W. T. Bactericidal activity of bovine fetal serum against smooth and rough strains of Escherichia coli. Am J Vet Res. 1972 Apr;33(4):731–740. [PubMed] [Google Scholar]
- Barta O., Barta V., Miniats O. P., Ingram D. G. Bactericidal activity of conventional and germfree pig serum against smooth and rough strains of Escherichia coli. Am J Vet Res. 1971 Jul;32(7):1077–1082. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Carroll E. J. Bactericidal activity of bovine serums against coliform organisms isolated from milk of mastitic udders, udder skin, and environment. Am J Vet Res. 1971 May;32(5):689–701. [PubMed] [Google Scholar]
- Carroll E. J. In vitro bactericidal reactions of serums and milks obtained from cows inoculated with selected serum-resistant and serum-sensitive coliform bacteria. Am J Vet Res. 1974 Feb;35(2):205–211. [PubMed] [Google Scholar]
- Carroll E. J., Jain N. C., Schalm O. W., Lasmanis J. Experimentally induced coliform mastitis: inoculation of udders with serum-sensitive and serum-resistant organisms. Am J Vet Res. 1973 Sep;34(9):1143–1146. [PubMed] [Google Scholar]
- Carroll E. J., Schalm O. W., Lasmanis J. Experimental coliform (Aerobacter aerogenes) mastitis: bacteria and host factors in virulence and resistance. Am J Vet Res. 1969 Oct;30(10):1795–1804. [PubMed] [Google Scholar]
- Castellino F. J., Fish W. W., Mann K. G. Structural studies on bovine lactoferrin. J Biol Chem. 1970 Sep 10;245(17):4269–4275. [PubMed] [Google Scholar]
- Harmon R. J., Schanbacher F. L., Ferguson L. C., Smith K. L. Changes in lactoferrin, immunoglobulin G, bovine serum albumin, and alpha-lactalbumin during acute experimental and natural coliform mastitis in cows. Infect Immun. 1976 Feb;13(2):533–542. doi: 10.1128/iai.13.2.533-542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon R. J., Schanbacher F. L., Ferguson L. C., Smith K. L. Concentration of lactoferrin in milk of normal lactating cows and changes occurring during mastitis. Am J Vet Res. 1975 Jul;36(7):1001–1007. [PubMed] [Google Scholar]
- Jain N. C., Schalm O. W., Lasmanis J. Experimentally induced coliform (Aerobacter aerogenes) mastitis in normal cows and in cows made neutropenic by an equine anti-bovine leukocyte serum. Am J Vet Res. 1971 Dec;32(12):1929–1935. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. J., Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966 Nov;21(6):538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite B., Oram J. D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967 Oct 28;216(5113):328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Reiter B., Brock J. H., Steel E. D. Inhibition of Escherichia coli by bovine colostrum and post-colostral milk. II. The bacteriostatic effect of lactoferrin on a serum susceptible and serum resistant strain of E. coli. Immunology. 1975 Jan;28(1):83–95. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanbacher F. L., Smith K. L. Formation and role of unusual whey proteins and enzymes: relation to mammary function. J Dairy Sci. 1975 Jul;58(7):1048–1062. doi: 10.3168/jds.S0022-0302(75)84678-9. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Conrad H. R., Porter R. M. Lactoferrin and IgG immunoglobulins from involuted bovine mammary glands. J Dairy Sci. 1971 Oct;54(10):1427–1435. doi: 10.3168/jds.S0022-0302(71)86043-5. [DOI] [PubMed] [Google Scholar]
- Steel E. D. Adsorption in vitro to Escherichia coli of antibodies and other proteins in bovine serum and colostrum and its effects on the production of E. coli agglutinins. Immunology. 1975 Jul;29(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Role of iron in host-parasite interactions. J Infect Dis. 1971 Oct;124(4):401–410. doi: 10.1093/infdis/124.4.401. [DOI] [PubMed] [Google Scholar]
- Young I. G., Cox G. B., Gibson F. 2,3-Dihydroxybenzoate as a bacterial growth factor and its route of biosynthesis. Biochim Biophys Acta. 1967 Jul 25;141(2):319–331. doi: 10.1016/0304-4165(67)90106-7. [DOI] [PubMed] [Google Scholar]



