Abstract
Purpose of review
The purpose of this article is to examine the contemporary data linking testosterone therapy in overweight and obese men with testosterone deficiency to increased lean body mass, decreased fat mass, improvement in overall body composition and sustained weight loss. This is of paramount importance because testosterone therapy in obese men with testosterone deficiency represents a novel and a timely therapeutic strategy for managing obesity in men with testosterone deficiency.
Recent findings
Long-term testosterone therapy in men with testosterone deficiency produces significant and sustained weight loss, marked reduction in waist circumference and BMI and improvement in body composition. Further, testosterone therapy ameliorates components of the metabolic syndrome. The aforementioned improvements are attributed to improved mitochondrial function, increased energy utilization, increased motivation and vigor resulting in improved cardio-metabolic function and enhanced physical activity.
Summary
The implication of testosterone therapy in management of obesity in men with testosterone deficiency is of paramount clinical significance, as it produces sustained weight loss without recidivism. On the contrary, alternative therapeutic approaches other than bariatric surgery failed to produce significant and sustained outcome and exhibit a high rate of recidivism. These findings represent strong foundations for testosterone therapy in obese men with testosterone deficiency and should spur clinical research for better understanding of usefulness of testosterone therapy in treatment of underlying pathophysiological conditions of obesity.
Keywords: BMI, obesity, testosterone therapy, waist circumference, weight loss
INTRODUCTION
Obesity impacts quality of life and shortens life expectancy. Obesity is a chronic condition that cannot be ameliorated simply with lifestyle behavior alone [1,2]. Obesity contributes to insulin resistance, type 2 diabetes (T2DM) and is associated with a host of comorbidities and therefore represents a healthcare crisis. Lifestyle changes produce modest weight loss in the early stages of weight management strategies, but a high rate of recidivism is observed. Treatment of obesity necessitates evidence-based medical interventions [3,4]. Although lifestyle modifications are highly recommended, as integral part of strategies designed for treatment and management of obesity [5–7], in most patients, such strategies are not always successful in the long term because of high rate of recidivism, in part due to lack of adherence to prescribed regimen [8–11]. The limited benefits of the current approved drugs, together with the undesirable adverse side-effects of such agents in long-term management of obesity have contributed to reduced adherence rates and to discontinuation of use [2]. Efforts to target patient education and increase awareness are warranted.
Desirable outcomes in management of overweight and obesity necessitates development and utilization of new well tolerated and efficacious agents, which can be used in combination with lifestyle changes to achieve weight loss. Contemporary approaches to management of obesity include lifestyle modifications [12] and pharmaco-therapeutic agents, such as incretin and glucagon-like peptide-1 (GLP-1) receptor agonists [13], enzyme inhibitors (dipeptidyl peptidase inhibitors) [14], angiopoietin-like proteins [15] and bariatric surgery [16]. Furthermore, a number of Food and Drug Administration (FDA)-approved drugs for treatment of obesity have serious adverse side-effects and were taken off the market [12,17]. Thus, limited approaches to manage obesity are available except lifestyle changes, which produce moderate effects on weight loss and in most cases are unsustainable. Here, we summarize recent findings related to long-term testosterone therapy that produces improvement in body composition and more specifically weight loss, reduction in waist circumference and BMI. We propose use of testosterone therapy as a new strategy for managing overweight and obesity in men with testosterone deficiency.
Box 1.

no caption available
CLINICAL CHALLENGES IN MANAGEMENT OF OBESITY
Clinicians face complex challenges in their fight to treat and manage obesity. These include a lack of well tolerated and effective pharmaco-therapeutic agents and patients monitoring to safeguard against serious adverse side-effects. Further, FDA-approved pharmaco-therapeutic agents offer only modest benefits in some but not all patients. A more difficult challenge is patients’ adherence and compliance and the perceived moderate effects and the undesirable adverse side-effects that contribute to the limited utility of the available drugs [2]. A number of centrally acting drugs intended to regulate appetite were employed, however, agents, such as fenfluramine, dexfenfluramine, sibutramine, venlafaxine, rimonabant, phentermine diethylpropion; phendimetrazine and benzphentamine, were withdrawn from the market because of adverse side-effects or lack of efficacy in treatment of obesity [2].
NEW APPROACHES FOR TREATMENT OF OBESITY IN MEN WITH TESTOSTERONE DEFICIENCY (HYPOGONADISM)
The prevalence of testosterone deficiency increases with comorbidities, such as insulin resistance and T2DM, obesity, hypertension, and cardiovascular disease (CVD) ranging from 30 to 50% [18–21]. Mulligan et al.[18] reported that approximately 52.4% of all obese men had testosterone levels below 300 ng/dl (10.4 nmol/l). Similarly, Luconi et al.[22] suggested that approximately 75% of men with obesity grade III awaiting bariatric surgery had hypogonadism. In our study of 255 hypogonadal men, we noted 71% of men were obese and 14.1% had obesity grade III, using a testosterone cut-off of 12.1 nmol/l [23▪▪]. Testosterone levels are reduced with increased waist circumference and obesity [17,24] and approximately 40% of obese nondiabetic men and 50% of obese diabetic men aged above 45 years have low free testosterone [18,25]. The concomitant presence of obesity and diabetes is associated with an additional increase in prevalence of testosterone deficiency approaching 34% [18,19,25,26].
Testosterone therapy in men with testosterone deficiency (hypogonadism) has profound effects on body composition, resulting in reduced fat mass, increased lean body mass (LBM) (Table 1) [27,43,44,45▪,46▪▪,47–53] and significant reduction in anthropometric parameters, such as weight, waist circumference and BMI [20,23▪▪,27,37,39,42,43,48,54–56,57▪,58▪▪–61▪▪,62▪,63▪,64–66] (Table 2). The effects of testosterone therapy on increased LBM and reduced fat mass and the changes in anthropometric parameters were consistently reported in most studies [20–22,23▪▪,24–44,45▪,46▪▪,47–56,57▪,58▪▪–61▪▪,62▪,63▪,64–66], irrespective of testosterone formulations used or duration of testosterone treatment (Tables 1 and 2). On the basis of the consistent findings of testosterone therapy, which demonstrated significant reductions in total body fat mass and increases in LBM [23▪▪,27–44,45▪,46▪▪,47–56,57▪,58▪▪–61▪▪,62▪,63▪,64–66] as well as improvement in weight loss and reductions in waist circumference and BMI (Tables 1 and 2), Allan and Mclachlan [68] proposed the use of testosterone therapy in men with testosterone deficiency for management of obesity. Saad et al.[69] further proposed testosterone therapy as a new potential intervention strategy for managing obesity in hypogonadal men (testosterone deficiency). The data from recent studies with long-term testosterone therapy in men with testosterone deficiency, using testosterone formulations which result in sufficient circulating physiological testosterone levels and good patient adherence, reported significant and sustained weight loss, reduced BMI and waist circumference [46▪▪,58▪▪–61▪▪,70]. These findings were corroborated by studies in which testosterone therapy produced significant and sustained weight loss [54–56,57▪,58▪▪–61▪▪,62▪,63▪,64–67] (Table 2).
Table 1.
Testosterone therapy increases lean body mass and reduces total body fat mass in men with testosterone deficiency
| Study | Testosterone formulation | Treatment period | Lean body mass | Fat mass |
| Marin et al. [27] | Gel | 9 months | ↑ | ↓ |
| Snyder et al. [28] | Patch | 36 months | ↑ | ↓ |
| Kenny et al. [29] | Patch | 12 months | ↑ | ↓ |
| Crawford et al. [30] | Mixed esters | 12 months | ↑ | ↓ |
| Ferrando et al. [31] | TE | 6 months | ↑ | ↓ |
| Steidle et al. [32] | Gel | 3 months | ↑ | ↓ |
| Wittert et al. [33] | Oral TU | 12 months | ↑ | ↓ |
| Casaburi et al. [34] | TE | 3 months | ↑ | ↓ |
| Page et al. [35] | TE | 36 months | ↑ | ↓ |
| Kapoor et al. [20] | Mixed esters | 3 months | ↑ | ↓ |
| Bhasin et al. [36] | TE | 5 months | ↑ | ↓ |
| Kapoor et al. [37] | Mixed esters | 3 months | ↑ | ↓ |
| Bhasin et al. [38] | Gel | 6 months | ↑ | ↓ |
| Svartberg et al. [39] | Injectable TU | 12 months | ↑ | ↓ |
| Allan et al. [40] | Patch | 12 months | ↑ | ↓ |
| Srinivas-Shankar et al. [41] | Gel | 6 months | ↑ | ↓ |
| Aversa et al. [42] | Injectable TU | 24 months | ↑ | ↓ |
| Aversa et al. [43] | Injectable TU | 12 months | ↑ | ↓ |
| Behre et al. [44] | Gel | 6 months | ↑ | ↓ |
| Finkelstein et al. [45▪] | Gel | 4 months | ↑ | ↓ |
| Francomano et al. [46▪▪] | Injectable TU | 60 months | ↑ | ↓ |
| Bouloux et al. [47] | Oral TU | 12 months | ↑ | ↓ |
| Pexman-Fieth et al. [48] | Gel | 6 months | ↑ | ↓ |
| Juang et al. [49] | Gel | 3 months | ↑ | ↓ |
| Rodriguez-Tolra et al. [50] | Gel/Injectable TU | 24 months | ↑ | ↓ |
| Frederiksen et al. [51] | Gel | 6 months | ↑ | ↓ |
| Emmelot-Vonk et al. [52] | Oral TU | 6 months | ↑ | ↓ |
| Borst et al. [53] | TE | 12 months | ↑ | ↓ |
TE, testosterone enanthate; TU, testosterone undecanoate
Table 2.
Effects of testosterone therapy on weight loss, waist circumference and BMI
| Study | Testosterone formulation | Treatment period | Weight loss | Waist circumference | Body mass index |
| Marin et al. [27] | Gel | 9 months | ND | ↓ | ND |
| Kapoor et al. [20,37] | Mixed esters | 3 months | ND | ↓ | ND |
| Svartberg et al. [39] | Injectable TU | 12 months | ND | ↓ | ND |
| Heufelder et al. [54] | Gel | 12 months | ND | ↓ | ND |
| Aversa et al. [42] | Injectable TU | 24 months | ND | ↓ | ND |
| Aversa et al. [43] | Injectable TU | 12 months | ND | ↓ | ND |
| Kalinchenko et al. [55] | Injectable TU | 7 months | ↓ | ↓ | ↓ |
| Aversa et al. [56] | Injectable TU | 36 months | ND | ↓ | ND |
| Zitzmann et al. [57▪] | Injectable TU | 9–12 months | ND | ↓ | ND |
| Francomano et al. [46▪▪] | Injectable TU | 60 months | ↓ | ↓ | ↓ |
| Francomano et al. [58▪▪] | Injectable TU | 12 months | ↓ | ↓ | ↓ |
| Haider et al. [59▪▪] | Injectable TU | 12–72 months | ↓ | ↓ | ↓ |
| Haider et al. [60▪▪] | Injectable TU | 12–72 months | ↓ | ↓ | ↓ |
| Saad et al. [23▪▪] | Injectable TU | 12–60 months | ↓ | ↓ | ↓ |
| Yassin and Doros [61▪▪] | Injectable TU | 12–60 months | ↓ | ↓ | ↓ |
| Pexman-Fieth et al. [48] | Gel | 6 months | ↓ | ↓ | ↓ |
| Hackett et al. [62▪,63▪] | Injectable TU | 7 and 20 months | ↓ | ↓ | ↓ |
| Bhattacharya et al. [64,65] | Gel | 12 months | ND | ↓ | ND |
| Garcia et al. [66] | Injectable TU | 24 months | ND | ↓ | ND |
| Zitzmann et al. [72] | Injectable TU | 12–192 months | ↓ | ↓ | ↓ |
ND, no data; TU, testosterone undecanoate.
EFFECTS OF TESTOSTERONE THERAPY ON OBESE MEN WITH TESTOSTERONE DEFICIENCY AND VARIOUS GRADES OF OBESITY
Obesity is categorized into three grades on the basis of patients’ BMI. BMI ranging from 30 to 34.9 kg/m2 falls in grade I, whereas BMI ranging from 35 to 39.9 kg/m2 falls in grade II and BMI more than 40 kg/m2 is categorized as grade III. Data reported from three registries [23▪▪,59▪▪–61▪▪], in which long-term testosterone therapy was evaluated in men with testosterone deficiency and varying grades of obesity, suggested that testosterone therapy is effective in producing weight loss in all three grades of obesity. As shown in Fig. 1, long-term testosterone therapy produced significant progressive, sustained weight loss without recidivism in men with testosterone deficiency with various grades of obesity, [23▪▪,59▪▪–61▪▪]. This reduction in weight was also associated with marked and significant reduction in waist circumference (Fig. 2) [23▪▪,59▪▪–61▪▪]. These findings are consistent with those reported in Table 2 and strongly support the concept that testosterone therapy in men with testosterone deficiency represents a novel and useful therapeutic strategy for treatment and management of obesity in hypogonadal obese men.
FIGURE 1.
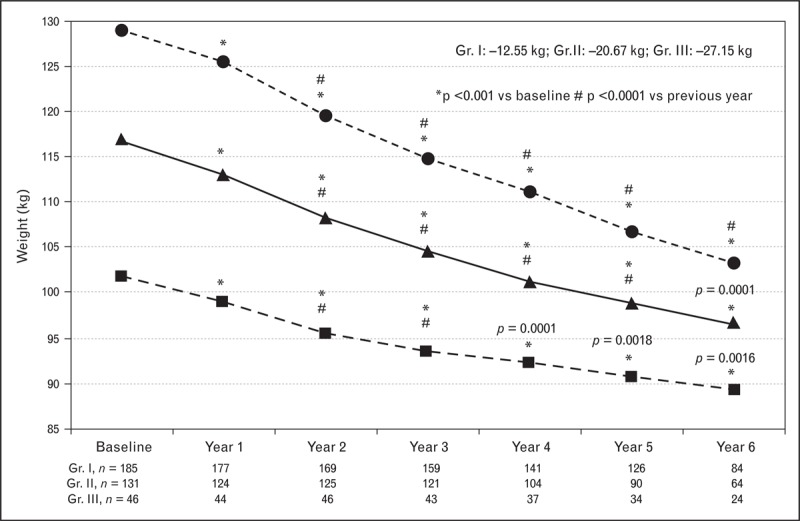
Testosterone therapy in men with testosterone deficiency and differing grade of obesity produces significant and sustained weight loss. Hypogonadal men (n = 362) with obesity grade I (Gr. I, n = 185, mean age: 58.39 ± 8.04 years), grade II (Gr. II, n = 131, mean age: 60.62 ± 5.56 years) and grade III (Gr. III, n = 46, mean age: 60.28 ± 5.39 years) treated with testosterone undecanoate injections for up to 6 years. Weight expressed in kilogram. Adapted with permission from [60▪▪].
FIGURE 2.
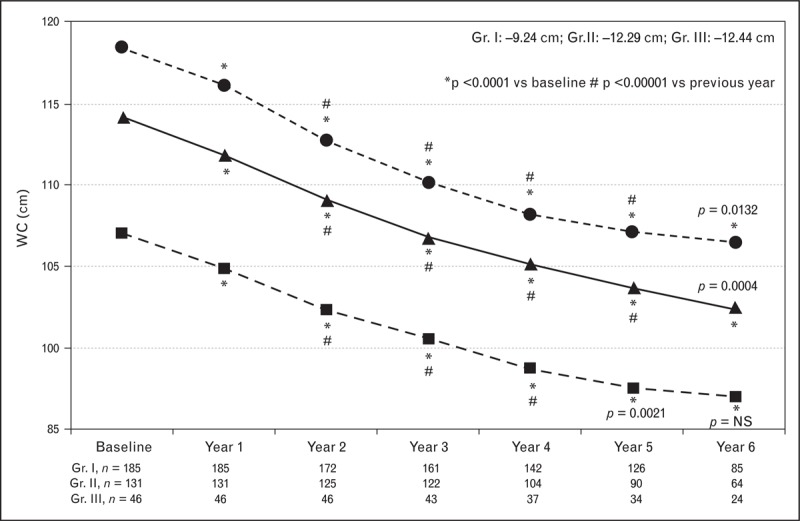
Testosterone therapy in men with testosterone deficiency and differing grade of obesity produces marked and sustained reductions in waist circumference. Waist circumference (cm) in 362 hypogonadal men with obesity grade I (Gr. I, n = 185, mean age: 58.39 ± 8.04 years), grade II (Gr. II, n = 131, mean age: 60.62 ± 5.56 years) and grade III (Gr. III, n = 46, mean age: 60.28 ± 5.39 years) Treated with testosterone undecanoate injections for up to 6 years. Adapted from [60▪▪].
TESTOSTERONE THERAPY IN MEN WITH TESTOSTERONE DEFICIENCY IS ASSOCIATED WITH WEIGHT LOSS
Testosterone therapy is associated with weight loss [23▪▪,42,56,58▪▪,59▪▪] and obesity is associated with reduced testosterone levels [68,73–77,78▪,79]. The potential mechanisms involved in low testosterone levels in obesity encompass complex mechanisms, including increased levels of sex hormone binding globulin (SHBG), low or inappropriate normal levels of luteinizing hormone, adipocyte dysfunction, androgen resistance and insulin resistance. Loss of androgen receptor function increases the number of adipocytes and the accumulation of visceral fat [80]. Low baseline testosterone predicts obesity in men [81] and normalization of physiological testosterone levels reduces the activity of lipoprotein lipase and tryglycerides [77]. Testosterone treatment results in improved insulin sensitivity, lipid oxidation and reduction in fat mass with concomitant gain in fat free mass. Camacho et al.[78▪] reported that weight loss is associated with increased testosterone levels, a finding supported by Corona et al.[79]. Intervention measures, such as diet and exercise or surgical treatment of obesity, results in increased testosterone levels [79,82,83].
Effects of testosterone therapy on myogenesis and adipogenesis
Androgens regulate myogenesis and inhibit adipogenesis [67,84–88]. Maneschi et al.[89] reported that testosterone therapy preserves visceral adipose tissue function, and testosterone deficiency results in derangement and dysfunction of visceral adipose tissue metabolism. Men with testosterone deficiency and obesity have reduced testosterone levels and weight loss produces increased total testosterone levels [79,78▪].
Effects of testosterone therapy on carbohydrate, protein and fat metabolism
Testosterone regulates carbohydrates, proteins and fat metabolism [79,89] and testosterone therapy in men with testosterone deficiency results in normalization of glucose utilization and increased lipid oxidation [90]. Testosterone deficiency affects energy production and utilization and therefore upsets this physiological balance resulting in storage of lipids and increased adipogenesis and altering mitochondrial function [89–94]. Obesity contributes to premature cardiac aging via disrupted mitochondrial biogenesis and function and is an independent risk factor for development of heart failure [92–94]. Testosterone therapy ameliorates this dysfunction.
Effects of testosterone therapy in men with testosterone deficiency on fatigue, motivation, vigor and physical activity
Yu and Traish [95] suggested that testosterone deficiency contributes to fatigue via alterations of mitochondrial function and energy production and utilization. Testosterone treatment of 1053 hypogonadal men produced increased quality of life with reduced fatigue, increased libido and erectile function and reduced waist circumference [48]. These findings are supported by several other studies in which testosterone therapy resulted in improved motivation, vigor, energy, and reduces fatigue concomitant with significant reduction in waist circumference and improvement in quality of life [44,47,48,57▪,95]. Healthy volunteers, who received a single injection of testosterone undecanoate experienced reduction in fatigue-inertia significantly versus placebo [96]. The increased motivation, energy and reduced fatigue in response to testosterone therapy significantly contribute to more physical activity, thus resulting in increased energy utilization associated with increased muscle mass and improved mitochondrial function. This, in part, explains the observed weight loss with testosterone therapy in men treated for long durations with appropriate testosterone formulations producing physiological levels [58▪▪–61▪▪,72]. In contrast, androgen deprivation therapy (ADT) used in management of hormone-dependent prostate cancer results in fatigue and reduces energy and motivation [95,97].
Effects of testosterone therapy on fat deposition and vascular health
Weight gain is attributed to 88% increased fat mass, and weight loss is associated with 72% decrease in fat mass and 28% decrease in LBM [98]. One of the key observations in testosterone therapy is that testosterone increases LBM, thus increasing resting energy expenditure. Testosterone therapy in men with testosterone deficiency with or without diet and physical activity not only produced reduction in fat mass but also improvements in cardiometabolic function and reduced carotid intima media thickness (CIMT), epicardial fat and trunk fat [46▪▪,58▪▪,70]. Also reduction in CIMT was noted with testosterone but not with diet and physical exercise alone. Reduction in CIMT with testosterone therapy was previously demonstrated [42,71]. Testosterone therapy for 18 weeks reduced ectopic and liver fat in obese men [99▪], suggesting that testosterone therapy has a protective effect on the cardiovascular system and reduces the risk of CVD. Testosterone therapy for 12 months in men with testosterone deficiency and spinal cord injury significantly improved LBM and resting energy and percentage basal energy expenditure [100]. The predictors of weight regain are reduced levels of testosterone, retinol binding protein 4, SHBG and MetS [101,102▪]. These findings support a role for testosterone therapy in management of obesity.
TESTOSTERONE THERAPY AMELIORATES METABOLIC SYNDROME COMPONENTS, IMPROVES SEXUAL FUNCTION, ENERGY, MOOD, MOTIVATION AND QUALITY OF LIFE
Testosterone therapy ameliorated components of MetS [62▪,63▪,64,65,103▪▪,104]. Testosterone therapy significantly improved Homeostasis Model Assessment (HOMA)-insulin resistance, CIMT and hsCRP, TNF-α, weight, BMI and waist circumference [39,42,43,46▪▪,55,58▪▪,70]. A controlled 5-year study in men with MetS showed significant decreases in weight, waist circumference, BMI, HbA1c, HOMA-insulin resistance, total cholesterol, low density lipoprotein (LDL)-cholesterol, triglycerides, hsCRP, systolic and diastolic blood pressure, and an increase in HDL [46▪▪,70]. Long-term testosterone therapy in men with testosterone deficiency produced a significant reduction in total cholesterol, LDL cholesterol, triglycerides, and increased HDL. Testosterone treatment reduced fasting glucose, HbA1c, the nonspecific inflammatory marker hsCRP and liver enzymes aspartate aminotransferase and alanine aminotransferase suggesting improvement in hyperglycemia and a reduction in the inflammatory response [23▪▪,46▪▪,58▪▪–61▪▪,70,89,105–107]. Further, testosterone therapy reduces inflammation, improves erectile function and increases vigor and reduces fatigue. These changes in physical and behavioral activity result in improved quality of life.
CHALLENGES AND LIMITATIONS OF TESTOSTERONE THERAPY IN OBESE MEN WITH TESTOSTERONE DEFICIENCY
Among the challenges of testosterone therapy is the myth that testosterone causes prostate cancer (PCa). Although this myth has been debunked [108,109], the fear of physicians from litigation has presented a huge challenge to testosterone therapy in men with testosterone deficiency [110,111]. Recently, another challenge was raised suggesting that testosterone therapy causes myocardial infarctions (MIs), stroke and death [112–114]. Although these reports suffer from serious methodological flaws and poor scientific evidence-based medicine, the purported information that testosterone therapy is harmful has confounded the knowledge gained from more than 4 decades of experience with testosterone therapy, and is in direct contradiction with this large body of actual patient data [115]. A large number of studies have shown that testosterone therapy does not increase the risk of MI, stroke or death [115]. On the contrary, testosterone deficiency is considered a risk of CVD [115]. Jespersen et al.[116] reported ADT is associated with greater risk for MI and stroke in men with PCa. Keating et al.[117] showed that ADT is associated with worsening of diabetes control and increased despite the use of other diabetes medications.
EXPERT OPINION
One may argue that testosterone therapy has been around for more than 75 years and no data have been reported on the effects of testosterone on weight loss, until recently. So, why now and how could this be explained? Several key reasons explain this deficit in the literature. First, most studies reported on testosterone therapy were of very short duration and this does not permit necessary tissue remodeling and changes in LBM and fat mass, which require longer time periods. Second, testosterone formulations used in many prior studies did not provide sustained physiological levels of testosterone and in most cases the circulating testosterone levels were suboptimal, thus resulting in incomplete responses. Third, in addition, poor patients’ compliance to testosterone therapy is of paramount importance in the effectiveness of testosterone therapy. These factors explain, in part, the neutral effects of testosterone therapy on weight observed in some studies. Schoenfeld et al.[118] showed that adherence rates to testosterone therapy are variable depending on formulations and therefore differing outcomes are expected.
It should be noted that testosterone deficiency is associated with a shift in fuel metabolism from lipid oxidation toward glucose utilization [119] and testosterone therapy [51,120] increased muscle mass and lipid oxidation in aging men. Furthermore, higher endogenous circulating testosterone levels were associated with reduced loss of LBM in elderly men [121] and testosterone therapy in frail men preserves muscle thickness [122]. Thus, it is not surprising that in obese men with testosterone deficiency, long-term testosterone therapy with formulations that achieve physiological levels, along with adequate adherence, produced significant and sustained weight loss, concomitant with reduction in waist circumference and BMI (Table 2, Figs 1 and 2) [23▪▪,27–44,45▪,46▪▪,47–56,57▪,58▪▪–61▪▪,62▪,63▪,64–66,72]. Further, long-term testosterone therapy in men with testosterone deficiency produced improvements in cardio-metabolic function, ameliorated MetS components, reduced fatigue, increased vigor and energy and improved quality of life [62▪,63▪,64–66,103▪▪,104–107,123]. We suggest that testosterone therapy offers well tolerated and effective treatment of obesity in men with testosterone deficiency and this novel approach provides a unique opportunity to manage obese men. Other therapeutic targets for the treatment of obesity have been proposed including hypothalamic malonyl-CoA and CPT1c [124,125] and GLP-1, oxyntomodulin, peptide YY, gastric inhibitory peptide and ghrelin [126]. These targets may prove useful in addition to testosterone therapy.
CONCLUSION
Lifestyle modifications are considered a cornerstone in combating obesity. However, this is difficult to maintain over the long term, and the ability to achieve modest weight loss with lifestyle modification is limited, at best. Pharmacotherapy coupled with lifestyle modification provides an alternative to combating obesity with lifestyle changes alone. We propose that testosterone therapy in obese men with testosterone deficiency offers a well tolerated and effective therapy and produces sustained and significant weight loss. Testosterone therapy increases LBM, reduces fat mass and produces sustained and significant weight loss, reduction in waist circumference and BMI. We believe that testosterone therapy in obese men with testosterone deficiency is a unique and effective therapeutic approach to management of obesity. The fact that this therapy has been used over the past 7 decades to treat hypogonadism (testosterone deficiency) and is proven to be well tolerated and effective should be an added tool to the armament for the war on obesity.
Acknowledgements
This work was solely supported by the Department of Urology, Boston University School of Medicine, Boston, MA02118, USA. I would like to express my sincere appreciation to my colleagues Dr Andre T Guay and Dr Mohit Khera for their thoughtful reading of the manuscript and for their helpful and constructive comments.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Taubes G. Treat obesity as physiology, not physics. Nature 2012; 492:155. [DOI] [PubMed] [Google Scholar]
- 2.Gadde KM. Current pharmacotherapy for obesity: extrapolation of clinical trials data to practice. Expert Opin Pharmacother 2014; 15:809–822 [DOI] [PubMed] [Google Scholar]
- 3.Colagiuri S. Diabesity: therapeutic options. Diabetes Obes Metab 2010; 12:463–473 [DOI] [PubMed] [Google Scholar]
- 4.Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review). J Diabetes Complications 2013; 27:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the obesity Society. J Am Coll Cardiol 2014; 63:2985–3023 [DOI] [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the obesity Society. Circulation 2014; 129:S102–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyer VA. U. S. Preventive Services Task Force Screening for and management of obesity in adults: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157:373–378 [DOI] [PubMed] [Google Scholar]
- 8.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes 1989; 13:123–136 [PubMed] [Google Scholar]
- 9.Ayyad C, Andersen T. Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obes Rev 2000; 1:113–119 [DOI] [PubMed] [Google Scholar]
- 10.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005; 29:1153–1167 [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Sternberg JA, Letizia KA, et al. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 1989; 13 Suppl 2:39–46 [PubMed] [Google Scholar]
- 12.Dyson PA. The therapeutics of lifestyle management on obesity. Diabetes Obes Metab 2010; 12:941–946 [DOI] [PubMed] [Google Scholar]
- 13.Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012; 36:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raz I, Hanefeld M, Xu L, et al. Sitagliptin Study 023 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571 [DOI] [PubMed] [Google Scholar]
- 15.Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS J 2011; 278:559–564 [DOI] [PubMed] [Google Scholar]
- 16.Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013; 258:628–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chilton M, Dunkley A, Carter P, et al. The effect of antiobesity drugs on waist circumference: a mixed treatment comparison. Diabetes Obes Metab 2014; 16:237–247 [DOI] [PubMed] [Google Scholar]
- 18.Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007; 30:911–917 [DOI] [PubMed] [Google Scholar]
- 20.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906 [DOI] [PubMed] [Google Scholar]
- 21.Dhindsa S, Prabhakar S, Sethi M, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:5462–5468 [DOI] [PubMed] [Google Scholar]
- 22.Luconi M, Samavat J, Seghieri G, et al. Determinants of testosterone recovery after bariatric surgery: is it only a matter of reduction of body mass index? Fertil Steril 2013; 99:1872–1879 [DOI] [PubMed] [Google Scholar]
- 23▪▪.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013; 21:1975–1981 [DOI] [PubMed] [Google Scholar]; This study is one of two observational registry studies demonstrating progressive, sustainable, clinically meaningful weight loss in hypogonadal men treated with testosterone for up to 5 years.
- 24.Svartberg J, von Mühlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. The Tromsø study. Eur J Epidemiol 2004; 19:657–663 [DOI] [PubMed] [Google Scholar]
- 25.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corona G, Mannucci E, Mansani R, et al. Organic, relational and psychological factors in erectile dysfunction in men with diabetes mellitus. Eur Urol 2004; 46:222–228 [DOI] [PubMed] [Google Scholar]
- 27.Marin P, Holmang S, Gustafsson C, et al. Androgen treatment of abdominally obese men. Obes Res 1993; 1:245–251 [DOI] [PubMed] [Google Scholar]
- 28.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999; 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- 29.Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001; 56:M266–272 [DOI] [PubMed] [Google Scholar]
- 30.Crawford BA, Liu PY, Kean MT, et al. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 2003; 88:3167–3176 [DOI] [PubMed] [Google Scholar]
- 31.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 2002; 282:E601–607 [DOI] [PubMed] [Google Scholar]
- 32.Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003; 88:2673–2681 [DOI] [PubMed] [Google Scholar]
- 33.Wittert GA, Chapman IM, Haren MT, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 2003; 58:618–625 [DOI] [PubMed] [Google Scholar]
- 34.Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170:870–878 [DOI] [PubMed] [Google Scholar]
- 35.Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl 2005; 26:85–92 [PubMed] [Google Scholar]
- 36.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005; 90:678–688 [DOI] [PubMed] [Google Scholar]
- 37.Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2007; 156:595–602 [DOI] [PubMed] [Google Scholar]
- 38.Bhasin S, Parker RA, Sattler F, et al. AIDS Clinical Trials Group, Protocol A5079 Study Team Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab 2007; 92:1049–1057 [DOI] [PubMed] [Google Scholar]
- 39.Svartberg J, Agledahl I, Figenschau Y, et al. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res 2008; 20:378–387 [DOI] [PubMed] [Google Scholar]
- 40.Allan CA, Strauss BJ, Burger HG, et al. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 2008; 93:139–146 [DOI] [PubMed] [Google Scholar]
- 41.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2010; 95:639–650 [DOI] [PubMed] [Google Scholar]
- 42.Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503 [DOI] [PubMed] [Google Scholar]
- 43.Aversa A, Bruzziches R, Francomano D, et al. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest 2010; 33:776–783 [DOI] [PubMed] [Google Scholar]
- 44.Behre HM, Tammela TL, Arver S, et al. European Testogel® Study Team, Giltay EJ, Gooren LJ A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012; 15:198–207 [DOI] [PubMed] [Google Scholar]
- 45▪.Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013; 369:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study experimentally showed that mid-normal levels of testosterone are required to prevent an increase in fat mass.
- 46▪▪.Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol 2014; 2014:527470. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was the first long term study (5-years duration) with a control group showing progressive, sustained and clinically meaningful reductions in weight and waist circumference.
- 47.Bouloux PM, Legros JJ, Elbers JM, et al. Study 43203 Investigators Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: a 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 2013; 16:38–47 [DOI] [PubMed] [Google Scholar]
- 48.Pexman-Fieth C1, Behre HM, Morales A, et al. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male 2014; 17:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juang PS, Peng S, Allehmazedeh K, et al. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med 2014; 11:563–573 [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Tolrà J, Torremadé Barreda J, del Rio L, et al. Effects of testosterone treatment on body composition in males with testosterone deficiency syndrome. Aging Male 2013; 16:184–190 [DOI] [PubMed] [Google Scholar]
- 51.Frederiksen L, Højlund K, Hougaard DM, et al. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr) 2012; 34:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emmelot-Vonk MH, Verhaar HJJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men. J Am Med Assoc 2008; 299:39–52 [DOI] [PubMed] [Google Scholar]
- 53.Borst SE, Yarrow JF, Conover CF, et al. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab 2014; 306:E433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009; 30:726–733 [DOI] [PubMed] [Google Scholar]
- 55.Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010; 73:602–612 [DOI] [PubMed] [Google Scholar]
- 56.Aversa A, Bruzziches R, Francomano D, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male 2012; 15:96–102 [DOI] [PubMed] [Google Scholar]
- 57▪.Zitzmann M, Mattern A, Hanisch J, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med 2013; 10:579–588 [DOI] [PubMed] [Google Scholar]; This was the largest study in a mixed hypogonadal population from 22 countries including Asian patients showing a significant reduction of waist circumference.
- 58▪▪.Francomano D, Bruzziches R, Barbaro G, et al. Effects of testosteroneundecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest 2014; 37:401–411 [DOI] [PubMed] [Google Scholar]; This study was the first controlled study in hypogonadal men with obesity grade III showing significant benefits of testosterone therapy in addition to diet and exercise; the study also showed that some effects can be achieved by testosterone therapy but not by diet and exercise alone. The study also showed that discontinuation of interventions resulted in reversal of some but not all of the achieved effects.
- 59▪▪.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with ‘diabesity’: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014; 2014:683515. [DOI] [PMC free article] [PubMed] [Google Scholar]; This observational 6-year long study examined the effects of testosterone therapy in obese hypogonadal men with type 2 diabetes; this study showed that beneficial effects on weight, waist circumference and glycaemic control were progressive and sustainable over the full observation time.
- 60▪▪.Haider A, Yassin A, Doros G, et al. Reductions of weight and waist size in 362 hypogonadal men with obesity grades I to III under long-term treatment with testosterone undecanoate (TU): observational data from two registry studies. Endocr Rev 2014; 35:SUN-0895 [Google Scholar]; This study is a pooled analysis from two observational registries demonstrating progressive, sustainable, clinically meaningful weight loss in hypogonadal men with three grades of obesity receiving up to 6-years testosterone therapy.
- 61▪▪.Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes 2013; 3:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is one of two observational registries demonstrating progressive, sustainable, clinically meaningful weight loss in hypogonadal men receiving up to 5-year testosterone therapy.
- 62▪.Hackett G, Cole N, Bhartia M, et al. BLAST Study Group Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med 2014; 11:840–856 [DOI] [PubMed] [Google Scholar]; This study is the longest study to date in hypogonadal men with type 2 diabetes showing reductions in weight and waist circumference.
- 63▪.Hackett G, Cole N, Bhartia M, et al. Blast Study Group The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract 2014; 68:203–215 [DOI] [PubMed] [Google Scholar]; This study is a controlled study demonstrating that reaching target testosterone levels is essential to achieve meaningful results.
- 64.Bhattacharya RK, Khera M, Blick G, et al. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim Registry in the US (TRiUS). BMC Endocr Disord 2011; 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharya RK, Khera M, Blick G, et al. Testosterone replacement therapy among elderly males: the Testim Registry in the US (TRiUS). Clin Interv Aging 2012; 7:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia JA, Sanchez PE, Fraile C, Escovar P. Testosterone undecanoate improves erectile dysfunction in hypogonadal men with the metabolic syndrome refractory to treatment with phosphodiesterase type 5 inhibitors alone. Andrologia 2011; 43:293–296 [DOI] [PubMed] [Google Scholar]
- 67.Miller KK. Androgen deficiency: effects on body composition. Pituitary 2009; 12:116–124 [DOI] [PubMed] [Google Scholar]
- 68.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes 2010; 17:224–232 [DOI] [PubMed] [Google Scholar]
- 69.Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev 2012; 8:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Francomano D, Ilacqua A, Bruzziches R, et al. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology 2014; 83:167–173 [DOI] [PubMed] [Google Scholar]
- 71.Zitzmann M, Vorona E, Wenk M, et al. Testosterone administration decreases carotid artery intima media thickness as indicator of vascular damage in middle-aged overweight men. J Androl 2008; 29 Suppl.:54–55 [Google Scholar]
- 72.Zitzmann M, Saad F, Kliesch S. Long-term treatment with testosterone undecanoate injections leads to sustained weight loss and improvement of metabolic syndrome parameters in 381 hypogonadal men. J Sex Med 2014; 11 Suppl. 1:7 [Google Scholar]
- 73.Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818 [DOI] [PubMed] [Google Scholar]
- 74.Glass AR, Swerdloff RS, Bray GA, et al. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab 1977; 45:1211–1219 [DOI] [PubMed] [Google Scholar]
- 75.Travison TG, Araujo AB, Kupelian V, et al. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 2007; 92:549–555 [DOI] [PubMed] [Google Scholar]
- 76.Haring R, Ittermann T, Volzke H, et al. Prevalence, incidenc and risk factors of testosterone deficiency in a population-based cohort of men:results from the study of health in Pomerania. Aging Male 2010; 13:247–257 [DOI] [PubMed] [Google Scholar]
- 77.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab 2011; 96:2341–2353 [DOI] [PubMed] [Google Scholar]
- 78▪.Camacho EM, Huhtaniemi IT, O’Neill TW, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol 2013; 168:445–455 [DOI] [PubMed] [Google Scholar]; This study showed that changes in weight have a greater effect on testosterone than age.
- 79.Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 2013; 168:829–843 [DOI] [PubMed] [Google Scholar]
- 80.Rana K, Fam BC, Clarke MV, et al. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab 2011; 301:E767–E778 [DOI] [PubMed] [Google Scholar]
- 81.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese–American men. Int J Obes Relat Metab Disord 2000; 24:485–491 [DOI] [PubMed] [Google Scholar]
- 82.Facchiano E, Scaringi S, Veltri M, et al. Age as a predictive factor of testosterone improvement in male patients after bariatric surgery: preliminary results of a Monocentric Prospective Study. Obes Surg 2013; 23:167–172 [DOI] [PubMed] [Google Scholar]
- 83.Khoo J, Piantadosi C, Duncan R, et al. Comparing effectsof a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 2011; 8:2868–2875 [DOI] [PubMed] [Google Scholar]
- 84.Chazenbalk G1, Singh P, Irge D, et al. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013; 78:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003; 144:5081–5508 [DOI] [PubMed] [Google Scholar]
- 86.Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 2006; 147:141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhasin S. Regulation of body composition by androgens. J Endocrinol Invest 2003; 26:814–822 [DOI] [PubMed] [Google Scholar]
- 88.Bhasin S, Taylor WE, Singh R, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci 2003; 58:M1103–1110 [DOI] [PubMed] [Google Scholar]
- 89.Maneschi E, Morelli A, Filippi S, et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol 2012; 215:347–362 [DOI] [PubMed] [Google Scholar]
- 90.Høst C, Gormsen LC, Christensen B, et al. Independent effects of testosterone on lipid oxidation and VLDL-TG production: a randomized, double-blind, placebo-controlled, crossover study. Diabetes 2013; 62:1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Traish A, Abdallah B, Yu G. Androgen deficiency and mitochondrial dysfunction: implications for fatigue, muscle dysfunction, insulin resistance, diabetes, and cardiovascular disease. Horm Mol Biol Clin Invest 2011; 8:431–444 [DOI] [PubMed] [Google Scholar]
- 92.Abel ED. Obesity stresses cardiac mitochondria even when you are young. J Am Coll Cardiol 2011; 57:586–589 [DOI] [PubMed] [Google Scholar]
- 93.Niemann B, Chen Y, Teschner M, et al. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am CollCardiol 2011; 57:577–585 [DOI] [PubMed] [Google Scholar]
- 94.Yin X, Lanza IR, Swain JM, et al. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 2014; 99:E209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu G, Traish A. Induced testosterone deficiency: from clinical presentation of fatigue, erectile dysfunction and muscle atrophy to insulin resistance and diabetes. Horm Mol Biol Clin Invest 2011; 8:425–430 [DOI] [PubMed] [Google Scholar]
- 96.O’Connor DB, Archer J, Wu FCW. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab 2004; 89:2837–2845 [DOI] [PubMed] [Google Scholar]
- 97.Cary KC, Singla N, Cowan JE, et al. Impact of androgen deprivation therapy on mental and emotional well being in men with prostate cancer: analysis from the CaPSURE™ Registry. J Urol 2014; 191:964–970 [DOI] [PubMed] [Google Scholar]
- 98.Pourhassan M, Bosy-Westphal A, Schautz B, et al. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr 2014; 99:779–791 [DOI] [PubMed] [Google Scholar]
- 99▪.Hoyos CM, Yee BJ, Phillips CL, et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol 2012; 167:531–541 [DOI] [PubMed] [Google Scholar]; This is the first randomized controlled trial to demonstrate by diagnostic imaging techniques that testosterone reduces liver fat content.
- 100.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 2011; 43:574–579 [DOI] [PubMed] [Google Scholar]
- 101.McInnes KJ, Smith LB, Hunger NI, et al. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes 2012; 61:1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102▪.Wang P, Menheere PP, Astrup A, et al. Metabolic syndrome, circulating RBP4, testosterone, and SHBG predict weight regain at 6 months after weight loss in men. Obesity (Silver Spring) 2013; 21:1997–2006 [DOI] [PubMed] [Google Scholar]; This study showed that testosterone as well as three other factors controlled by or associated with testosterone are predictors of weight regain following weight loss in men.
- 103▪▪.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract 2014; 68:314–329 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first long-term registry study showing that testosterone therapy up to 5 years improves all metabolic syndrome components in a sustainable manner.
- 104.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011; 34:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283 [DOI] [PubMed] [Google Scholar]
- 106.Corona G, Isidori AM, Buvat J, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med 2014; 11:1577–1592 [DOI] [PubMed] [Google Scholar]
- 107.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health 2013; 31:103–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320 [DOI] [PubMed] [Google Scholar]
- 109.Morgentaler A. Goodbye androgen hypothesis, hello saturation model. Eur Urol 2012; 62:765–767 [DOI] [PubMed] [Google Scholar]
- 110.Gooren LJ, Behre HM, Saad F, et al. Diagnosing and treating testosterone deficiency in different parts of the world. Results from global market research. Aging Male 2007; 10:173–181 [DOI] [PubMed] [Google Scholar]
- 111.Khera M, Crawford D, Morales A, et al. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol 2014; 65:115–123 [DOI] [PubMed] [Google Scholar]
- 112.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013; 310:1829–1836 [DOI] [PubMed] [Google Scholar]
- 114.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of nonfatal myocardial infarction following testosterone therapy prescription in men. PLoS One 2014; 9:e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Traish AM, Guay AT, Morgentaler A. Death by testosterone? We think not!. J Sex Med 2014; 11:624–629 [DOI] [PubMed] [Google Scholar]
- 116.Jespersen CG, Nørgaard M, Borre M. Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population-based cohort study. Eur Urol 2014; 65:704–709 [DOI] [PubMed] [Google Scholar]
- 117.Keating NL, Liu PH, O’Malley AJ, et al. Androgen-deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol 2014; 65:816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. J Sex Med 2013; 10:1401–1409 [DOI] [PubMed] [Google Scholar]
- 119.Høst C, Gormsen LC, Hougaard DM, et al. Acute and short term chronic testosterone fluctuations effects on glucose homeostasis, insulin sensitivity and adiponectin: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab 2014; 99:E1088–1096 [DOI] [PubMed] [Google Scholar]
- 120.Frederiksen L, Højlund K, Hougaard DM, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol 2012; 166:469–476 [DOI] [PubMed] [Google Scholar]
- 121.LeBlanc ES, Wang PY, Lee CG, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 2011; 96:3855–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atkinson RA, Srinivas-Shankar U, Roberts SA, et al. Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J Gerontol A Biol Sci Med Sci 2010; 65:1215–1219 [DOI] [PubMed] [Google Scholar]
- 123.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract 2013; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 124.Wolfgang MJ, Lane MD. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J 2011; 278:552–558 [DOI] [PubMed] [Google Scholar]
- 125.Suwa A, Shimokawa T. Emerging targets for the treatment of obesity. FEBS J 2011; 278:551. [DOI] [PubMed] [Google Scholar]
- 126.Tharakan G, Tan T, Bloom S. Emerging therapies in the treatment of ‘diabesity’: beyond GLP-1. Trends Pharmacol Sci 2011; 32:8–15 [DOI] [PubMed] [Google Scholar]


