Abstract
Leucine-rich repeat containing G protein-coupled receptor 4 (LGR4) promotes the Wnt signaling through interaction with R-spondins or norrin. Using PCR amplification from rat ovarian cDNAs, we identified a naturally occurring Lgr4 splice variant encoding only the ectodomain of Lgr4, which was named Lgr4-ED. Lgr4-ED can be detected as a secreted protein in the extracts from rodent and bovine postnatal gonads, suggesting conservation of Lgr4-ED in mammals. Recombinant Lgr4-ED purified from the conditioned media of transfected 293T cells was found to dose-dependently inhibit the LGR4-mediated Wnt signaling induced by RSPO2 or norrin, suggesting that it is capable of ligand absorption and could have a potential role as an antagonist. Intraperitoneal injection of purified recombinant Lgr4-ED into newborn mice was found to significantly decrease the testicular expression of estrogen receptor alpha and aquaporin 1, which is similar to the phenotype found in Lgr4-null mice. Administration of recombinant Lgr4-ED to superovulated female rats can also decrease the expression of estrogen receptor alpha, aquaporin 1, LH receptor and other key steroidogenic genes as well as bring about the suppression of progesterone production. Thus, these findings suggest that endogenously expressed Lgr4-ED may act as an antagonist molecule and help to fine-tune the R-spondin/norrin-mediated Lgr4-Wnt signaling during gonadal development.
Introduction
Genomic studies of leucine-rich repeat containing G protein-coupled receptors (LGRs) from diverse species have indicated that LGRs can be subdivided into three groups [1], [2]. The ligands for the group A and group C LGRs are glycoprotein hormones and relaxin/insulin-like peptides, respectively. Intriguingly, several studies have demonstrated that the group B LGRs are able to interact with R-spondins [3], [4], whereas our recent study has shown that they are also able to bind to bursicon-like molecules such as bursicon and norrin [5], [6].
The mammalian group B LGRs have recently gained prominence as potential stem cell markers and they seem to play crucial roles in maintaining stem cell functions in diverse tissues. For example, LGR4 is required for maintenance of stem cells in the intestine, mammary gland and prostate [7]–[9]. LGR5 is a marker of stem cells located in the crypts of the gastrointestinal tracts [10], [11], the nascent nephrons of the kidney [12], and the hair follicles [13]. LGR6-positive stem cells in the hair follicles have been found to be capable of generating all cell lineages of the skin [14].
In addition to their vital roles in stem cells, studies using mutant animal models have also shown that the group B LGRs are essential during mammalian development. For example, it has been demonstrated that the Lgr4 gene displays a very wide expression, with stronger signals being present in the kidney, adrenal gland, bone/cartilage, gastrointestinal tracts, heart, reproductive tracts and nervous systems [15], [16]. As a result of this wide distribution, the phenotypes of Lgr4-null mice are quite complicated. Disruption of the Lgr4 gene in mice on the C57B1/6J x Swiss Webster background led to perinatal lethality and intrauterine growth retardation; these effects were associated with pronounced decreases in the weights of the kidney and liver [16]. In contrast, Lgr4-null mice that have a CD1 background are viable; nevertheless, male Lgr4-null mice are sterile and have a number of major defects affecting their reproductive tracts; these include the dilated rete testis and absence of sperms in the epididymis [17]. Further studies have suggested that Lgr4 plays pivotal roles in regulating the expression of estrogen receptor alpha (Esr1) and aquaporin (Aqp1), remodeling of basement membrane, and regional differentiation of the male reproductive tracts via epithelial-mesenchymal interactions [17], [18].
In addition to the testis, the Lgr4 gene is also abundantly expressed in ovarian follicles and has an even higher expression level in corpus luteum [15]. However, the expression profiles of Lgr4 in the postnatal gonads as well as how the Lgr4 signaling affects postnatal ovarian development have not yet been well characterized. In the present study, we identified a naturally occurring Lgr4 splice variant encoding only the extracellular ectodomain of Lgr4 and named it as Lgr4-ED. The recombinant Lgr4-ED protein was further generated to characterize its potential roles in the postnatal testis and ovary.
Materials and Methods
Ethics statement
All animals were housed under a controlled humidity, temperature, and light regimen in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Yang-Ming University. Animal treatments and sacrifice were approved by the Institutional Animal Care and Use Committee of the National Yang-Ming University (Permit Number: 1021229). All efforts were made to minimize animal suffering.
Animal treatments and progesterone assays
C57B1/6J mice and Sprague-Dawley rats were obtained from the laboratory animal center (National Yang-Ming University, Taipei, Taiwan) and randomly allocated into groups. For testing the Lgr4-ED effects on mouse testes, neonatal male mice were intraperitoneally injected with 10 µg of the purified Lgr4-ED protein every week and the testes were harvested on day 22. For time-course analyses of Lgr4 expression in the superovulation model, immature female rats (26-day old) were primed with 15 IU pregnant mare serum gonadotropin (PMSG) at 0900–1000 A.M. and received an intraperitoneal injection of 10 IU human chorionic gonadotropin (hCG) 48 h later. For Lgr4-ED injection in the superovulation model, 20 µg of the purified Lgr4-ED protein was co-injected with PMSG intraperitoneally and the ovaries were harvested at 48 h after injection.
For analyzing the changes of progesterone, the harvested ovaries were weighed and homogenized in cold PBS. The protein contents in collected supernatants were measured for normalization using the Micro BCA protein assay kit (Pierce Biotechnology). The amounts of progesterone in the supernatants were measured by ELISA using the specific anti-progesterone antibody (Sigma).
Cloning of the Lgr4 splice variant and generation of the recombinant Lgr4-ED protein
The Lgr4 splice variant was identified during PCR amplification of Lgr4 from the mature rat ovarian cDNA by Dr. Masataka Kudo using primers 5-TTGGAGAGTCTAACCTTG-3 and 5-TTAATAGCACTAAGGTCACAG-3. The cDNA construct encoding the Lgr4-ED protein was kindly provided by Dr. Aaron Hsueh at Stanford University. To facilitate recombinant protein purification, the construct was designed by adding a FLAG epitope tag at the N-terminus. The constructed plasmid was purified and then transfected into human 293T cells using LipofectAMINE 2000 (Life Technologies). Transfected cells were selected by the Zeocin-containing medium. The selected cells were allowed to reach confluence and then cultured for 72 h in the serum-free medium. Conditioned media were collected, filtrated and then subjected to anti-FLAG M1 affinity gel (Sigma) for protein purification. Measurement of the protein content was carried out by Micro BCA protein assay kit (Pierce Biotechnology). The purity and biochemical characteristics of the purified protein were analyzed by electrophoresis on a 10% SDS polyacrylamide gel.
cDNA isolation and real-time PCR quantification
For cDNA preparation, the total RNAs of harvested tissues or cells were extracted using the TRIzol reagent according to manufacturer instructions (Life Technologies). Five micrograms of total RNA were then reverse-transcribed using RevertAid First Strand cDNA Synthesis kit (Fermentas, Hanover, MD) with the oligo-dT primer. For quantitative TaqMan real-time PCR, a QuantiTect Probe PCR kit (QIAGEN Sciences) was used. The primer pairs and fluorescent probes for each gene were listed as follows: Rat Lgr4 forward, CAGTGCCCAGTGAAGCCATTC; rat Lgr4 reverse, TGTTGTCATCCAGCCACAGA. Mouse Lgr4 forward, CAGTACCCAGTGAAGCCATTC; rat Lgr4 reverse, TGTTGTCATCCAGCCACAGA; rat and mouse Lgr4 probe, TAGATGCCAACCATATTACCTCAGTCC. Rat and mouse Actb forward, CTCTGTGTGGATTGGTGGCTC; rat and mouse Actb reverse CTGCTTGCTGATCCACATCTG; rat and mouse Actb probe CCTGGCCTCACTGTCCACCTTCC. Rat Lhr forward, GTATCAGCAATTACATGAAGGTCAG; rat Lhr reverse, AGATCCTAATGTAGCAAGCACAGAT; rat Lhr probe, CTTAATCCTCAACGTGGTGGCCTTC. Rat Star forward, AGATGAAGTGCTAAGTAAGGTGGTG; rat Star reverse, CCAGTTCTTCATAGAGTCTGTCCAT; rat Star probe, TCTAGCAGCACCTCCAGTCGGAACA. Rat Hsd3b forward, AGACCATCCTAGATGTCAA-TCTGAA; rat Hsd3b reverse, CAGGATGATCTTCTTGTAGGAGT; rat Hsd3b probe, TCTACTGCAGCACAGTTGACGTTGC. Rat Cyp11a1 forward, CCAAGTTCAACCTCATCCTGA; rat Cyp11a1 reverse, GTGTGACTGCAGCCTGCAAT; rat Cyp11a1 probe,CTTCAACTTCCAGCCTCTCAAGCAG.
For the quantitative SYBR Green real-time PCR, SYBR Green PCR Master Mix kit (Life Technologies) was used and the primer pairs for each gene were listed as follows: Rat and mouse Esr1 forward, GCTGCGCAAGTGTTACGAA; rat and mouse Esr1 reverse, CATTTCGGCCTTCCAAGTCAT. Rat and mouse Esr2 forward, GAGGCAGAAAGTAGCCGGAA; rat and mouse Esr2 reverse, CGTGAGAAAGAAGCATCAGGA. Rat Aqp1 forward, ATCACCTCCTCCCTGCTCGA; rat Aqp1 reverse, AGTGTAGTCAATGGCCAGCA. Mouse Aqp1 forward, ATCACCTCCTCCCTAGTCGA; mouse Aqp1 reverse, AGTGTAGTCAATCGCCAGCA. Rat and mouse Actb forward, CTCTGTGTGGATTGGTGGCTC; rat and mouse Actb reverse TTGCGCAAGTTAGGTTTTGTC.
Immunoblotting and immunohistochemical analyses
The rabbit anti-Esr1 antibody was purchased from Santa Cruz Biotechnology. The mouse anti-FLAG monoclonal antibody was from Sigma-Aldrich Corp. The rabbit anti-Lgr4 antibodies were from Abcam and Dr. Aaron Hsueh. To extract full-length Lgr4 from transfected 293T cells, the cells were lysed in ice-cold RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM dithiothreitol, 1% NP40, 0.1% SDS) supplemented with protease inhibitor cocktails (Roche). The lysate was pelleted by centrifugation at 16,000 g for 15 min at 4°C and the supernatant was mixed with 5X sample buffer and incubated at 37°C for 1 hr before analyzed by SDS-PAGE. The bovine follicular fluid was collected from a local slaughterhouse. Rat ovaries and mouse testes were homogenized in ice-cold PBS. Following centrifugation, protein amounts in the supernatants were quantified by Micro BCA protein assay kit (Pierce Biotechnology) before subjecting to electrophoresis and immunoblotting assays.
For immunohistochemical analyses, the ovaries and testes harvested from treated animals were fixed in Bouin’s fixative and embedded. Tissue sections were probed with specific primary antibodies. Substitution for the primary antibodies with the rabbit preimmune serum or normal IgG served as the negative controls. Staining was visualized using the HRP-conjugated secondary antibody followed by the Nova Red kit (DakoCytomation) or Alexa Fluor 488-conjugated secondary antibody (Life Technologies) followed by observation under a fluorescent microscope.
Wnt reporter luciferase assays
TOPFLASH–luciferase assays were performed by transfecting human LGR4 or LGR4 and human norrin plasmids as described previously [5]. Briefly, for RSPO2 treatment, 293T cells were seeded into 24-well plates and transfected with human LGR4 (0.05 µg) and pCMV-β-Gal (0.01 µg) plasmids using LipofectAMINE 2000 (Life Technologies). At 24 h after transfection, cells were further treated with or without WNT3A (1 nM) and RSPO2 (3 nM) for another 16 h in serum-free medium. For norrin expression, 293T cells were transfected with LGR4 (0.05 µg), pCMV-β-Gal (0.01 µg) and/or increasing amounts of norrin plasmid using LipofectAMINE 2000 (Life Technologies). At 24 h after transfection, cells were further cultured in serum-free medium for another 16 h. Luciferase activities were determined using luciferase assay kits (Promega) and normalized using β-galactosidase activities. All experiments were performed at least three times in triplicate.
Data analysis
For comparisons of significant difference, the values were subjected to analysis by the Student’s t-test. Significance was accepted at P<0.05 and is indicated by asterisks in the figures unless noted otherwise.
Results
Identification of a Lgr4 splice variant encoding only the ectodomain of Lgr4 protein
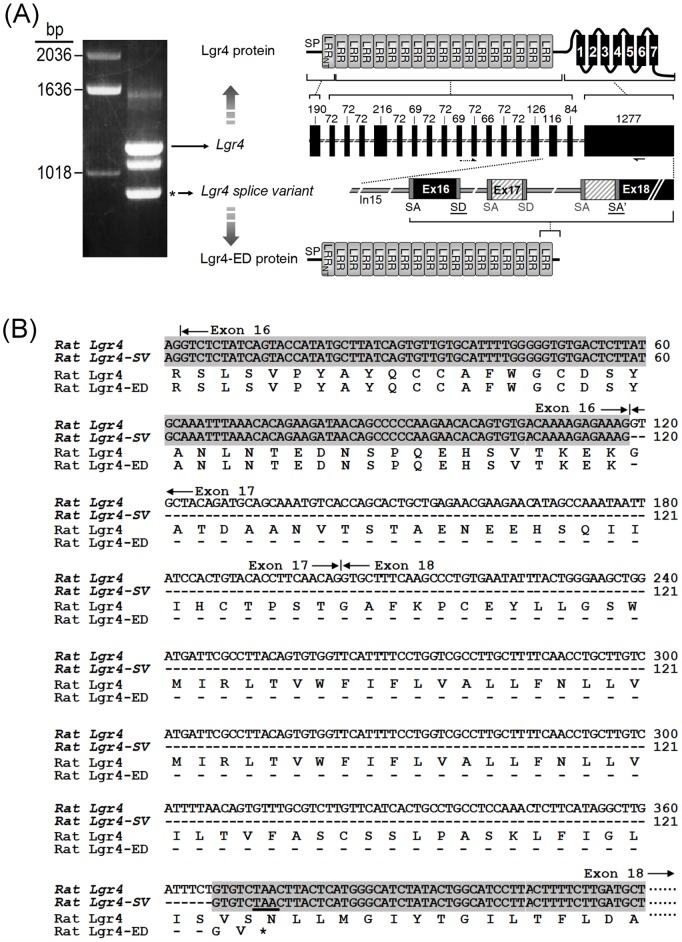
During PCR amplification of the Lgr4 fragment from mature rat ovaries, a previously uncharacterized splice form was found (Fig. 1A, left panel). The splice variant was then cloned and sequenced. Genomic structure analysis indicated that this splice form consists of the first 16 exons of Lgr4 (Fig. 1A, right panel). Sequencing data indicated that alternative splicing had resulted in deletion of exon 17 and recreation of a previously unidentified exon/intron junction in exon 18 of the Lgr4 gene, thus leading to a frame-shift and the introduction of an early termination codon before the translation of seven-transmembrane domains of Lgr4 (Fig. 1B). Consequently, this splice variant encodes the first 500 amino acids of Lgr4, including the signal peptide, N-flanking cysteine-rich and leucine-rich repeat sequence, 17 leucine-rich repeat motifs and C-flanking cysteine-rich sequence (Fig. 1A, right panel). The resulting protein contains only the extracellular portion of Lgr4 and thus was named Lgr4 ectodomain, Lgr4-ED.
Figure 1. Gene structure of Lgr4 and the amino acid sequence of Lgr4-ED.
(A) Cloning and gene organization of rat Lgr4 and rat Lgr4 splice variant. Left panel: both fragments of full-length Lgr4 and Lgr4 variant can be amplified from the mature rat ovarian cDNA by using a forward primer spanning exon 10 and 11 and a reverse primer in exon 18. Right panel: exons are represented by boxes; introns are shown by the lines between exons. The numbers indicate the base pairs in each exon. Primer locations were indicated by arrows at the bottom of axons. The Lgr4 variant is spliced via selection of the splice donor (SD) site of exon 16 and an alternative splice acceptor (SA’) of exon 18; this results in the deletion of exon 17 and the initial 164 bps of exon 18 (slash boxes). The deduced domain structure from each exon of the full-length Lgr4 and Lgr4-ED proteins are shown. SP, signal peptide; LRRnt; leucine rich repeat N-terminal domain; LRR; leucine rich repeat domain; TM, transmembrane domain. (B) Comparison of nucleotide sequences and deduced amino acids between rat Lgr4 and rat Lgr4 splice variant (SV). Dashed lines indicate gaps introduced in the sequence for optimal alignment.
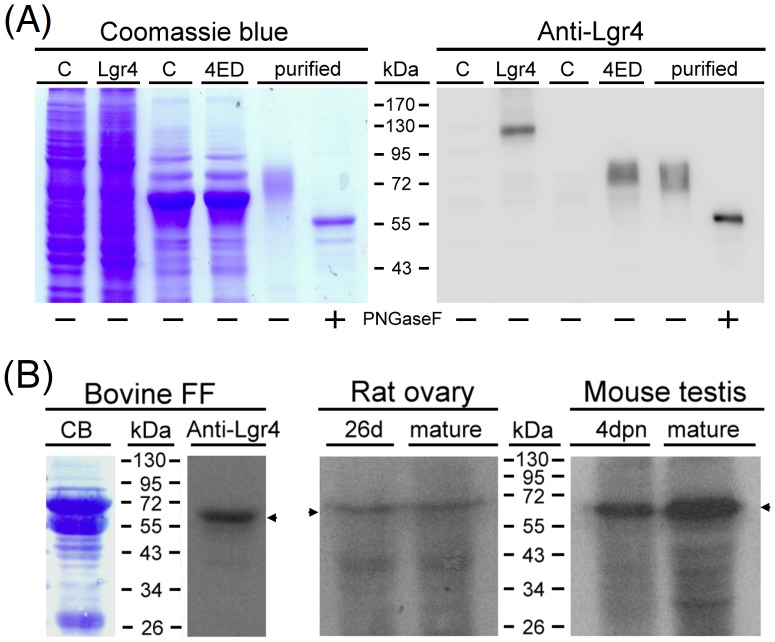
Lgr4-ED was predicted to be a secreted protein due to the lack of seven-transmembrane domains of the original protein. To confirm this, the cDNA corresponding to full-length rat Lgr4 or Lgr4-ED was constructed into pcDNA3.1 to allow mammalian cell expression. Western blot analyses indicated that the Lgr4 antibody can specifically recognize the full-length Lgr4 protein migrated as a band around 130 kDa. The recombinant Lgr4-ED protein was capable of being secreted from transfected 293T cells and had a molecular weight around 70 kDa (Fig. 2A, right panel). We further purified the recombinant FLAG-tagged Lgr4-ED protein from the conditioned media using affinity chromatography. Under reducing conditions, purified Lgr4-ED migrated as a band around 70 kDa (Fig. 2A, left panel, lane 5). The protein migrated faster after treatment with N-glycosidase F (Fig. 2A, left panel, lane 6), indicating the N-linked glycosylation of the recombinant Lgr4-ED protein.
Figure 2. Recombinant and endogenous expression of the Lgr4-ED proteins.
(A) SDS-PAGE analysis of full-length rat Lgr4 in transfected 293T cells and recombinant N-terminal FLAG-tagged rat Lgr4-ED (4ED) secreted by transfected 293T cells. C: cells transfected with control pcDNA3.1. Purified Lgr4-ED was further treated without or with PNGase F to characterize its glycosylated nature. The gels were stained using Coomassie blue (left panel) or probed with the anti-Lgr4 antibody (right panel). To adjust the band signals, 1.5 µg and 0.015 µg of purified Lgr4-ED before or after de-glycosylation was loaded for Coomassie blue staining and Western blotting, respectively. (B) Detection of the endogenous Lgr4-ED protein signals in different species. Bovine follicular fluid (FF) and the homogenized supernatants from rat ovaries and mouse testes were subjected to SDS-PAGE and Western analysis against Lgr4. CB, Coomassie blue staining; dpn, days postnatal.
To further confirm the endogenous translation of Lgr4-ED, protein samples from the gonads of various species, including the follicular fluid collected from bovine ovaries and the supernatants from tissue homogenates of rat ovaries and mouse testes at different ages, were used (Fig. 2B). Western analysis using the anti-Lgr4 antibody showed a specific band around 70 kDa in the bovine follicular fluid. This signal did not overlap with prominent albumin bands as compared to the protein migration positions visualized by Coomassie blue staining, suggesting that the Lgr4 antibody was specific. In homogenates prepared from immature or mature rat ovaries and immature or mature mouse testes, signals of a similar molecular size were also detected. Taken together, these findings suggest that the Lgr4-ED protein is naturally secreted by both types of gonads of various mammals.
Functional tests of Lgr4-ED
We further evaluated the bioactivity of the recombinant Lgr4-ED protein. Lgr4 has been demonstrated to be a receptor for R-spondins that mediate the Wnt signal enhancement [3], [4]. The combination of WNT3A with RSPO2 led to a significant increase of the Wnt signaling as measured by the TOPFLASH reporter assay. In contrast, exogenous addition of the recombinant Lgr4-ED suppressed this response in a concentration-dependent manner (Fig. 3A). In addition, norrin is also able to stimulate the Wnt signaling mediated by human LGR4 [5], whereas such a stimulation can be reversed by co-expression of Lgr4-ED (Fig. 3B). These findings suggest that the recombinant Lgr4-ED protein acts as a functional antagonist capable of neutralizing the Wnt signaling induced by R-spondins and norrin.
Figure 3. Antagonist effects of Lgr4-ED.
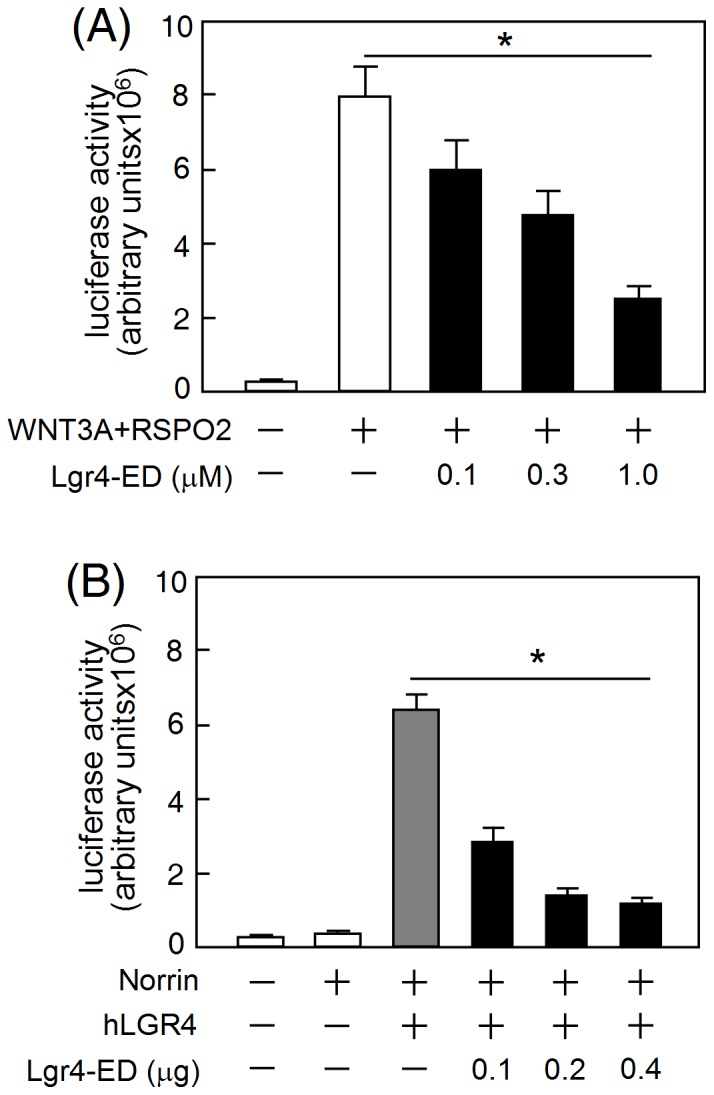
(A) For the R-spondin assays, HEK293T cells were transfected with plasmids encoding TOPFLASH, CMV-β-Gal and human LGR4. At 24 h after transfection, cells were further treated with WNT3A, RSPO2 and graded amounts of purified Lgr4-ED as indicated. (B) For the norrin assays, HEK293T cells were co-transfected with TOPFLASH, CMV-β-Gal, LGR4, norrin and increasing amounts of rat Lgr4-ED plasmids as indicated. After recovery in serum-containing medium for 24 h, the cells were incubated in the serum-free medium for 16 h before performing luciferase assays. Luciferase data were normalized against the β-galactosidase levels and are shown as the mean ± SD of triplicates (*, P<0.05). Three additional experiments were performed and all three produced similar results.
Testicular and ovarian expression of Lgr4
To determine the testicular cell types that express Lgr4, the testes from mice at 7 days of age were digested and the cell types were further separated using magnetic activated cell sorting (MACS) based on antibodies against Thy1 antigens expressed in spermatogonial stem cells [19]. As shown in Fig. 4A, the Lgr4 mRNA is mainly detected in the Thy1− somatic cell-enriched fraction but not in the Thy1+ spermatogonial stem cell-enriched germ cell fraction.
Figure 4. Expression of Lgr4 in the postnatal gonads.
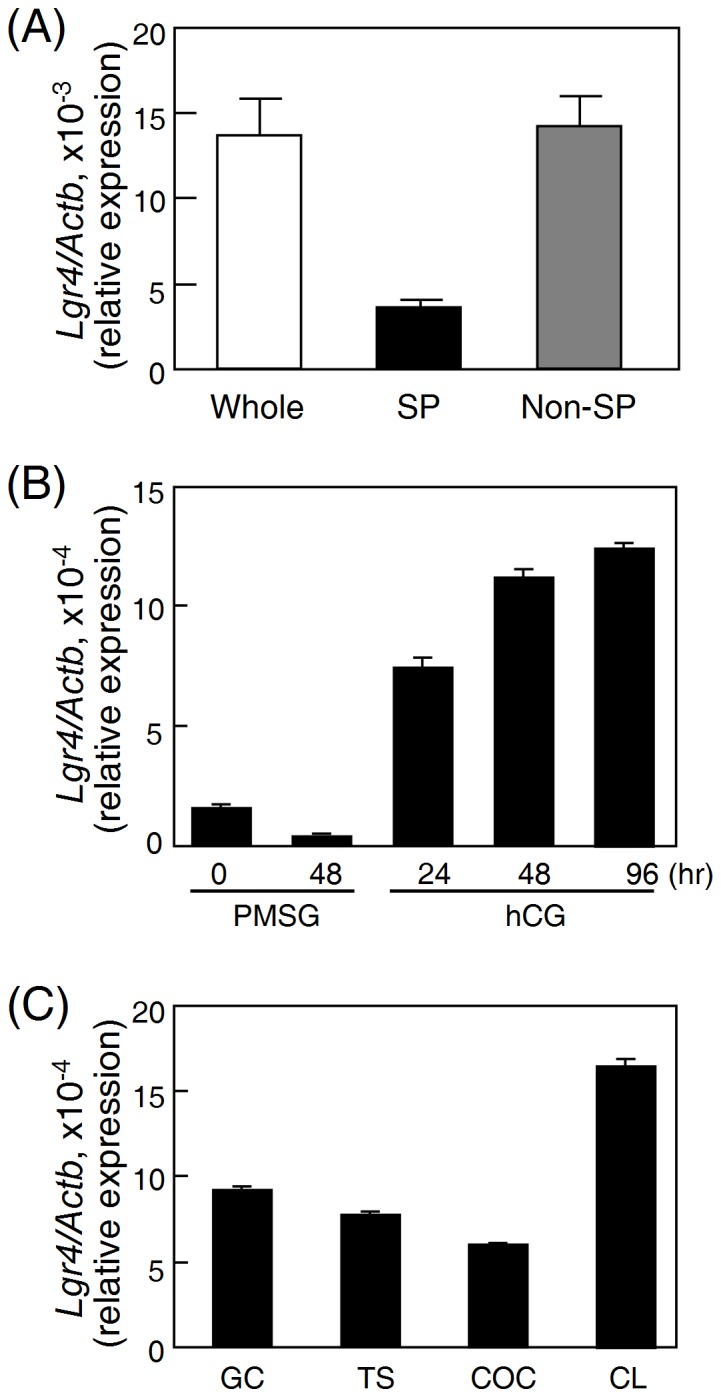
(A) Comparison of Lgr4 expression in different testicular cell types. The whole testes from mice at 7 days postnatal and the isolated spermatogonia (SP) and non-spermatogonia (non-SP) were used for real-time quantification of the Lgr4 expression levels. (B) Female rats at 26 d of age were treated with PMSG, followed 48 h later by hCG for different intervals. The ovaries at indicated intervals were harvested for real-time quantification of the Lgr4 expression level. (C) Comparison of Lgr4 expression in different ovarian cell types by real-time PCR quantification. Granulosa cells (GC), theca shells (TS), and cumulus-oocyte complexes (COC) were isolated from rat ovaries (26 days old), whereas corpora lutea (CL) were obtained from PMSG-primed rats at 72 h after hCG treatment. Data were normalized using β-actin levels and are expressed as the mean ± S.D.
To determine the expression of Lgr4 in the ovary, the ovaries were collected from superovulated immature rats primed with PMSG followed by hCG for different intervals. Quantitative real-time PCR analyses showed that the Lgr4 mRNA expression was elevated and remained at high levels 24 h after hCG injection (Fig. 4B). Subsequent analyses of the Lgr4 transcript in various ovarian compartments, including granulosa cells, theca shells, cumulus-oocyte complexes and corpora lutea, indicated that Lgr4 is widely expressed in these different ovarian cell types with corpora lutea showing the highest expression level (Fig. 4C).
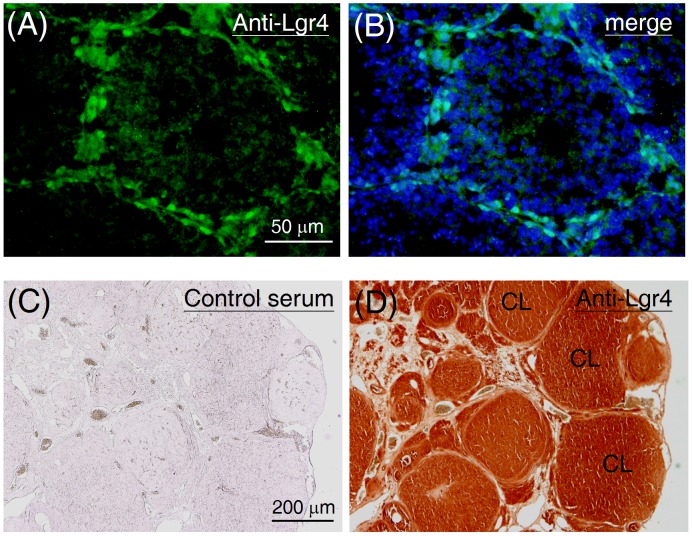
In addition, immunohistochemical analyses were also carried out to confirm the distribution of the Lgr4 protein. In mouse testes, the Lgr4 positive cells were mainly located around the periphery of the seminiferous tubules (Fig. 5A and 5B). Considering the Lgr4 mRNA profile above and the cell morphology shown in the immunohistochemical staining, this data suggests that the Lgr4 protein is probably expressed in peritubular myoid cells but not in spermatogonia or Sertoli cells. In the ovaries harvested from adult rats (8 weeks old), a strong Lgr4 immunoreactivity was detected in corpora lutea (Fig. 5C and 5D), consistent with its mRNA profile.
Figure 5. Distribution of the Lgr4 protein in the postnatal gonads.
To assess the testicular distribution of Lgr4, testicular sections from mice at 5 weeks old were probed with the anti-Lgr4 antibody followed by fluorescein isothiocyanate-conjugated secondary antibody. Data are shown in parallel without (A) or with (B) a DAPI merged image. To assess the ovarian distribution of Lgr4, ovarian sections from mature rats (8 weeks old) were probed either (C) with preimmune rabbit serum or (D) with the anti-Lgr4 antibody. The sections were then visualized using a horseradish peroxidase system. CL, corpus luteum.
Lgr4-ED treatment decreases the expression of estrogen receptors and aquaporin 1 in the postnatal testis
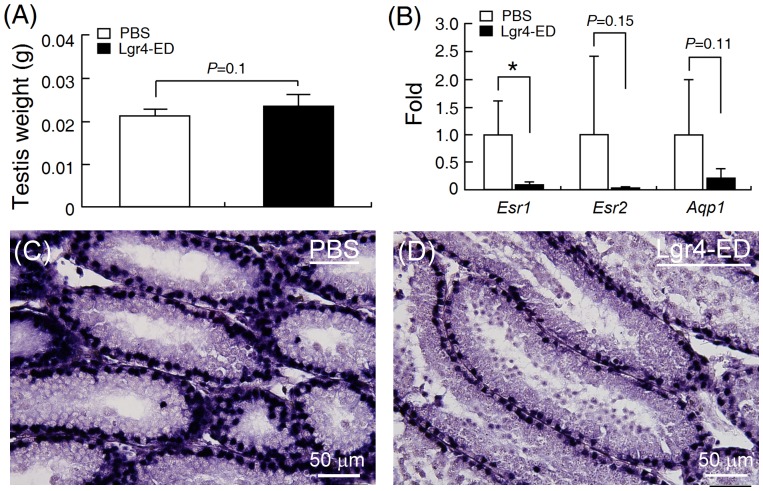
It has been shown that Lgr4 knockout leads to swelling and liquid accumulation in mouse testes as a consequence of down-regulation of the expression of estrogen receptor alpha (Esr1) and aquaporin (Apq1) [17], [18]. To test whether Lgr4-ED is a functional antagonist on testicular development in vivo, newborn male mice were intraperitoneally injected with Lgr4-ED on day 1, 8 and 15 after birth and their testes were harvested on day 22 for analyses. We found that Lgr4-ED administration did not affect the survival, body weights (controls: 8.4±1.3 g; treated: 8.5±1.4 g, n = 6) and testicular weights (Fig. 6A) of the treated mice. However, Lgr4-ED treatment was found to suppress the mRNA expression of estrogen receptors and Aqp1, with Esr1 showing the most significant decrease (Fig. 6B). To confirm this, immunochemical staining against Esr1 was carried out and it was found that the Esr1 protein signal was significantly decreased in the testes of mice after Lgr4-ED injection (Figs. 6C and 6D). Taken together, our results suggest that Lgr4-ED administration can produce some of the phenotypes associated with Lgr4-knockout mice and this is likely via neutralizing the actions of endogenous Lgr4 ligands by Lgr4-ED.
Figure 6. The recombinant Lgr4-ED protein affects postnatal development of the testis.
Neonatal male mice were injected intraperitoneally with either controlled saline or purified recombinant Lgr4-ED (10 µg/animal) on postnatal day 1, 8 and 15. Mice were then sacrificed on day 22 and (A) the testicular weights were measured. (B) The expression levels of Esr1, Esr2, Aqp1 in the harvested testes were compared by real-time PCR. Data were normalized against β-actin levels and represent the mean ± SEM (n = 6). Asterisks indicate P<0.05. (C and D) In addition, the testes were also subjected to immunohistochemical staining to detect the Esr1 protein and visualized using an alkaline phosphatase staining system.
Lgr4-ED treatment suppresses ovarian development and steriodogenesis
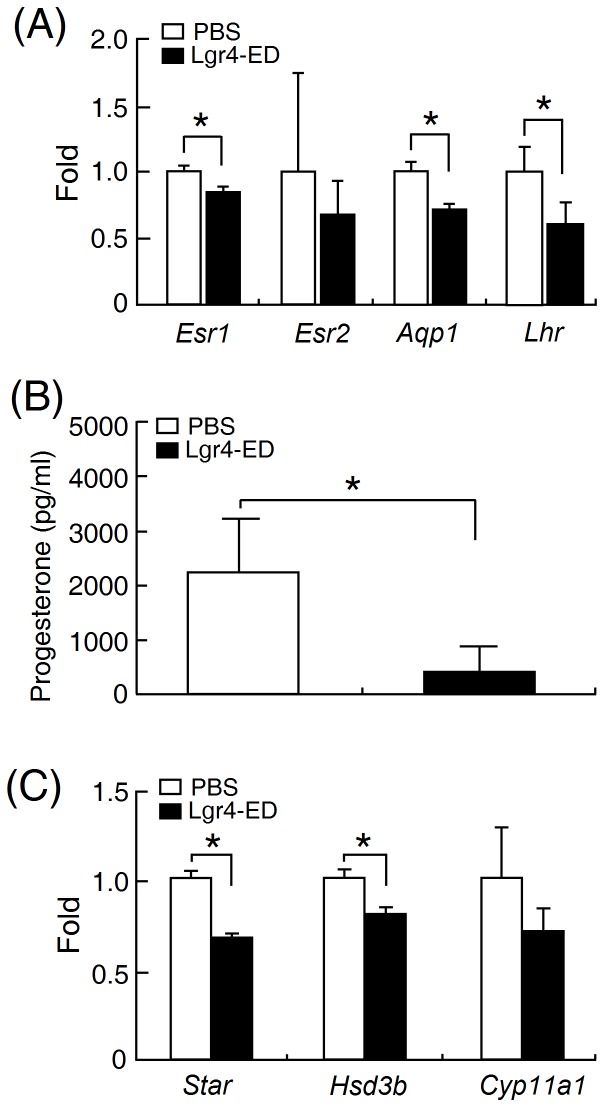
To test the Lgr4-ED effects on ovarian development and steriodogenesis, immature female rats were intraperitoneally injected with PMSG together with or without the recombinant Lgr4-ED protein and their ovaries were then collected 2 days after injection. Although no significant changes in the ovarian weight (control: 0.031±0.0015 g; treated: 0.039±0.0041 g, n = 7) were detected, Lgr4-ED administration did significantly decrease the expression levels of Esr1, Aqp1 and Lhr (Fig. 7A). In addition, Lgr4-ED injection also led to the suppression of PMSG-induced progesterone production (Fig. 7B) and this was accompanied by decreases in the transcript levels of various steroidogenic enzymes including steroidogenic acute regulatory protein and 3-β-hydroxysteroid dehydrogenase (Fig. 7C).
Figure 7. The recombinant Lgr4-ED protein decreases gonadotropin-driven ovarian development and steroidogenesis.
Immature female rats (26 days old) were intraperitoneally injected with PMSG in the presence or absence of 20 µg Lgr4-ED. The ovaries were harvested two days later for assessing the expression levels of (A) Esr1, Esr2, Aqp1 and Lhr. β-actin was used for normalization. (all bars; n = 7). In addition, (B) the progesterone amounts and (C) the transcript levels of various steroidogenic genes in the ovaries were evaluated (n = 4). Data are shown as the mean ± SEM. Asterisks indicate P<0.05.
Discussion
In the testis, undifferentiated spermatogonia are discontinuously scattered around the basement of seminiferous tubules. Although Lgr4 has been proposed as a stem cell marker in many tissues [7]–[9], our staining results showed that the Lgr4 signal is mainly located in the connected cells that form a continuous sheet surrounding the basement membrane of seminiferous tubules (Fig. 5A), suggesting that Lgr4 is unlikely to be located in spermatogonia. Real-time PCR quantification of isolated testicular cells further supported this hypothesis (Fig. 4A). Indeed, a previous study has also proposed that the Lgr4 transcript is probably expressed in the myoepithelial cells of seminiferous tubules [15]. Using a gene trap approach, Qian et al. further demonstrated that the Lgr4 transcript is selectively located in peritubular myoid cells but not in spermatogonia of postnatal mouse testes [20]; these findings are consistent with our results.
By disrupting the Lgr4 gene with a trap vector carrying the β-galactosidase tracing enzyme, Lgr4 in mouse ovaries has been proposed to be expressed in corpus lutea but not in other follicular compartments [15]. Although our results of mRNA quantification and protein staining in rat ovaries indicated that corpus lutea did express the highest level of Lgr4, we also detected moderate levels of the Lgr4 transcript in other ovarian cells isolated from antral follicles (Fig. 4C). The stage difference between the appearance of Lgr4 mRNA in rat ovaries and the appearance of translated β-galactosidase protein signal in mouse ovaries may be explained by the fact that different species were used or by effects of as yet uncharacterized regulatory factors that are induced after luteogenesis to control the initiation of translation of the Lgr4 mRNA. In addition, sequence difference between endogenous Lgr4 and the tracing LacZ-containing cassette may also result in different effects on post-transcriptional or translational regulation.
Our findings indicating the presence of a Lgr4 splice variant encoding only the Lgr4 ectodomain is novel for the group B LGRs. Although it is well known that splicing errors may happen in the posttranscriptional processing of premRNAs derived from complex genes, these erroneous mRNAs, which encode potentially toxic polypeptide fragments, are generally eliminated in the cytoplasm rapidly; this will lead to undetectable levels of the corresponding proteins [21]. Our findings showing the consistent presence of the Lgr4-ED protein signal in mouse, rat and bovine gonads strongly suggest this Lgr4 transcript variant is unlikely to be due to a random splicing error (Fig. 2). Therefore, this naturally occurring Lgr4 splice variant seems to be highly conserved across a range of mammalian species and thus the translated product may play important, but as yet uncharacterized, roles in modulation of the Lgr4 signaling.
Indeed, alternative splicing of G protein-coupled receptor (GPCR) genes has been demonstrated to be involved in the signal modulation of physiological processes and diseases. For GPCRs with a relatively large N-terminal ectodomain, alternative splicing may generate truncated receptors lacking the transmembrane region and these then serve as dominant-negative antagonists to full-length receptors. Not only found in our study of Lgr4, alternative splice variants encoding only the extracellular region of GPCRs have also been reported for several members of the LGR family. For example, in the group A LGRs, a truncated soluble luteinizing hormone receptor lacking the transmembrane region and a putative soluble fragment of follicle-stimulating hormone receptor have been isolated from the turkey ovary and the ovine testis, respectively [22], [23]. Furthermore, a human thyroid-stimulating hormone receptor mRNA variant encoding only the extracellular ligand-binding domain has also been found and reported to play a potential role in thyroid physiology and/or autoimmune thyroid disease [24]. Among the group C LGRs, an alternative Lgr7 transcript in rodents that encodes a secreted protein containing the low-density lipoprotein class A module has been identified. Expression of this truncated fragment significantly decreases the relaxin-induced signaling of Lgr7 [25]. Beyond the LGR family, splice variants containing only the extracellular region of receptors have also been reported for other GPCRs that have a large ectodomain, such as corticotropin-releasing hormone receptor [26], metabotropic glutamate receptors [27], [28] and gamma-aminobutyric acid B receptor [29]. In the majority of these cases, the truncated proteins seem to act as molecules that are able to bind but are unable to bring about signaling; this allows fine-tuning of the full-length receptor signals.
Lgr4 has been reported to bind R-spondins or norrin; thereby, the forming complex can further enhance the Wnt signaling [3]–[5]. Using reporter assays, we demonstrated that the recombinant Lgr4-ED can indeed dampen the Wnt/β-catenin signaling in vitro (Fig. 3). Interestingly, balance of the Wnt/β-catenin signaling has been demonstrated to be crucial for normal development of the male and female reproductive systems. For examples, deficiency in the Wnt signaling, including in Wnt4, Wnt5a, and Wnt7a, will result in severe malformation of the genitals and infertility [30]–[32], whereas hyperactivation of the Wnt/β-catenin pathway can also lead to germ cell apoptosis and male infertility [33], [34]. Thus, the endogenous expression of Lgr4-ED may act as a decoy molecule that aids modulation of the strength of the Wnt/β-catenin signaling in order to maintain appropriate development conditions for the gonads.
Lgr4-null mice show strong dilation of the rete testis and efferent ducts due to defects in liquid reabsorption. These phenotypes are accompanied by down-regulation of steroid receptors, water transporters and ion transporters, including estrogen receptor, androgen receptor, aquaporin 1, aquaporin 9, Na+-K+-ATPase and sodium/hydrogen exchanger 3 [17], [35]. Of interest, not only showing the antagonizing effect against the Wnt/β-catenin signaling in vitro (Fig. 3), injection of the recombinant Lgr4-ED into mice can also down-regulate the expression of Esr1 and Aqp1 in the testis in vivo (Fig. 6). Although there is still no consensus regarding the testicular expression and localization of Esr1 in different species and previous studies on Lgr4-null mice also indicated the reduction of Esr1 immunostaining was mainly observed in the epididymis and efferent ducts but not in the testis, several recent reports have clearly demonstrated that both mRNA and corresponding protein of Esr1 can be detected in the Sertoli cells in mouse and rat testes [36]–[38]. In addition, the Esr1-knockut male mice are sterile and show atrophic testes with a loss of germ cells in the dilated seminiferous tubules [39], consistent with the phenotypes observed in Lgr4-knock male mice [17], [20]. Therefore, the testicular effects of Lgr4 on controlling the Esr1 expression might potentially explain the consequence of infertility in Lgr4-null mice. In addition, although expression of Aqp1 has been reported to be abundant in the epididymis, several studies have also demonstrated it can be detected locally in some specific cells of the testis. For example, the Aqp1 protein signal can be detected in the endothelium of the blood vessels in the testicular interstitium of cats [40]. Similar results have also been reported in monkeys, rats and mice [41], [42]. These might explain why we can observe the expressional change of Aqp1 in Lgr4-ED treated mice. Taken together, these findings suggest that Lgr4-ED will be useful as a tool when exploring the possible physiological functions of Lgr4 in various organs.
Taking advantage of this possibility, we used Lgr4-ED to study the potential roles of Lgr4 in the ovary. Although Lgr4-null female mice also show significant reduction in fertility [35], no obvious histological change has ever been reported in the ovary. By using superovulated female rats for the Lgr4-ED injection, we showed that Lgr4-ED had suppressive effects on the PMSG induction of Lhr expression and on progesterone production in the ovary (Fig. 7). However, we also noticed that changes of steroidogenic gene levels in Lgr4-ED treated animals are not consistent with the dramatic down-regulation of progesterone production. This might be explained by the following reasons. It has been known that the levels of steroidogenic genes can be elevated immediately in the gonadotropin-primed superovulated rodents but then gradually decrease along with the time. Taking Star as an example, the Star transcript in the superovulated rats has been reported to reach the highest level at 6 hr after PMSG injection and return to almost the basal level after two days [43]. However, for clearly comparing the difference of progesterone levels between control and treatment groups, we designed to harvest the ovaries at 48 hr after treatment to provide enough time for progesterone production and accumulation. This may lead us to miss the best time point for quantification of these genes. In addition, down-regulation of Lhr, steroidogenic genes and other yet uncharacterized candidates in Lgr4-ED treated animals may also cause synergetic effects on suppression of progesterone production.
Taken together, our findings suggest that the Lgr4 signaling may help to accelerate the ovarian luteinization process. However, there is a need to consider the possibility that the lack of apparent phenotypes in the Lgr4-null mouse ovaries may be due to signal compensation by other ovary-expressed receptors such as Lgr5, Lgr6, and frizzled, which are also involved in similar downstream to activate the Wnt/β-catenin signaling as Lgr4. One possibility to explain our data is that administration of the recombinant Lgr4-ED protein may absorb all the potential Wnt activators, such as R-spondins and norrin, and thus there will be a magnification of Lgr4-ED’s ovarian effects because of the interruption of other potential Wnt/β-catenin signaling driven by other group B LGRs and frizzled proteins. This hypothesis suggests that there is a need to characterize the detailed expression profiles of all the group B LGR members and to identify their ligand signatures across the various ovarian compartments; this will greatly help our understanding of their interplay and relationships.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Science Council (NSC99-2628-B-010-014-MY3 and NSC102-2320-B-010-026) in Taiwan for CWL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, et al. (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14: 1257–1271. [DOI] [PubMed] [Google Scholar]
- 2. Luo CW, Hsueh AJ (2006) Genomic analyses of the evolution of LGR genes. Chang Gung Med J 29: 2–8. [PubMed] [Google Scholar]
- 3. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108: 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Lau W, Barker N, Low TY, Koo BK, Li VS, et al. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297. [DOI] [PubMed] [Google Scholar]
- 5. Deng C, Reddy P, Cheng Y, Luo CW, Hsiao CL, et al. (2013) Multi-functional norrin is a ligand for the LGR4 receptor. J Cell Sci 126: 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo CW, Dewey EM, Sudo S, Ewer J, Hsu SY, et al. (2005) Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc Natl Acad Sci U S A 102: 2820–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mustata RC, Van Loy T, Lefort A, Libert F, Strollo S, et al. (2011) Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep 12: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Dong J, Li D, Lai L, Siwko S, et al. (2013) Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells 31: 1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo W, Rodriguez M, Valdez JM, Zhu X, Tan K, et al.. (2013) Lgr4 is a key regulator of prostate development and prostate stem cell differentiation. Stem Cells. [DOI] [PMC free article] [PubMed]
- 10. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 11. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, et al. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36. [DOI] [PubMed] [Google Scholar]
- 12. Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, et al. (2012) Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep 2: 540–552. [DOI] [PubMed] [Google Scholar]
- 13. Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 14. Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, et al. (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389. [DOI] [PubMed] [Google Scholar]
- 15. Van Schoore G, Mendive F, Pochet R, Vassart G (2005) Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol 124: 35–50. [DOI] [PubMed] [Google Scholar]
- 16. Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, et al. (2004) Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol 18: 2241–2254. [DOI] [PubMed] [Google Scholar]
- 17. Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, et al. (2006) Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol 290: 421–434. [DOI] [PubMed] [Google Scholar]
- 18.Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, et al.. (2006) LGR4 Regulates the Postnatal Development and Integrity of Male Reproductive Tracts in Mice. Biol Reprod. [DOI] [PubMed]
- 19. Kubota H, Avarbock MR, Brinster RL (2003) Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 100: 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian Y, Liu S, Guan Y, Pan H, Guan X, et al. (2013) Lgr4-mediated Wnt/beta-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development 140: 1751–1761. [DOI] [PubMed] [Google Scholar]
- 21. Pulak R, Anderson P (1993) mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev 7: 1885–1897. [DOI] [PubMed] [Google Scholar]
- 22. You S, Kim H, Hsu CC, El Halawani ME, Foster DN (2000) Three different turkey luteinizing hormone receptor (tLH-R) isoforms I: characterization of alternatively spliced tLH-R isoforms and their regulated expression in diverse tissues. Biol Reprod 62: 108–116. [DOI] [PubMed] [Google Scholar]
- 23. Khan H, Yarney TA, Sairam MR (1993) Cloning of alternately spliced mRNA transcripts coding for variants of ovine testicular follitropin receptor lacking the G protein coupling domains. Biochem Biophys Res Commun 190: 888–894. [DOI] [PubMed] [Google Scholar]
- 24. Graves PN, Tomer Y, Davies TF (1992) Cloning and sequencing of a 1.3 KB variant of human thyrotropin receptor mRNA lacking the transmembrane domain. Biochem Biophys Res Commun 187: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 25. Scott DJ, Tregear GW, Bathgate RA (2005) LGR7-truncate is a splice variant of the relaxin receptor LGR7 and is a relaxin antagonist in vitro. Ann N Y Acad Sci 1041: 22–26. [DOI] [PubMed] [Google Scholar]
- 26. Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, et al. (1999) A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol 13: 2189–2202. [DOI] [PubMed] [Google Scholar]
- 27. Valerio A, Ferraboli S, Paterlini M, Spano P, Barlati S (2001) Identification of novel alternatively-spliced mRNA isoforms of metabotropic glutamate receptor 6 gene in rat and human retina. Gene 262: 99–106. [DOI] [PubMed] [Google Scholar]
- 28. Malherbe P, Kratzeisen C, Lundstrom K, Richards JG, Faull RL, et al. (1999) Cloning and functional expression of alternative spliced variants of the human metabotropic glutamate receptor 8. Brain Res Mol Brain Res 67: 201–210. [DOI] [PubMed] [Google Scholar]
- 29. Schwarz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, et al. (2000) Characterization of gamma-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J Biol Chem 275: 32174–32181. [DOI] [PubMed] [Google Scholar]
- 30. Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405–409. [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126: 1211–1223. [DOI] [PubMed] [Google Scholar]
- 32. Parr BA, McMahon AP (1998) Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395: 707–710. [DOI] [PubMed] [Google Scholar]
- 33. Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D (2008) Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in sertoli cells. Biol Reprod 79: 475–485. [DOI] [PubMed] [Google Scholar]
- 34. Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, et al. (2010) Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod 82: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li XY, Lu Y, Sun HY, Wang JQ, Yang J, et al. (2010) G protein-coupled receptor 48 upregulates estrogen receptor alpha expression via cAMP/PKA signaling in the male reproductive tract. Development 137: 151–157. [DOI] [PubMed] [Google Scholar]
- 36. Han J, Park M, Kim JH, Kim A, Won M, et al. (2009) Increased expression of the testicular estrogen receptor alpha in adult mice exposed to low doses of methiocarb. J Appl Toxicol 29: 446–451. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Zhu J, Li X, Li S, Lan Z, et al.. (2014) Expression of Genomic Functional Estrogen Receptor 1 in Mouse Sertoli Cells. Reprod Sci. [DOI] [PMC free article] [PubMed]
- 38. Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, et al. (2008) 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod 78: 101–114. [DOI] [PubMed] [Google Scholar]
- 39. Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, et al. (1996) Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137: 4796–4805. [DOI] [PubMed] [Google Scholar]
- 40. Arrighi S, Aralla M (2014) Immunolocalization of aquaporin water channels in the domestic cat male genital tract. Reprod Domest Anim 49: 17–26. [DOI] [PubMed] [Google Scholar]
- 41. Fisher JS, Turner KJ, Fraser HM, Saunders PT, Brown D, et al. (1998) Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 139: 3935–3945. [DOI] [PubMed] [Google Scholar]
- 42. Lu DY, Li Y, Bi ZW, Yu HM, Li XJ (2008) Expression and immunohistochemical localization of aquaporin-1 in male reproductive organs of the mouse. Anat Histol Embryol 37: 1–8. [DOI] [PubMed] [Google Scholar]
- 43. Mizutani T, Sonoda Y, Minegishi T, Wakabayashi K, Miyamoto K (1997) Molecular cloning, characterization and cellular distribution of rat steroidogenic acute regulatory protein (StAR) in the ovary. Life Sci 61: 1497–1506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.