Abstract
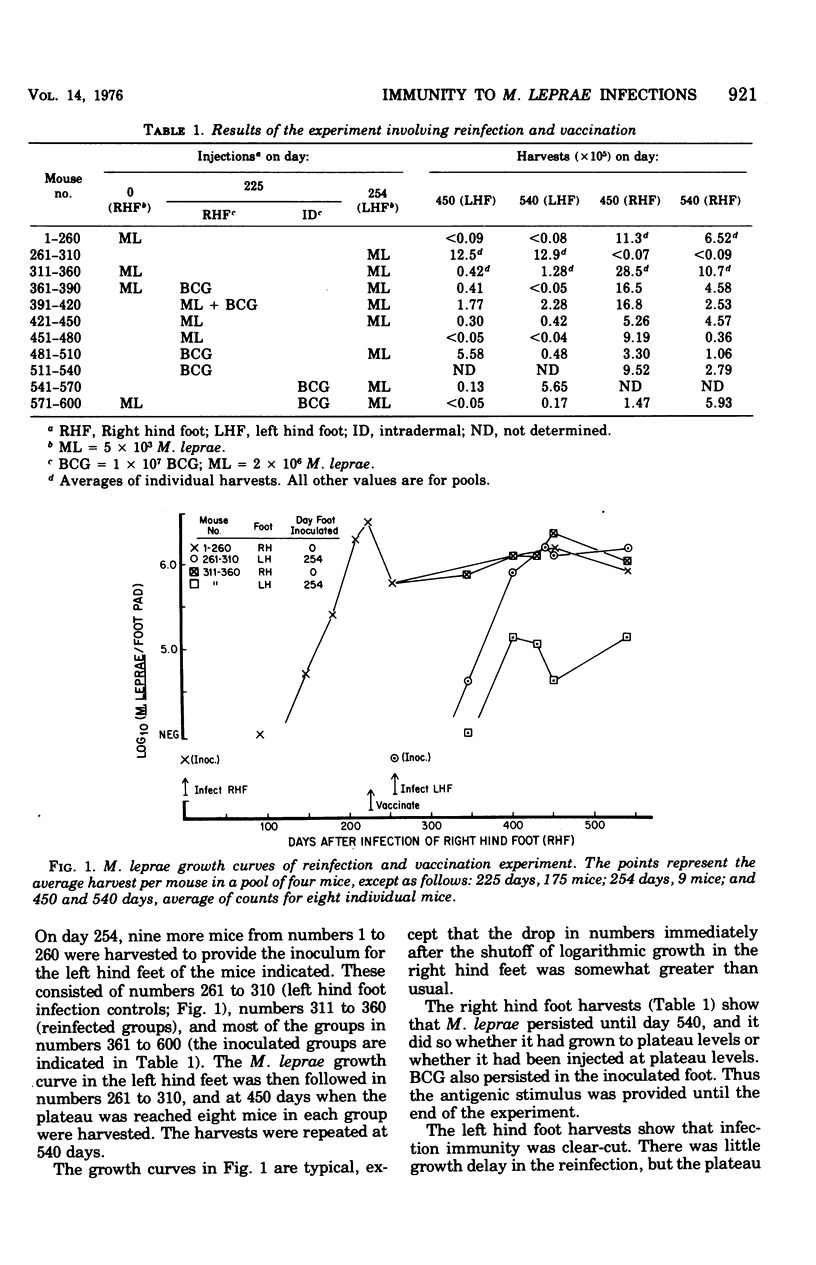
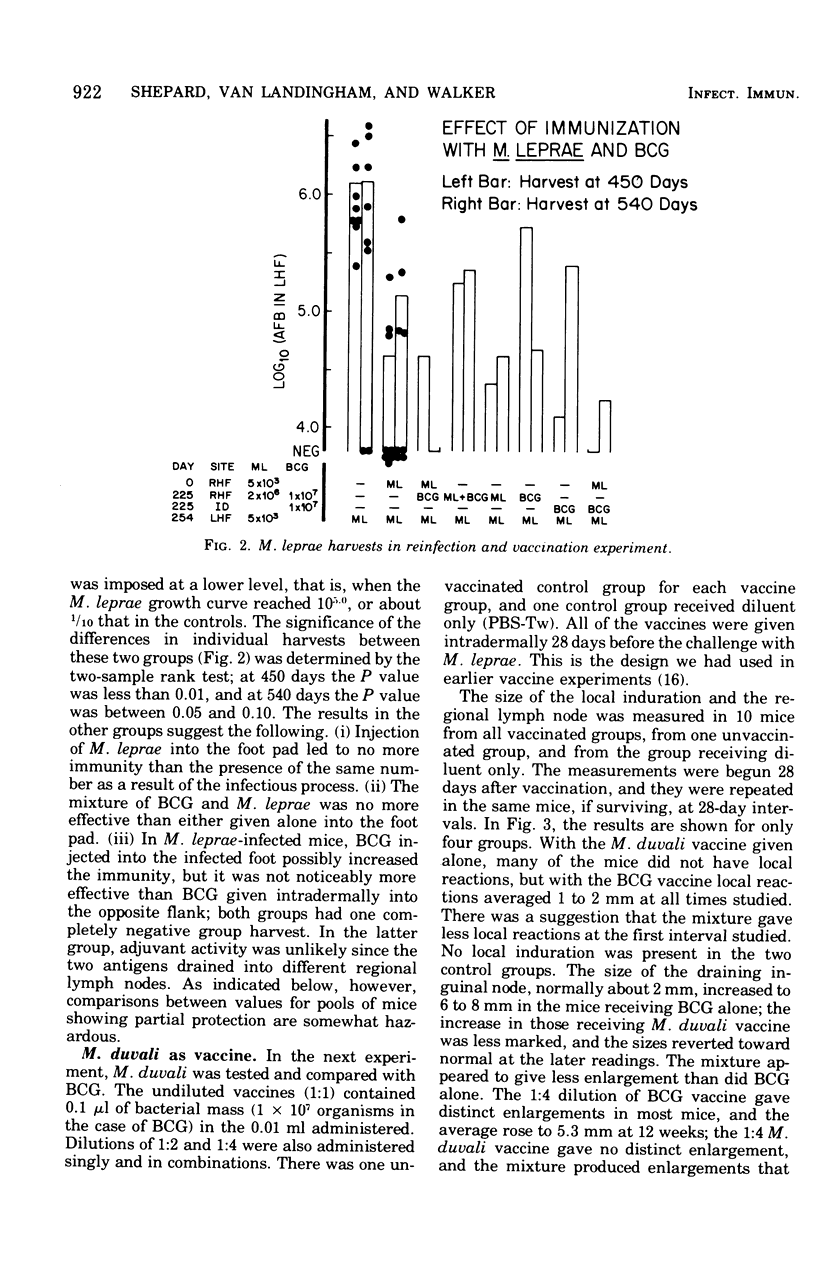
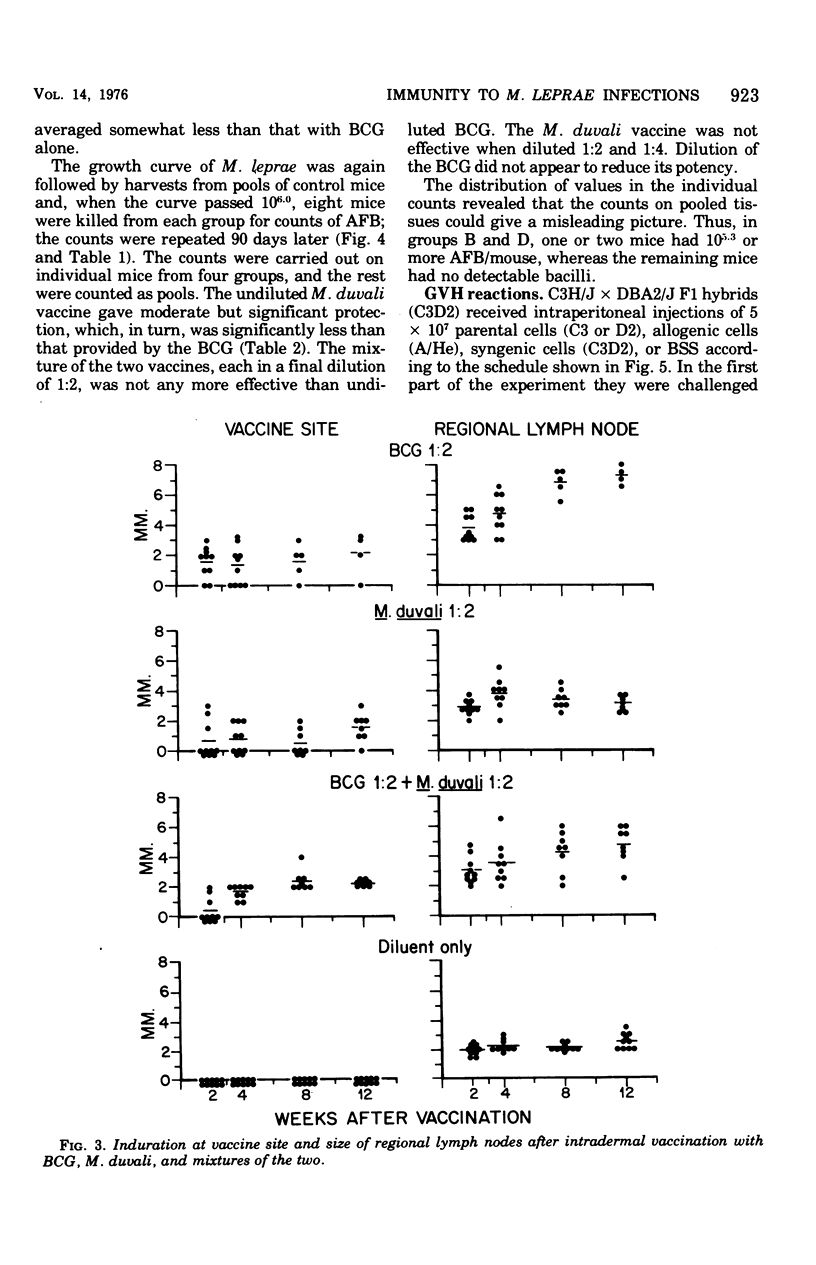
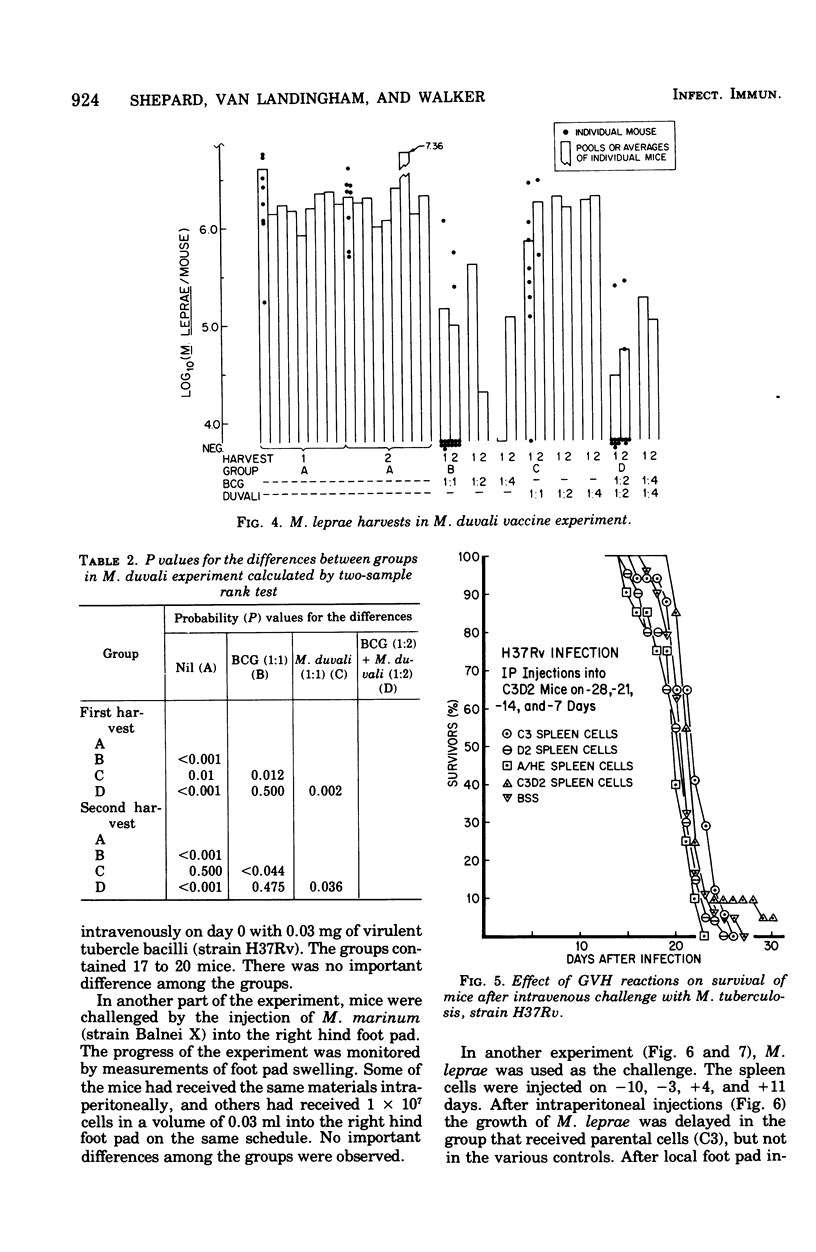
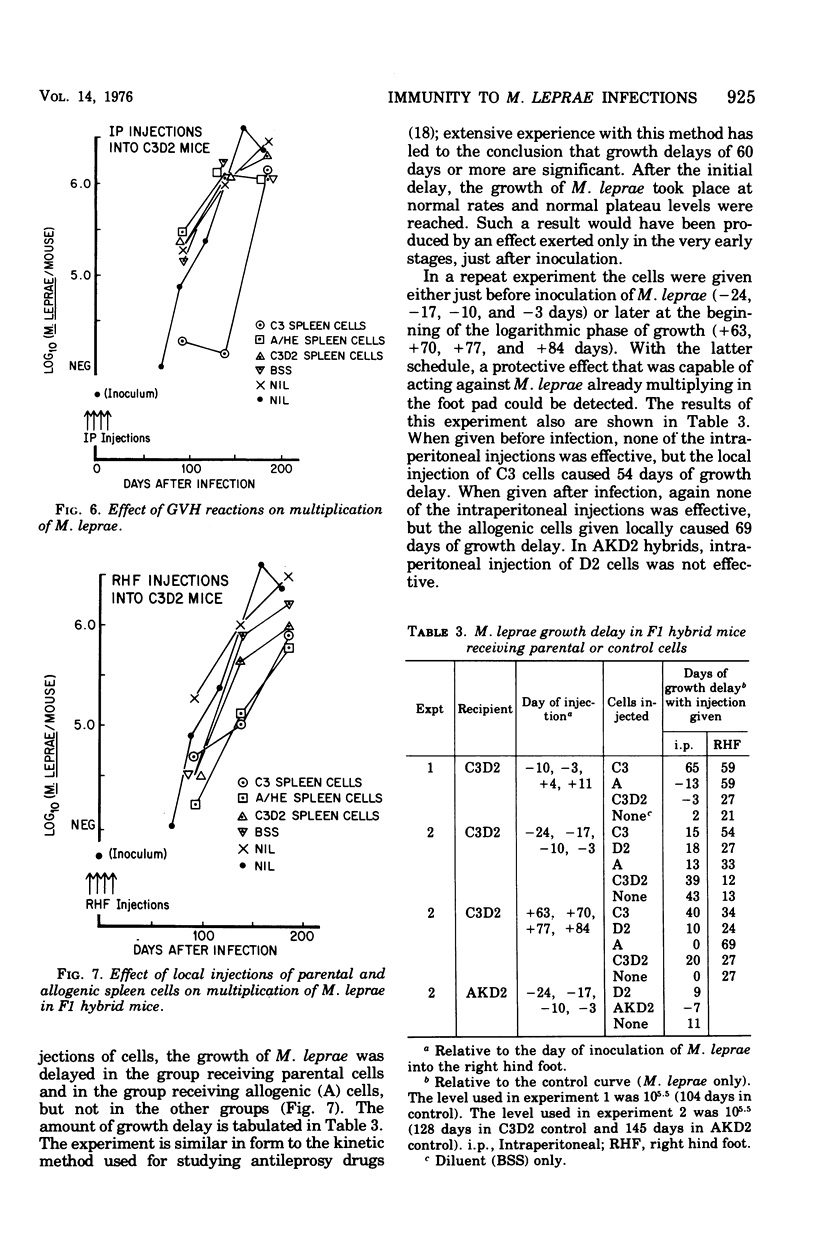
Infections of mice with Mycobacterium leprae in one rear foot pad immunized them against a second infection in the other rear foot pad. Purified bacilli harvested from the first infection also produced immuniy when injection into the foot pads of previously uninfected mice. Injections of BCG afforded similar protection, but had no adjuvant effect on M. leprae. M. duvali, a cultivable mycobacterium that is reported to be more closely related antigenically to M. leprae than BCG is, provided much less protection against M. leprae challenge than BCG did. Moreover, when M. duvali was mixed with BCG, it was not any more effective than BCG alone. Graft-versus-host reactions, induced by injections of parental spleen cells into F1 hybrids, provided no protection against M. tuberculosis and M. marinum challenge. They gave moderate protection against M. leprae in one experiment but not in another with a different schedule. Allogenic spleen cells had a protective effect when injected locally into the infected foot pad. The effect produced by these injections of spleen cells was a delay in the appearance of bacterial growth; however, there was no decrease in the rate of logarithmic growth when it did appear and no reduction in the eventual plateau level.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ J. M., HANKS J. H. Enhancement of resistance to murine leprosy by BCG plus specific antigen. Int J Lepr. 1956 Jan-Mar;24(1):65–73. [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Askenase P. W., Gershon M. D. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med. 1975 Sep 1;142(3):732–747. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann E., Gleichmann H., Schwartz R. S. Immunologic induction of malignant lymphoma: genetic factors in the graft-versus-host model. J Natl Cancer Inst. 1972 Sep;49(3):793–804. [PubMed] [Google Scholar]
- Han S. H., Weiser R. S., Kau S. T. Prolonged survival of skin allografts in leprosy patients. Int J Lepr Other Mycobact Dis. 1971 Jan-Mar;39(1):1–6. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Levy L., Remington J. S. Resistance to Mycobacterium leprae in Mice Infected with Toxoplasma gondii and Besnoitia jellisoni. Infect Immun. 1974 Nov;10(5):1068–1071. doi: 10.1128/iai.10.5.1068-1071.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L., Ng H., Evans M. J., Krahenbuhl J. L. Susceptibility of thymectomized and irradiated mice to challenge with several organisms and the effect of dapsone on infection with Mycobacterium leprae. Infect Immun. 1975 May;11(5):1122–1132. doi: 10.1128/iai.11.5.1122-1132.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L. Superinfection in mice previously infected with Mycobacterium leprae. Infect Immun. 1975 May;11(5):1094–1099. doi: 10.1128/iai.11.5.1094-1099.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C., GUINTO R. S. IMMUNOLOGICAL IDENTIFICATION OF FOOT-PAD ISOLATES AS MYCOBACTERIUM LEPRAE BY LEPROMIN REACTIVITY IN LEPROSY PATIENTS. J Exp Med. 1963 Aug 1;118:195–204. doi: 10.1084/jem.118.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C., MCRAE D. H. MYCOBACTERIUM LEPRAE IN MICE: MINIMAL INFECTIOUS DOSE, RELATIONSHIP BETWEEN STAINING QUALITY AND INFECTIVITY, AND EFFECT OF CORTISONE. J Bacteriol. 1965 Feb;89:365–372. doi: 10.1128/jb.89.2.365-372.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. VACCINATION AGAINST EXPERIMENTAL INFECTION WITH MYCOBACTERIUM LEPRAE. Am J Epidemiol. 1965 Mar;81:150–163. doi: 10.1093/oxfordjournals.aje.a120504. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Chang Y. T. Effect of DDS on established infections with Mycobacterium leprae in mice. Int J Lepr Other Mycobact Dis. 1967 Jan-Mar;35(1):52–57. [PubMed] [Google Scholar]
- Shepard C. C. Further experience with the kinetic method for the study of drugs against Mycobacterium leprae in mice. Activities of DDS, DFD, ethionamide, capreomycin and PAM 1392. Int J Lepr Other Mycobact Dis. 1969 Oct-Dec;37(4):389–397. [PubMed] [Google Scholar]
- Shepard C. C., Habas J. A. Relation of infection to tissue temperature in mice infected with Mycobacterium marinum and Mycobacterium leprae. J Bacteriol. 1967 Mar;93(3):790–796. doi: 10.1128/jb.93.3.790-796.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. Hereditary Characteristic that Varies Among Isolates of Mycobacterium leprae. Infect Immun. 1971 Jan;3(1):121–126. doi: 10.1128/iai.3.1.121-126.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Ribi E. Cell walls from Mycobacterium tuberculosis (BCG) as vaccine against Mycobacterium leprae infections in mice. Proc Soc Exp Biol Med. 1968 Feb;127(2):517–521. doi: 10.3181/00379727-127-32729. [DOI] [PubMed] [Google Scholar]
- Shepard C. C. Temperature optimum of Mycobacterium leprae in mice. J Bacteriol. 1965 Nov;90(5):1271–1275. doi: 10.1128/jb.90.5.1271-1275.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C. Vaccination of mice against M. leprae infection. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):222–226. [PubMed] [Google Scholar]
- Stewart-Tull D. E., Davies M. Adjuvant activity of Mycobacterium leprae. Infect Immun. 1972 Dec;6(6):909–912. doi: 10.1128/iai.6.6.909-912.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



