Abstract
Purpose of review
To provide an overview of the burden of norovirus disease in healthcare settings and the factors responsible for outbreaks in these institutions; to assess progress on interventions aimed at reducing the burden of norovirus disease.
Recent findings
Norovirus outbreaks in healthcare settings are driven by confluence of viral diversity, the built environment, and host factors. Some of these characteristics may be modifiable and the target of successful interventions.
Summary
Most norovirus outbreaks in hospital and residential care institutions are associated with a particular genotype, known as GII.4. The persistence of norovirus is associated with strain diversity, which is driven by immune evasion and viral adaptation to interaction with a variety of human histo-blood group antigens. The healthcare environment presents serious challenges for control, both because of the physical structure of the built space and the high levels of contact among patient populations who may have compromised hygiene. Increased vulnerability among the populations in healthcare institutions is likely to be multifactorial and may include the following: nutritional status, immunodeficiency or senescence, chronic inflammation, and microbiome alterations. Current control measures are based on general infection control principles, and treatment is mainly supportive and nonspecific. Vaccines and antiviral agents are being developed with promising results, but none are currently available.
Keywords: hospital, norovirus, risk factors, transmission
INTRODUCTION
Noroviruses are endemic in the human population, affect people of all ages, and are recognized as the leading cause of infectious intestinal disease across the age range [1–3]. Norovirus outbreaks are common in many settings but predominate where there are high levels of contact and potentially compromised hygiene, such as populations in hospitals and nursing homes. In addition to the health impacts, healthcare-associated outbreaks pose a significant operational and economic burden to health systems [4,5▪,6]. In otherwise healthy populations norovirus gastroenteritis is generally mild and self–limiting, but there is increasing evidence that it may lead to long-term sequelae [7,8] and contribute to excess mortality in the elderly and the immunocompromised [9–16] who are inordinately affected in healthcare-associated outbreaks. At present, there are no specific interventions rigorously proven to prevent transmission and/or disease [17–19,20▪].
The factors that facilitate sustained transmission in health and long-term care settings are likely to be the result of a combination of the built environment, behavior patterns associated with patients, visitors and staff, the characteristics of the norovirus strains, and/or host-related factors that influence susceptibility to disease [21,22] (Fig. 1). Noroviruses are a highly diverse set of RNA viruses, but genogroup 2 genotype 4 (GII.4) strains overwhelmingly cause these healthcare outbreaks, with elderly patients and the immunocompromised being the most frequently and/or severely affected. Health and residential-care institution-associated outbreaks occur all year round; however, they peak in the winter months, coinciding with other winter pressures on the healthcare system [23,24]. Noroviruses are likely to be introduced into these settings from the community through infected patients, visitors and/or staff, who may either be asymptomatic, presymptomatic (i.e., incubating) or symptomatic at the time of admission. Following introduction, the hospital environment can facilitate transmission. Although healthcare outbreaks are clearly linked to disease in the community, they are also distinct in terms of the intensity of their seasonality, overwhelming predominance of a single genotype and impacts on a vulnerable population. Here, we review the recent data on the viral, environmental, and host factors that are associated with the high disease burden and the challenges in controlling norovirus in healthcare settings.
FIGURE 1.
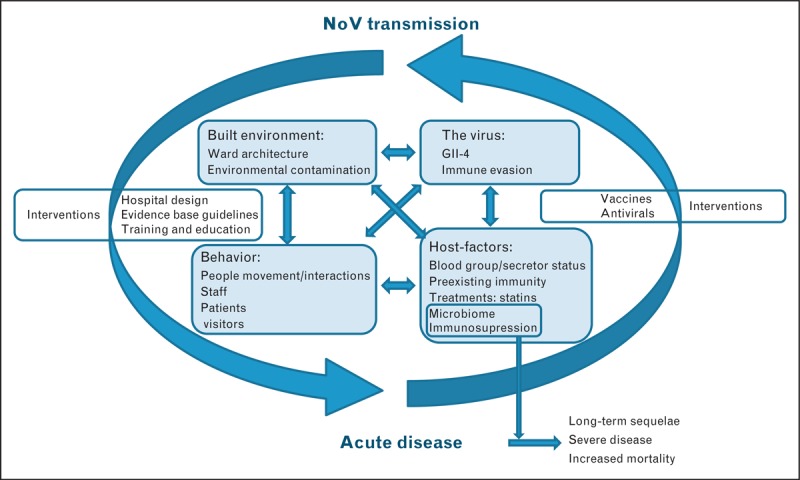
Interrelationships among the main drivers of healthcare associated norovirus outbreaks and potential intervention points.
Box 1.
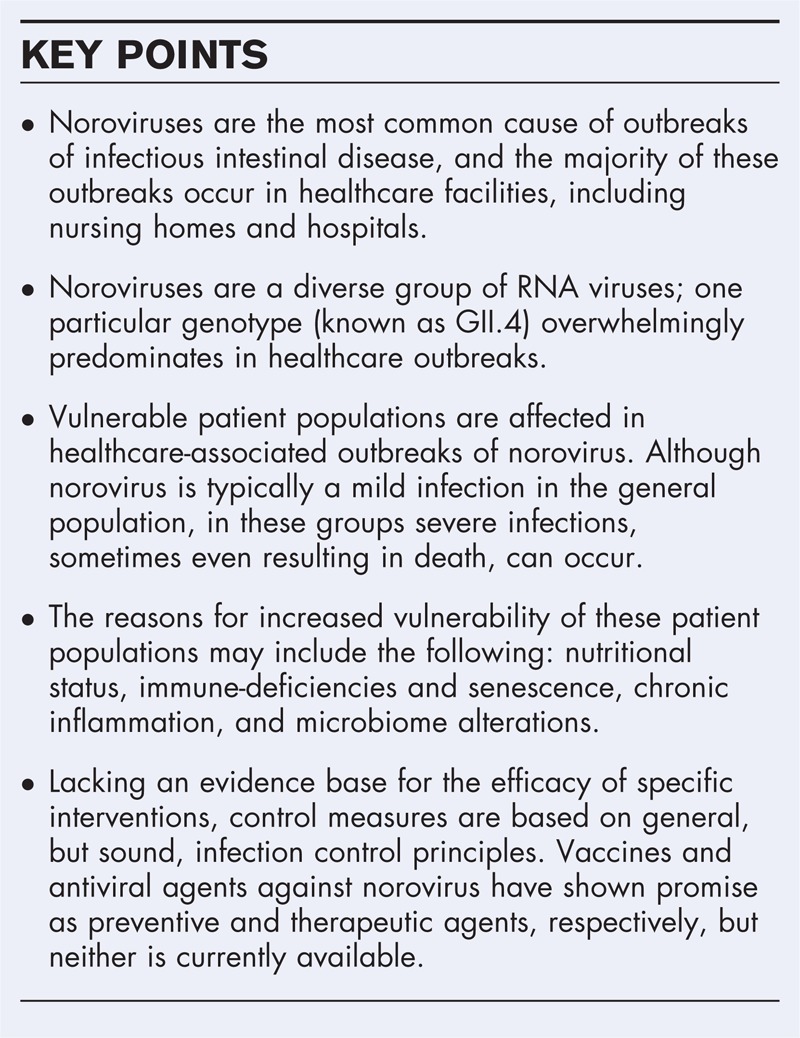
no caption available
THE VIRUS AND ROUTES OF TRANSMISSION
Noroviruses are a highly diverse group of single-stranded RNA viruses. There are two genogroups (I and II), comprising 9 and 22 genotypes, respectively [25], that principally cause disease in humans. Despite this great diversity, norovirus outbreaks in healthcare settings are caused overwhelmingly by GII.4 strains. These viruses also are associated with more severe outcomes, even after accounting for the more vulnerable case-mix that they tend to infect in healthcare settings [26]. The emergence of particular GII.4 variants correlates with periodic increases in the number of outbreaks and the overall magnitude of the annual norovirus epidemic wave. This phenomenon, similar to the antigenic shift seen with influenza viruses, is thought to be due to the emergence of antigenic variants for which there is little or no population immunity [27–31].
Norovirus infections in hospitals and nursing homes are associated with high attack rates (median 50%, range 9–78%) and may be protracted, with a mean duration of 16 days (range 3–44) and 19 days (range 6–92) in nursing homes and hospitals, respectively, according to one systematic review [17]. Noroviruses are easily transmitted by the fecal–oral route through direct contact with infected individuals and contaminated surfaces, and by aerosol dispersal following vomiting episodes that subsequently lead to contamination of the surrounding environment [32,33]. Widespread environmental contamination occurs during outbreaks in healthcare settings, but its precise origin and contribution to spread remain poorly understood [34,35]. Detection of norovirus genetic material on environmental surfaces has been correlated with ongoing and recurring outbreaks in several settings, including healthcare institutions [36,37]. High viral loads in faeces and vomitus during and after the acute phase of infection, the low infectious dose and the short incubation time associated with noroviruses are the key factors associated with transmission in semiclosed environments [38,39▪]. Spatial proximity to a symptomatic case has been identified as an important factor for the propagation of norovirus infections [22,40,41]. Although there are both symptomatic and asymptomatic infections among patients and staff, it appears that symptomatic patients are the main drivers of transmission [40].
THE HEALTHCARE ENVIRONMENT, PREVENTION AND OUTBREAK CONTROL
Although the role of ward closures, specific cleaning regimes and case isolation in controlling norovirus in healthcare institutions continues to be debated, the evidence to date suggests that the best strategy for preventing the spread of norovirus infections in hospitals is likely to be by preventing direct contact between infected and susceptible patients. The introduction of norovirus into the hospital environment from the community may be practically inevitable; curtailing spread in the hospital could be significantly curtailed through the isolation of patients in single occupancy rooms while receiving care. As proximity to a symptomatic case is a driver of norovirus outbreaks, transmission of norovirus infections is likely to be promoted in an environment in which care is provided in wards with high patient density with limited physical barriers and shared toilet facilities, coupled with patient movement between assessment units and final inpatient destination wards. In nursing homes, density of room occupancy is likely less of a driver of transmission; residents are more mobile and self-sufficient and gather in communal use rooms, all of which can facilitate norovirus transmission.
Hospital systems (i.e., acute care facilities) across the developed world, including those in Europe, Japan, Australia, and Canada, are commonly affected by norovirus outbreaks. The United States is an exception to this. Although long-term care facilities are the predominant setting for outbreaks in the United States (>60% of all norovirus outbreaks), less than 5% of reported outbreaks are in acute care hospital settings [42]. The large difference in rates of reported hospital outbreaks between the Unites States and other affluent countries may be suggestive of a lower incidence in the United States, but a survey of infection preventionists found noroviruses to be the number one cause of infectious disease outbreaks in United States hospitals [43], so the degree of under-reporting from hospital outbreaks remains a question.
The main approaches to preventing and controlling norovirus outbreaks, common across several national guidelines, include promotion of hand hygiene, patient isolation (separation of symptomatic patients) and cohorting (grouping of patients based on symptoms), staff exclusion from work, visitor restrictions, enhanced environmental cleaning and disinfection, and closures of units. The specifics of these control measures are beyond the scope of this review; published guidelines should be consulted for further details [18,19,44–46]. Areas of controversy include the effectiveness of alcohol-based hand sanitizers and closures of affected units to new admissions. Despite widespread use, the evidence on the effectiveness of alcohol-based hand sanitizers is inconclusive [47,48], so they should be used in addition to, not instead of, hand washing during outbreaks. Some studies suggest that ward closure is effective at reducing cases and the duration of outbreaks [4]. Because it is a costly and disruptive intervention, ward closure remains controversial and guideline documents do not consistently recommend it for all outbreaks [44].
HOST FACTORS ASSOCIATED WITH POPULATIONS IN HEALTHCARE SETTINGS
Both host genetic factors and acquired immunity play a role in norovirus susceptibility. Genetic resistance to norovirus infection is related to human histo-blood group antigen (HBGA) genotype. Individuals who express HBGA on cell surfaces and in body fluids, termed secretors, are generally susceptible to a wider range of norovirus strains whereas nonsecretor individuals tend to be significantly more resistant to norovirus infections [27,49]. However, susceptibility and resistance patterns differ according to norovirus strain [50]. The ability of norovirus-specific antibodies to bind to norovirus capsid sites involved in attachment to HBGA is believed to correlate with protection [28,31,51▪▪,52,53,54▪▪].
Predominance of GII.4 strains may be related to both the ability of this genotype to evade herd immunity through continuous evolution, but also because of its ability to attach to a wider range of cellular host receptors that are present in the majority of the population [55].
In immunocompromised patients, norovirus can cause chronic dehydrating diarrhea, leading to severe disease complications and sometimes mortality (reported to be up to 25%) [12,14–16,56▪▪]. The evolution of GII.4 strains within a long-term shedding immunocompromised patient has been observed to lead to the generation of antigenically distinct strains, supporting the hypotheses that long-term shedders in healthcare settings may serve as a source for the emergence of epidemic strains [57▪▪].
Although children have the highest incidence in the community [58], among hospital in-patients the elderly suffer a longer duration of illness with more severe symptoms, contributing to excess mortality [9,10]. Immunosenescence may be one contributory factor; this consequence of aging is increasingly recognized as a major risk factor leading to increases in inflammation, autoimmunity, cancer, susceptibility to gastrointestinal infections, and poor response to vaccines, which is particularly acute among the elderly in residential care [59,60]. Another risk factor may be ongoing statin use, which has been implicated as a risk factor for norovirus disease [11]. Consistent with this are in-vitro and in-vivo experiments that have demonstrated that statins can increase norovirus pathogenicity and reduce the infectious dose required to cause disease in animal models [61▪,62]. Considering the increasing and widespread use of these types of drugs in an aging population globally, a better understanding of the relationship between statins and the risk of norovirus infection and disease and of age related waning immunity is needed.
Another area gaining interest is the interaction between the gut microbiota and noroviruses [63▪]. Disruption of the gut microbiota following norovirus infection has been described in some patients independent of age, resulting in a loss of diversity and increased Proteobacteria, which may potentially lead to an increased risk of complications, such as postinfection irritable bowel syndrome [7,64]. Microbiota composition changes significantly with age [65]; a decrease of bifidobacteria, which are thought to play an immune-modulatory role and represent important components of a ‘healthy’ gut microbiota, is known to be associated with the aging process. Among the elderly, the microbiota associated with those in long-term care is less diverse than among those that remain in the community, and that the loss of the ‘community’-like microbiota is associated with ill-health [66]. Kuss et al.[67] demonstrated that the gut flora directly impacts on infectivity and pathogenicity of viruses by facilitating entry and infection through direct virus-bacteria interactions, and the recent observation that norovirus can bind to HBGA-like molecules present in certain gut bacteria provide an interesting avenue to explore the relationship between microbiota composition and norovirus infection, with a potential to inform new therapeutic approaches [63▪]. Therefore, nutritional status, immunosenescence, inflammation, the microbiome and even whether an individual lives in the community or in an institution may all be associated with aging and susceptibility to norovirus. As such, a holistic approach may be required to better understand host factors associated with norovirus disease, and ultimately to inform the design of therapy and prevention.
Other healthcare associated infections, such as Clostridium difficile diarrhea, are associated with altered microbiota composition characterized by a loss of diversity [68]; repopulation of the gut environment with ‘healthy’ microbiota can reverse chronic C. difficile diarrhea [69]. Recently, the acquisition of norovirus infection though fecal transplantation from an asymptomatic donor to a C. difficile chronically infected patient was reported [70], highlighting the risks of such therapies. However, new approaches using targeted gut colonization or bacteriotherapy show promise and may provide safer and adaptable future therapies for intestinal diseases and infections characterized by dysbiosis [71].
PROGRESS WITH ANTIVIRALS AND VACCINES
To date, there are no specific treatments available for norovirus disease and therapy is purely supportive, relying on rehydration. The increased recognition of the severity of norovirus disease and associated mortality in the immunocompromised and infirm has spurred interest in antivirals. Progress in this area is severely hampered by the lack of a cell culture system or appropriate animal models. The ability to produce norovirus virus like particles (VLPs) to use as a surrogate for the study of norovirus–ligand attachment does provide an indirect and more labor intensive approach to develop and evaluate specific therapies targeting viral entry. Carbohydrates and analogs that mimic the molecular structures of those recognized by noroviruses have been identified in recent years, shown to bind to VLPs in vitro and also inhibit binding of VLPs to jejunal biopsies [72]. Drugs and compounds that could potentially inhibit viral replication or virus protein synthesis can also be possible therapeutic agents, although at present the lack of an in vitro or an ideal surrogate system means progress in this area is slow [73]. Kaufman et al.[56▪▪] recently described the potential for antiviral therapies for the immunocompromised, and considered the wider population benefits that may be gained beyond the successful treatment of the individual patients in addition to proposing options for the design of clinical trials for evaluating the efficacy of potential antinorovirus therapies in immunocompromised patients.
Significant progress has been made in developing nonreplicating VLP-based vaccines against norovirus. These have shown to be immunogenic and to confer a significant degree of protection (48% against disease and 26% against infection) to challenge in volunteer studies [74]. Multivalent vaccines can induce broad mucosal and systemic blocking antibodies [75,76]. These promising results may, in the near future, lead to phase III clinical trials in different target populations [74]. One of the challenges that any norovirus vaccine must overcome is the need to elicit cross-reactive protection against the diverse population of norovirus genotypes and variant strains within genotypes. Norovirus GII.4 strains are the most prevalent, and their constant evolution is associated with epidemic waves every 2–4 years because of the emergence of antigenically novel strains that escape herd immunity [29–31]. Therefore, an efficacious norovirus vaccine must be able to protect against a variety of antigenically diverse variants of GII.4 noroviruses. If the strategy of employing consensus VLPs to provide protection against the evolving blockade epitopes is not successful, vaccines may need to be reformulated regularly, as is done for influenza vaccines, to incorporate novel antigens [53,77]. However, consensus or chimeric VLP approaches that require only the mutation of certain epitopes may provide a system that is more amenable for rapid production of vaccines that can adapt to emerging strains.
CONCLUSION
As such a wide range of the population is affected by norovirus, defining a target group for interventions, and vaccination in particular, is a challenge. Targeting a norovirus vaccine to protect the populations at higher risk of disease may include infants, the elderly, and the immunocompromised. The majority of outbreaks occur among the elderly in hospitals or in long-term residential care facilities; therefore, a vaccine to protect this population is a priority. Vaccination of infants will address the most common cause of pediatric hospitalization due to gastroenteritis in countries in which rotavirus vaccine is in use [5▪]. In addition, immunizing children could have important indirect benefits by limiting the transmission of norovirus in the general population. Before vaccines are ready to be rolled out for widespread use, there are still important questions that need to be answered such as the immunogenicity in infants and the elderly, which may be substantially reduced because of differences in exposure history and immune state and duration of protection.
As immunocompromised hospital patients may have chronic diarrhea and norovirus excretion, development of effective antiviral treatments and/or passive immunotherapy is a priority. Such therapies may not only benefit the affected patient directly, but may also benefit efforts to control transmission in the hospital, especially in transplant and oncology care units, in which most patients are immunosuppressed and at high risk of severe norovirus disease outcomes.
Given the high economic burden of nosocomial norovirus outbreaks, second only in the United Kingdom to the cost associated with urinary tract infections [4], a full cost–benefit analysis should inform refurbishment initiatives and future hospital design in that country, and perhaps others with similar hospital design structures. More generally, we advocate for a stronger evidence base for infection prevention and control of norovirus in healthcare settings, well designed controlled trials, or, where that is impractical or unethical, observational studies should be conducted on interventions, including ward closure, disinfection regimes, and cohorting strategies.
Acknowledgements
M.I.G. receives support from The Wellcome Trust and the National Institute for Health Research Health Protection Research Unit in Gastrointestinal Infections at the University of Liverpool.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19:1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam CC, Rodrigues LC, Viviani L, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012; 61:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastanaduy PA, Hall AJ, Curns AT, et al. Burden of norovirus gastroenteritis in the ambulatory setting – United States, 2001–2009. J Infect Dis 2013; 207:1058–1065 [DOI] [PubMed] [Google Scholar]
- 4.Lopman BA, Reacher MH, Vipond IB, et al. Epidemiology and cost of nosocomial gastroenteritis, Avon, England, 2002–2003. Emerg Infect Dis 2004; 10:1827–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Payne DC, Vinje J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that after the introduction of rotavirus vaccination programs, norovirus has become the main cause of moderate-to-severe pediatric gastroenteritis in the United States. This is an important reminder for those countries in which vaccines are being rolled as norovirus infection are rarely investigated in paediatric or sporadic cases of gastroenteritis.
- 6.Harris JP, Adams NL, Lopman BA, et al. The development of web-based surveillance provides new insights into the burden of norovirus outbreaks in hospitals in England. Epidemiol Infect 2014; 142:1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanini B, Ricci C, Bandera F, et al. Incidence of postinfectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol 2012; 107:891–899 [DOI] [PubMed] [Google Scholar]
- 8.Porter CK, Faix DJ, Shiau D, et al. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis 2012; 55:915–922 [DOI] [PubMed] [Google Scholar]
- 9.Harris JP, Edmunds WJ, Pebody R, et al. Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 2008; 14:1546–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavsson L, Andersson LM, Lindh M, Westin J. Excess mortality following community-onset norovirus enteritis in the elderly. J Hosp Infect 2011; 79:27–31 [DOI] [PubMed] [Google Scholar]
- 11.Rondy M, Koopmans M, Rotsaert C, et al. Norovirus disease associated with excess mortality and use of statins: a retrospective cohort study of an outbreak following a pilgrimage to Lourdes. Epidemiol Infect 2011; 139:453–463 [DOI] [PubMed] [Google Scholar]
- 12.Frange P, Touzot F, Debre M, et al. Prevalence and clinical impact of norovirus fecal shedding in children with inherited immune deficiencies. J Infect Dis 2012; 206:1269–1274 [DOI] [PubMed] [Google Scholar]
- 13.Haessler S, Granowitz EV. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 2013; 368:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munir N, Liu P, Gastanaduy P, et al. Norovirus infection in immunocompromised children and children with hospital-acquired acute gastroenteritis. J Med Virol 2014; 86:1203–1209 [DOI] [PubMed] [Google Scholar]
- 15.Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol 2010; 48:4303–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wingfield T, Gallimore CI, Xerry J, et al. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J Clin Virol 2010; 49:219–222 [DOI] [PubMed] [Google Scholar]
- 17.Harris JP, Lopman BA, O’Brien SJ. Infection control measures for norovirus: a systematic review of outbreaks in semi-enclosed settings. J Hosp Infect 2010; 74:1–9 [DOI] [PubMed] [Google Scholar]
- 18.Updated norovirus outbreak management and disease prevention guidelines. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports /Centers for Disease Control. 2011; 60(RR-3):1-18 Epub 2011/03/04 [PubMed] [Google Scholar]
- 19.MacCannell T, Umscheid CA, Agarwal RK, et al. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol 2011; 32:939–969 [DOI] [PubMed] [Google Scholar]
- 20▪.Harris JP, Adak GK, O’Brien SJ. To close or not to close? Analysis of 4 year's data from national surveillance of norovirus outbreaks in hospitals in England. BMJ Open 2014; 4:e003919. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article showed that in hospitals that close wards or bays to new admissions promptly the disruption and duration of norovirus outbreaks is shorter, and fewer patients are affected in comparison to those that delay ward closures.
- 21.Temime L, Opatowski L, Pannet Y, et al. Peripatetic health-care workers as potential superspreaders. Proc Natl Acad Sci USA 2009; 106:18420–18425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris JP, Lopman BA, Cooper BS, O’Brien SJ. Does spatial proximity drive norovirus transmission during outbreaks in hospitals? BMJ Open 2013; 3:e003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loveridge P, Cooper D, Elliot AJ, et al. Vomiting calls to NHS direct provide an early warning of norovirus outbreaks in hospitals. J Hosp Infect 2010; 74:385–393 [DOI] [PubMed] [Google Scholar]
- 24.Ahmed SM, Lopman BA, Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS One 2013; 8:e75922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green K. Knipe DM, Howley PM. Caliciviridae: the noroviruses. Fields virology Lippincott Williams & Wilkins, 6th ed.Philadelphia:2013 [Google Scholar]
- 26.Desai R, Hembree CD, Handel A, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis 2012; 55:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. Viral shape-shifting: norovirus evasion of the human immune system. Nat Rev Microbiol 2010; 8:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debbink K, Lindesmith LC, Donaldson EF, Baric RS. Norovirus immunity and the great escape. PLoS Pathog 2012; 8:e1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindesmith LC, Beltramello M, Donaldson EF, et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog 2012; 8:e1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindesmith LC, Costantini V, Swanstrom J, et al. Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J Virol 2013; 87:2803–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debbink K, Lindesmith LC, Donaldson EF, et al. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 2013; 208:1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans MR, Meldrum R, Lane W, et al. An outbreak of viral gastroenteritis following environmental contamination at a concert hall. Epidemiol Infect 2002; 129:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawyer LA, Murphy JJ, Kaplan JE, et al. 25- to 30-nm virus particle associated with a hospital outbreak of acute gastroenteritis with evidence for airborne transmission. Am J Epidemiol 1988; 127:1261–1271 [DOI] [PubMed] [Google Scholar]
- 34.Nenonen NP, Hannoun C, Svensson L, et al. Norovirus GII.4 detection in environmental samples from patient rooms during nosocomial outbreaks. J Clin Microbiol 2014; 52:2352–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CC, Wang YH, Wu CY, et al. A norovirus outbreak in a nursing home: norovirus shedding time associated with age. J Clin Virol 2013; 56:96–101 [DOI] [PubMed] [Google Scholar]
- 36.Boxman IL, Dijkman R, te Loeke NA, et al. Environmental swabs as a tool in norovirus outbreak investigation, including outbreaks on cruise ships. J Food Prot 2009; 72:111–119 [DOI] [PubMed] [Google Scholar]
- 37.Lopman B, Gastanaduy P, Park GW, et al. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol 2012; 2:96–102 [DOI] [PubMed] [Google Scholar]
- 38.Lee RM, Lessler J, Lee RA, et al. Incubation periods of viral gastroenteritis: a systematic review. BMC Infect Dis 2013; 13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Atmar RL, Opekun AR, Gilger MA, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 2014; 209:1016–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]; First experiemtal determination of the infectious dose for a norovirus strain.
- 40.Sukhrie FH, Teunis P, Vennema H, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis 2012; 54:931–937 [DOI] [PubMed] [Google Scholar]
- 41.Teunis P, Heijne JC, Sukhrie F, et al. Infectious disease transmission as a forensic problem: who infected whom? J R Soc Interface 2013; 10:20120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall AJ, Wikswo ME, Manikonda K, et al. Acute gastroenteritis surveillance through the national outbreak reporting system, United States. Emerg Infect Dis 2013; 19:1305–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhinehart E, Walker S, Murphy D, et al. Frequency of outbreak investigations in US hospitals: results of a national survey of infection preventionists. Am J Infect Control 2012; 40:2–8 [DOI] [PubMed] [Google Scholar]
- 44.Health Protection Agency, British Infection Association, Healthcare Infection Society, Infection Prevention Society, National Concern for Healthcare Infections, National Health Service Confederation. Guidelines for the management of norovirus outbreaks in acute and community health and social care settings. 2012 [Google Scholar]
- 45.Ireland NDSC. National guidelines on the management of outbreaks of norovirus infection in healthcare settings. https://www.hpsc.ie/hpsc/A-Z/Gastroenteric/ViralGastroenteritis/Publications/File,1194,en.pdf2004 [Google Scholar]
- 46.Australia CDN. Guidelines for the public health management of gastroenteritis outbreaks due to norovirus or suspected viral agents in Australia. https://www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdna-norovirus.htm/$File/norovirus-guidelines.pdf2010 [Google Scholar]
- 47.Park GW, Barclay L, Macinga D, et al. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII. J Food Prot 2010; 73:2232–2238 [DOI] [PubMed] [Google Scholar]
- 48.Blaney DD, Daly ER, Kirkland KB, et al. Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March. Am J Infect Control 2011; 39:296–301 [DOI] [PubMed] [Google Scholar]
- 49.Tan M, Jiang X. Norovirus–host interaction: implications for disease control and prevention. Expert Rev Mol Med 2007; 9:1–22 [DOI] [PubMed] [Google Scholar]
- 50.Tan M, Jiang X. Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev Mol Med 2014; 16:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪▪.Zhu S, Regev D, Watanabe M, et al. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog 2013; 9:e1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article, using the murine norovirus model the authors show that two murine norovirus strains which are genetically highly homologous can differ dramatically in their ability to induce protective immune responses, with one strain leading to a strong and cross-reactive response, whereas the other led to a weak and strain-specific response after primary infection. In addition, they showed that antibody and T cell (CD4+) responses are crucial for protection against secondary exposure, and specific viral proteins impact on immune responses in a strain specific manner. This has important implications for vaccine development and provides a potential model towards understanding the correlates of protection against norovirus disease.
- 52.Chen L, Wu D, Ji L, et al. Bioinformatics analysis of the epitope regions for norovirus capsid protein. BMC Bioinformatics 2013; 14 Suppl 4:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debbink K, Donaldson EF, Lindesmith LC, Baric RS. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in escape from human herd immunity. J Virol 2012; 86:1214–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Debbink K, Lindesmith LC, Donaldson EF, et al. Chimeric GII.4 norovirus virus-like particle based vaccines induce broadly blocking immune responses. J Virol 2014; 88:7256–7266 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that engineered chimeric norovirus VLP-based vaccines can provide broader protective response against multiple GII.4 strains. This provides a platform upon which a reformulation strategy that can respond to emerging norovirus GII-4 strains may be built.
- 55.Bull RA, White PA. Mechanisms of GII.4 norovirus evolution. Trends Microbiol 2011; 19:233–240 [DOI] [PubMed] [Google Scholar]
- 56▪▪.Kaufman SS, Green KY, Korba BE. Treatment of norovirus infections: moving antivirals from the bench to the bedside. Antiviral Res 2014; 105:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a compelling case for the need to develop antivirals for patient groups at risk of severe and chronic norovirus disease, and defines the parameters that need to be considered for designing clinical trials to evaluate potential antiviral compounds.
- 57▪▪.Debbink K, Lindesmith LC, Ferris MT, et al. Within host evolution results in antigenically distinct GII.4 noroviruses. J Virology 2014; 88:7244–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrated that the norovirus antigenic drift that occurs in chronically infected patients leads to norovirus strains with altered antigenic properties, demonstrating the potential long-term shedding as a driving force for the emergence of antibody escape mutant strains.
- 58.Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol 2010; 171:1014–1022 [DOI] [PubMed] [Google Scholar]
- 59.Fulop T, Pawelec G, Castle S, Loeb M. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis 2009; 48:443–448 [DOI] [PubMed] [Google Scholar]
- 60.Mabbott NA, Kobayashi A, Sehgal A, et al. Aging and the mucosal immune system in the intestine. Biogerontology 2014; 10.1007/s10522-014-9498-z[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61▪.Bui T, Kocher J, Li Y, et al. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J Gen Virol 2013; 94 (Pt 9):2005–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that in a pig model, cholesterol-lowering drugs potentiate norovirus infections resulting in a decrease in the infectious dose required to cause disease. The potential role of these drugs on increasing the risk of norovirus disease and disease severity in the elderly requires further investigation.
- 62.Jung K, Wang Q, Kim Y, et al. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One 2012; 7:e41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪.Miura T, Sano D, Suenaga A, et al. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol 2013; 87:9441–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]; These articles provide evidence that glycans derived from human or other mammalian hosts or from bacteria may provide effective antinorovirus compounds for blocking virus attachment to target cells, and neutralising infections.
- 64.Nelson AM, Walk ST, Taube S, et al. Disruption of the human gut microbiota following norovirus infection. PLoS One 2012; 7:e48224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hopkins MJ, Sharp R, Macfarlane GT. Variation in human intestinal microbiota with age. Dig Liver Dis 2002; 34 Suppl 2:S12–S18 [DOI] [PubMed] [Google Scholar]
- 66.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488:178–184 [DOI] [PubMed] [Google Scholar]
- 67.Kuss SK, Best GT, Etheredge CA, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011; 334:249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 2008; 197:435–438 [DOI] [PubMed] [Google Scholar]
- 69.Brandt LJ, Reddy SS. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45:S159–S167 [DOI] [PubMed] [Google Scholar]
- 70.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013; 108:1367. [DOI] [PubMed] [Google Scholar]
- 71.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog 2012; 8:e1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yazawa S, Yokobori T, Ueta G, et al. Blood group substances as potential therapeutic agents for the prevention and treatment of infection with noroviruses proving novel binding patterns in human tissues. PLoS One 2014; 9:e89071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arias A, Emmott E, Vashist S, Goodfellow I. Progress towards the prevention and treatment of norovirus infections. Future Microbiol 2013; 8:1475–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 2011; 365:2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LoBue AD, Lindesmith L, Yount B, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 2006; 24:5220–5234 [DOI] [PubMed] [Google Scholar]
- 76.Tamminen K, Lappalainen S, Huhti L, et al. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One 2013; 8:e70409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parra GI, Bok K, Taylor R, et al. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 2012; 30:3580–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]


