Abstract
Purpose of review
To discuss new insights regarding how sensitive (second-generation) thyroglobulin immunometric assays (Tg2GIMAs), (functional sensitivities ≤0.10 μg/L) necessitate different approaches for postoperative thyroglobulin monitoring of patients with differentiated thyroid cancer (DTC), depending on the presence of thyroglobulin autoantibodies (TgAbs).
Recent findings
Reliable low-range serum thyroglobulin measurement has both enhanced clinical utility and economic advantages, provided TgAb is absent (∼75% DTC patients). Basal [nonthyroid-stimulating hormone (TSH) stimulated] Tg2GIMA measurement obviates the need for recombinant human TSH stimulation because basal Tg2GIMA below 0.20 μg/L has comparable negative predictive value (>95%) to recombinant human TSH-stimulated thyroglobulin values below the cutoff of 2 μg/L. Now that radioiodine remnant ablation is no longer considered necessary to treat low-risk DTC, the trend and doubling time of low basal thyroglobulin values arising from postsurgical thyroid remnants have recognized prognostic significance. The major limitation of Tg2GIMA testing is interference by TgAb (∼25% DTC patients), causing Tg2GIMA underestimation that can mask disease. When TgAb is present, the trend in TgAb concentrations (measured by the same method) can serve as the primary (surrogate) tumor-marker and be augmented by thyroglobulin measured by a TgAb-resistant class of method (radioimmunoassay or liquid chromatography-tandem mass spectrometry).
Summary
The growing use of Tg2GIMA measurement is changing paradigms for postoperative DTC monitoring. When TgAb is absent, it is optimal to monitor the basal Tg2GIMA trend and doubling time (using the same method) in preference to recombinant human TSH-stimulated thyroglobulin testing. When TgAb is present, interference renders Tg2GIMA testing unreliable and the trend in serum TgAb concentrations per se (same method) can serve as a (surrogate) tumor-marker.
Keywords: differentiated thyroid cancer, thyroglobulin autoantibody interferences, thyroglobulin measurement
INTRODUCTION
Serum thyroglobulin is the primary biochemical tumor-marker used to monitor differentiated thyroid cancer (DTC) [1,2,3▪▪]. A global rise in the prevalence of DTC [4,5▪,6] is increasing the number of thyroidectomized patients needing lifelong monitoring for persistent or recurrent disease, typically involving periodic (6–12 months) serum thyroglobulin measurements augmented by anatomic imaging, as appropriate for recurrence risk. For decades, standard DTC treatment has involved total thyroidectomy followed by one or more doses of radioiodine (RAI) before long-term thyroid hormone suppression of thyroid-stimulating hormone (TSH), without regard to recurrence risk [1,2,7,8]. Yet most thyroid tumors are small (≤1.0 cm), belong to the papillary histotype (PTC) and have a low recurrence risk even when treated by surgery alone [9–11]. Increasingly, a more individualized, risk-stratified, approach to DTC diagnosis and management is being adopted [5▪,7,8,12] that limits RAI treatment to high-risk DTC (the minority of patients) [13,14▪,15]. As a consequence, more sensitive (second-generation) thyroglobulin immunometric assay (Tg2GIMA) measurements, which have an order of magnitude higher functional sensitivity (≤0.10 μg/l) than older (first-generation) tests (functional sensitivity ∼1.0 μg/l), are rapidly becoming the standard of care [3▪▪,16▪▪]. Over recent years, it has become clear that thyroglobulin assays need second-generation functional sensitivity in order to monitor the low basal (non-TSH stimulated) thyroglobulin concentrations arising from the intact surgical remnant of low-risk DTC patients who no longer receive routine RAI remnant ablation. Also that a basal Tg2GIMA below 0.20 μg/l has comparable negative predictive value to a recombinant human TSH (rhTSH)-stimulated thyroglobulin below the consensus cutoff of 2.0 μg/l [3▪▪,17–20]. Further, as with other biochemical tumor-marker tests such as calcitonin and carcinoembryonic antigen, the basal Tg2GIMA trend and doubling time (measured using the same thyroglobulin method, preferably in the same laboratory) have been shown to be important prognostic parameters [21–25,26▪]. The primary limitation of Tg2GIMA measurement is its high propensity for interference from both thyroglobulin autoantibodies (TgAb) [27–29,30▪▪] and heterophile antibodies, primarily human anti-mouse antibodies (HAMA) [31–33]. This review will focus on technical issues relating to current thyroglobulin methodology that impact the clinical utility of thyroglobulin testing and discuss how paradigms for postoperative DTC monitoring differ depending on the TgAb status of the patient.
Box 1.
no caption available
REVIEW OF FOUR DECADES OF THYROGLOBULIN METHODOLOGY
During the last four decades, thyroglobulin has been measured by three different classes of methodology [34]: Radioimmunoassay (RIA) used since the 1970s [29,35,36,37▪▪], Immunometric assay (IMA) used since the 1980s [29,36,37▪▪,38] and liquid chromatography-tandem mass spectrometry (LC-MS/MS) developed in 2008 [39–41,42▪]. These classes of thyroglobulin method differ fundamentally in functional sensitivity potential and propensity for interference from HAMA and TgAb [34].
Most thyroglobulin testing is made using IMA methods that can display ten-fold differences in functional sensitivity. A generational nomenclature system, similar to that used for TSH assays, has been adopted to distinguish between different assays because assay functional sensitivity is such a critical determinant of the clinical utility of thyroglobulin testing [3▪▪,19,43,44]. First-generation assays have functional sensitivity approximating 1.0 μg/L, whereas second-generation assays have functional sensitivity 0.10 μg/L or less. Both the RIA and LC-MS/MS class of method can achieve only first-generation functional sensitivity. Increasingly, laboratories are replacing first-generation tests with Tg2GIMA measurement because superior functional sensitivity allows basal (non-TSH stimulated) Tg2GIMA monitoring to replace the need for rhTSH stimulation [16▪▪,17–20,45–52] and facilitate the detection of the low thyroglobulin concentrations arising from postoperative thyroid remnants [53▪▪] and lymph node metastases secreting low levels of thyroglobulin [54,55]. Unfortunately, as with all IMA-class methods, Tg2GIMAs are highly prone to interference from both HAMA and TgAb, potentially limiting their clinical value [3▪▪,30▪▪]. Some RIA methods are still in use because this competitive methodology appears to convey more resistance to TgAb interference than IMA-class tests, although some interfering TgAbs undoubtedly cause some falsely high or low serum RIA values [29,56]. The new LC-MS/MS class of method should be free from TgAb and HAMA interferences. These methods employ extensive laboratory-specific specimen preparation using trypsin to break up serum thyroglobulin-TgAb complexes to release a conserved thyroglobulin peptide(s) for LC-MS/MS measurement [40,41,42▪]. However, as yet these methods have only been validated by correlation studies that have confirmed the underestimation typical of TgAb interference with Tg2GIMA and that thyroglobulin RIA values appear resistant, although the cause of undetectable LC-MS/MS and detectable thyroglobulin RIA values in some TgAb-positive and TgAb-negative sera needs further study [40,41]. Currently, LC-MS/MS clinical utility is compromised by suboptimal functional sensitivity (only first-generation), longer turnaround times than Tg2GIMA, limited availability and high instrumentation costs [42▪].
TECHNICAL LIMITATIONS OF THYROGLOBULIN METHODOLOGY
Three principle technical limitations currently negatively impact the clinical utility of thyroglobulin methods: first, suboptimal functional sensitivity; second, between-method discordances and third, interferences, primarily from TgAb, but also heterophilic antibodies, the most common being HAMA.
Functional sensitivity
Manufacturers and laboratories are unsure which parameter best describes a thyroglobulin assay's detection limit [34]. Immunoassay precision profiles are typically U-shaped, with low range precision and matrix bias determining the reliability for detecting low analyte concentrations [46,57]. This low range precision erodes as measurements are made over longer periods of time as a consequence of changes in reagent lots and calibrators, instrumentation factors and other intangible variables [58]. This deterioration in precision is particularly problematic for serial thyroglobulin measurements because postoperative DTC monitoring necessitates monitoring low thyroglobulin concentrations across long clinical intervals (6–12 months) between determinations. Functional sensitivity is the only parameter that represents the imprecision of low-range thyroglobulin measurement made under conditions representative of clinical practice. Specifically, functional sensitivity is defined as ‘the thyroglobulin concentration that can be measured in human serum with 20% between-run coefficient of variation over a 6–12 months period, during which time more than two reagent lots and more than two instrument calibrations should be used’ [3▪▪,34,44]. Functional sensitivity contrasts with the limit of quantitation calculation favored by many manufacturers and laboratories that is defined by the 20% coefficient of variation of runs made over short periods of time (days or weeks) without stipulations regarding the use of the clinically relevant matrix (human serum) or capturing any lot-to-lot variability [34]. It should be noted that because both thyroglobulin and TgAb are measured concurrently, the protocol for determining the functional sensitivity of thyroglobulin and TgAb assays is the same [44].
Between-method discordances
Restandardization against the Certified Reference Material, CRM-457, has greatly reduced differences between thyroglobulin assays over the last decade [59–61]. However, despite universal adoption of this standard, different methods can display a two-fold difference in the numeric thyroglobulin values reported for the same serum [29,45,46,57,62,63]. This is evident in Fig. 1 showing UK National External Quality Assessment Service for Thyroglobulin surveys for four TgAb-negative sera (A–D) that had thyroglobulin measured by each of 51 laboratories using 10 different methods. Thyroglobulin molecular heterogeneity is the likely cause of this method variability. Thyroglobulin is a large (660 kDa) dimeric glycoprotein that is heterogeneous with respect to differential thyroglobulin mRNA splicing, glycosylation and degree of iodination, also, the processes involved in thyroglobulin maturation, dimerization and molecular folding are complex and may become dysregulated in tumor tissue [64]. Given the conformational nature of thyroglobulin epitopes [65,66], it is not surprising that different immunoassays, employing different thyroglobulin antibody reagents, detect serum thyroglobulin isoforms with variable potency. The severe between-method discordances seen for tumor-derived thyroglobulins are reflected in abnormal ratios between the values reported by different methods that suggest different epitopes may be masked or exposed when the thyroglobulin structure is abnormal [62]. The magnitude of the between-method variability seen in Fig. 1 far exceeds the expected within-person thyroglobulin variability that approximates 15% [67]. In clinical practice, these between-method biases necessitate that postoperative thyroglobulin monitoring be made using the same manufacturers method and preferably the same laboratory [1,2,3▪▪,29,44,45].
FIGURE 1.
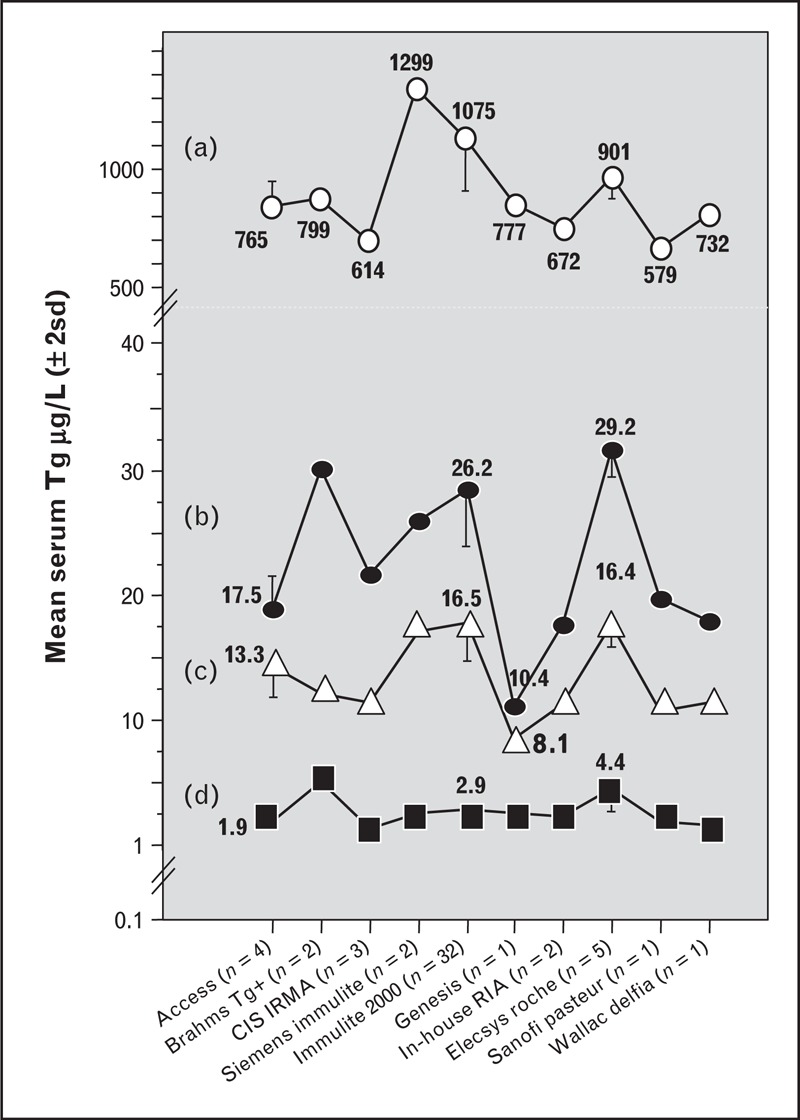
Between-method variability of current thyroglobulin methods. This figure shows the between-method variability of serum thyroglobulin values reported for four different sera (A–D) without TgAb, measured by 51 laboratories using 10 different methods. The +/−2 standard deviation limits are shown for methods with sufficient participating laboratories. The data were taken from the United Kingdom National External Quality Assessment Service for Thyroglobulin surveys with permission.
Interferences
TgAb interference is the most serious problem currently limiting the clinical utility of thyroglobulin testing [27,30▪▪], whereas interference caused by HAMA is less common [19,30▪▪,33]. Both types of interference primarily affect the IMA class of thyroglobulin method. TgAb causes underestimation that could mask the presence of disease [30▪▪,68], whereas HAMA typically causes thyroglobulin IMA overestimation [27,30▪▪]. The RIA class of thyroglobulin method is not affected by HAMA and appears resistant to TgAb interference, [27,36,37▪▪,56,69–73], whereas the new LC-MS/MS class of method should be free from both TgAb and HAMA interferences by virtue of trypsin digestion of antibody complexes and generation of a thyroglobulin-specific peptide that is the measurement parameter [40,41,42▪]. However, clinical studies are needed to show that abnormal polymorphic tumor thyroglobulins always generate the tryptic peptide necessary for LC-MS/MS detection [42▪].
Because the TgAb status of a patient can change (positive to negative or vice versa), current guidelines mandate that every thyroglobulin test have a TgAb measurement made directly by an immunoassay and not the indirect thyroglobulin recovery approach that often fails to detect interfering TgAb [2,3▪▪,29,30▪▪,34,44]. Unfortunately, TgAb detection by immunoassay is also problematic because current methods differ in sensitivity, specificity and the numeric values they report, despite claiming standardization against the same reference preparation (Medical Research Council 65/93). Many examples of the same serum being reported as ‘TgAb-positive’ by one method but ‘TgAb-negative’ by another have been reported [36,74–76]. Laboratories compound these problems by adopting different cutoff values to define a ‘positive’ TgAb result, even when they use the same manufacturer's test [29,30▪▪,36,37▪▪,48,75–79]. The selection of the TgAb cutoff impacts the reliability of TgAb detection, which is critical when a ‘negative’ TgAb result is used to authenticate a Tg2GIMA measurement [36]. The clinical consequences of a false-negative TgAb test would be that low or undetectable Tg2GIMA values might be reported that could mask disease [30▪▪,68]. In contrast, a false-positive TgAb test would unnecessarily decrease confidence in Tg2GIMA measurement and could prompt reflex testing by a less sensitive, but TgAb-resistant, class of thyroglobulin method (RIA or LC-MS/MS). One caution is that when laboratories use a ‘positive’ TgAb result to reflex thyroglobulin testing to different methods, the imprecision surrounding the TgAb cutoff value during DTC monitoring could lead to inappropriate changes in a patient's TgAb status (positive to negative or vice versa) that could prompt unnecessary reflexing between Tg2GIMA and RIA or LC-MS/MS testing.
FACTORS INFLUENCING SERUM THYROGLOBULIN CONCENTRATIONS
Because the source of thyroglobulin protein is thyroid tissue-specific and not tumor-specific, a number of factors will influence the interpretation of serum thyroglobulin concentrations [44]. These factors include the following: first, the mass of thyroid tissue present (normal thyroid remnant plus any tumor); second, the tissue's efficiency or inefficiency for thyroglobulin secretion; third, injury secondary to surgery, fine-needle aspiration biopsy, or RAI therapy can cause thyroglobulin release from damaged tissue that elevates serum thyroglobulin concentrations; fourth, the degree and chronicity of TSH stimulation that has a profound effect on serum thyroglobulin and fifth, the possibility of increased metabolic clearance of thyroglobulin-TgAb complexes that would alter the interpretation of the serum thyroglobulin concentrations in the presence of TgAb [80–83].
POSTOPERATIVE SERUM THYROGLOBULIN MONITORING
Serum thyroglobulin measurement only becomes a reliable tumor-marker after total thyroidectomy. Preoperative thyroglobulin measurement is considered to have limited value, although a number of studies report that an elevated preoperative serum thyroglobulin is a risk factor for nodular malignancy [84–87]. In addition, the relationship between the preoperative serum thyroglobulin and tumor burden may give some indication of the tumor's efficiency for thyroglobulin secretion and set a threshold for determining the significance of postoperative serum thyroglobulin changes [2].
Preoperative TgAb measurement may also have clinical utility. One report found nodular malignancy more likely when TgAb, but not thyroid peroxidase antibodies, were detected [88]. Most DTC patients with circulating TgAb have evidence of thyroid autoimmunity [66,89,90], although any causal relationship between Hashimoto's thyroiditis and PTC is unclear [90–92]. However, TgAb-positive PTC patients may be more likely to have extrathyroidal extension and a higher risk for persistent or recurrent disease than TgAb-negative PTC patients [14▪,93–95]. It follows that a preoperative TgAb measurement, and/or histologic evidence of lymphocytic infiltration in the surgical specimen, may be a risk factor and early indicator that postoperative Tg2GIMA measurement may be unreliable because of TgAb interference [90,96,97].
Optimal monitoring of differentiated thyroid cancers patients without thyroglobulin autoantibody
The majority (∼75%) of DTC patients either have no TgAb detected throughout their course or become TgAb-negative in the years following successful surgery [14▪,27,37▪▪,89,92,98–100]. With RAI treatment now being limited to high-risk DTC patients [13,14▪,15], most postoperative thyroglobulin testing will be made for low-risk PTC patients with low thyroglobulin concentrations arising from postsurgical thyroid remnants. This more conservative use of RAI, coupled with the growing use of Tg2GIMA measurement, is changing paradigms for postoperative serum thyroglobulin monitoring [5▪]. No longer can an ‘undetectable’ serum thyroglobulin be considered a useful criterion for the absence of disease because thyroglobulin that is ‘undetectable’ using an insensitive first-generation test may be ‘detectable’ when using more sensitive Tg2GIMA measurement [3▪▪,29,45,46,62]. Further, rhTSH stimulation should no longer be needed now that reliable basal Tg2GIMA measurement is available [16▪▪]. Studies have found that rhTSH typically stimulates basal thyroglobulin approximately ten-fold [19,46], so that the negative predictive value of a rhTSH-stimulated thyroglobulin value below the fixed cutoff of 1 to 2 μg/l is comparable to a basal Tg2GIMA value below 0.10–0.20 μg/L [16▪▪,49]. Although the low frequency of DTC recurrences impacts the ability to study positive predictive values (PPVs) [45], the PPV of an rhTSH-stimulated thyroglobulin above 1 to 2 μg/l appears comparable to a basal Tg2GIMA above 0.10–0.20 μg/l [20,49,101]. By considering the principle factors influencing serum thyroglobulin concentrations (thyroid tissue mass, injury and TSH), it is evident that the trend in basal Tg2GIMA, measured when TSH is suppressed, should reflect changes in thyroid tissue mass and thus provide a more sensitive parameter for disease than rhTSH-stimulated thyroglobulin testing. This is supported by a growing number of studies showing the prognostic utility of monitoring the basal Tg2GIMA trend and thyroglobulin doubling time [22,25,102], as is customary for other tumor-marker such as carcinoembryonic antigen and calcitonin [21].
The clinical utility of monitoring Tg2GIMA trends is illustrated in Figs. 2 and 3Figs 2 and 3. Figure 2 shows postoperative serum Tg2GIMA monitoring (TSH < 0.10 mIU/l at all points) of 18, non-RAI treated, TgAb-negative, PTC patients who displayed no evidence of disease at the end of more than 5 years follow-up. As previously reported, serum Tg2GIMA fell to a nadir of less than 0.05 to 0.5 μg/l during the first postoperative year (Fig. 2a) – the same thyroglobulin range as seen following thyroidectomy for medullary carcinoma [103] and consistent with the thyroglobulin secretion expected from the approximately 1 g of normal thyroid remnant remaining after thyroidectomy [53▪▪,104–106]. It should be noted that at a median of 0.17 postoperative years, serum Tg2GIMA was below 0.10 μg/l in 12 out of 18 (67%) cases, and even below the functional sensitivity limit of 0.05 μg/l in three out of 18 (17%) cases, affirming that without RAI treatment the normal thyroid remnant typically secretes a very low thyroglobulin level in the face of TSH suppression [53▪▪,104,105]. Figure 2b shows that serum Tg2GIMA values remain remarkably constant in the less than 0.05–0.5 μg/l range when TSH suppression is maintained during long-term follow-up. In fact, the median within-person percentage coefficient of variation of serum Tg2GIMA measurements made throughout 2–15 years of postoperative monitoring approximated 30%, illustrating the consistency of thyroglobulin secretion from normal thyroid remnant tissue. It is this consistency of remnant secretion that is why the basal Tg2GIMA doubling time (measured at a constant TSH level) is a sensitive parameter for detecting recurrence [22,25,102]. Figure 3 shows 25 years of postoperative Tg2GIMA monitoring (Beckman Access Tg2GIMA measurement of frozen archived specimens [37▪▪]) of a TgAb-negative PTC patient with persistent and recurrent disease. This case illustrates a number of points which are as follows: first, the high preoperative thyroglobulin (154 μg/L) suggested that serum thyroglobulin would be a sensitive postoperative tumor-marker; second, the ∼ten-fold thyroglobulin stimulation in response to thyroid hormone withdrawal prior to RAI suggested the tumor was responsive to TSH and supported the efficacy for TSH suppression; third, surgery was clearly a more effective treatment for metastatic PTC lymph nodes than RAI treatments; fourth, combined imaging modalities were needed to detect disease; fifth, persistent disease remained quiescent during TSH suppression for many years before an active recurrence manifested; sixth, a rising trend in basal Tg2GIMA (measured at constant TSH) suggested an increase in tumor mass because thyroglobulin secretion from normal remnant tissue (Fig. 2) remains constant. It was also likely that this patient had little or no normal remnant left after two doses of RAI and seventh, the doubling of basal Tg2GIMA during TSH suppression approximated 4 years – an interval indicating a good, long-term prognosis [22].
FIGURE 2.
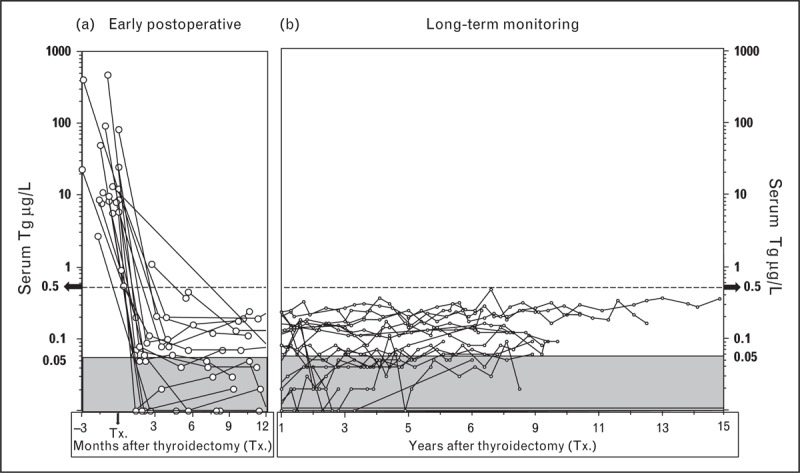
Basal serum (second-generation) thyroglobulin immunometric assay trends for disease-free, thyroglobulin autoantibody-negative papillary histotype (no radioiodine treatment). This figure shows serum basal Tg2GIMA measurements (Beckman Access analyses of frozen archived specimens [37▪▪]) made for 18 TgAb-negative PTC patients treated by thyroidectomy alone (no RAI treatment), maintained on long-term TSH suppression (<0.1 mIU/l), without evidence of recurrence at the end of follow-up. Panel (a) shows that during the early postoperative phase, all patients achieved a basal serum thyroglobulin below 0.5 μg/l by 6–12 months after thyroidectomy (Tx.). Panel (b) shows the stability of thyroglobulin secretion from normal remnant tissue when TSH is held constant (median within-person percentage coefficient of variation ∼30%). PTC, papillary histotype; RAI, radioiodine; Tg, thyroglobulin; TgAb, thyroglobulin autoantibody; TSH, thyroid-stimulating hormone.
FIGURE 3.
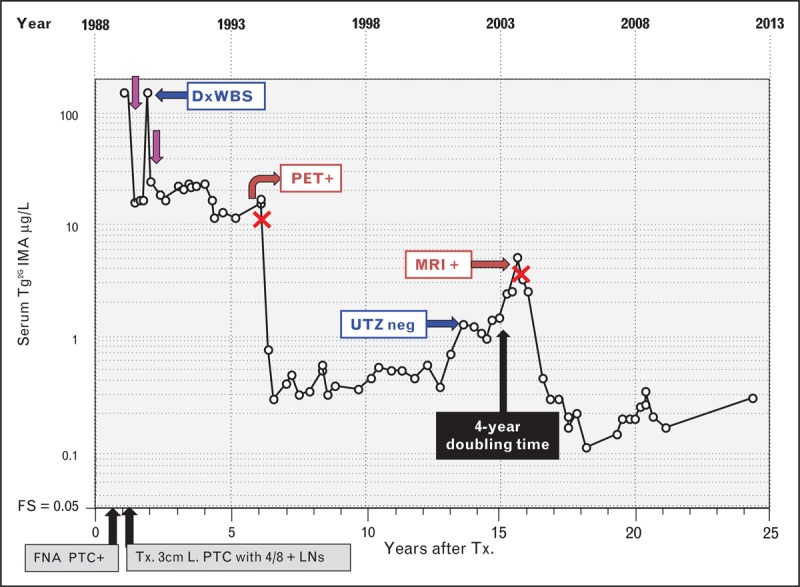
Serum (second-generation) thyroglobulin immunometric assay monitoring of a patient with persistent and recurrent papillary thyroid cancer (PTC) and negative thyroglobulin autoantibodies. This female patient presented in 1988 at age 42 with a 3-month history of an enlarging anterior neck mass. PTC was diagnosed by fine-needle aspiration biopsy (FNA) and treated by total thyroidectomy to remove a 3 cm tumor, together with 4 out of 8 PTC-positive lymph nodes. Preoperative serum thyroglobulin was elevated (154 μg/L) suggesting that postoperative serum thyroglobulin monitoring would be efficacious. Serum basal Tg2GIMA measurements made during 25 years of postoperative thyroglobulin monitoring are shown (Beckman Access Tg2GIMA measurement of frozen archived specimens [37▪▪]) during thyroid hormone therapy (TSH < 0.05 mIU/L). Following an initial RAI treatment (∼150 mCi) (purple arrows), persistent disease was suspected by inappropriately high thyroglobulin values (15–20 μg/L). Despite a negative diagnostic whole body scan (DxWBS), the elevated thyroglobulin prompted a second RAI treatment (150 mCi) that revealed foci of thyroid uptake and a submandibular lymph node on the post-treatment scan. However, the two RAI treatments had little effect on the elevated serum thyroglobulin, which remained persistently elevated for 5 years prompting computed tomography scans of the neck and chest (negative) followed by a PET scan that revealed a lymph node, confirmed by FNA as positive for PTC. During a left jugular dissection (surgeries indicated by red crosses), a 1.5 cm lymph node metastasis was removed producing a significant fall in serum Tg2GIMA into the 0.3–0.5 μg/L range, a level that was sustained for approximately 5-years. Thereafter, despite continued TSH suppression, Tg2GIMA rose with an approximate 4-year doubling time prompting an ultrasound investigation (negative) and later an MRI that detected a single 1.0 cm lymph node metastasis that was removed during a level 2, 3 and 5 neck dissection. Thereafter, serum Tg2GIMA values fell to the 0.20–0.40 μg/L range but remained detectable. The persistence of the detectable serum Tg2GIMA despite two doses of RAI suggests that disease may still be present. FNA, fine-needle aspiration; FS, functional sensitivity; LNs, lymph nodes; PTC, papillary histotype; RAI, radioiodine; Tg2GIMA, (second-generation) thyroglobulin immunometric assay; TSH, thyroid-stimulating hormone; Tx., thyroidectomy.
Optimal monitoring of patients with thyroglobulin autoantibody
A recent review of 1500 consecutive DTC patients with more than 2 years postoperative follow-up found that although 22.7% were currently TgAb-positive, an additional 12.7% had a past history of TgAb-positivity but had become TgAb-negative during the postoperative period [27,37▪▪,98,99]. Thus, approximately one-third of DTC patients have TgAb detected at some time in their course, and clearly the TgAb status of a patient can change over time necessitating TgAb measurement with every thyroglobulin test [1,2,14▪,16▪▪,30▪▪,44]. Although the most common change in TgAb status is positive to negative in response to successful treatment [27,37▪▪,98,99], the persistence of TgAb, a rising TgAb trend, the failure of TgAb to fall or a de-novo TgAb appearance is highly suspicious for active disease [7,14▪,37▪▪,89,92,100,107]. However, a rising TgAb concentration is not specific for recurrence but can also result from any thyroid injury causing thyroglobulin release (RAI treatment, lymph node biopsy or additional surgery) [90]. It is now apparent that TgAb concentrations respond to changes in the mass of thyroglobulin-secreting thyroid tissue so that the trend in TgAb concentrations can be used as a surrogate tumor-marker [7,14▪,30▪▪,37▪▪,65,89,90,98,100,107]. Thus, TgAb measurement is not merely a qualitative (positive versus negative) test for validating that a Tg2GIMA measurement is free from TgAb interference but acts as an indicator for thyroglobulin antigen sensed by the immune system. Unfortunately, given the difference in numeric values reported by different TgAb methods, and the time needed to establish the TgAb trend (TgAb half life ∼10 weeks [99]), it is critical that postoperative TgAb monitoring be performed using the same manufacturer's method [29,30▪▪,36,90]. Alternatively, when a change in TgAb method is necessary, the patient-specific ratio between the new versus the old TgAb test can be used to rebaseline TgAb testing to the new method, as previously described [37▪▪,90].
The clinical utility of monitoring TgAb trends as a surrogate tumor-marker is illustrated in Fig. 4, in which serum TgAb monitoring of four PTC patients without disease is shown in the upper panels, to contrast with four PTC patients with persistent or recurrent disease shown in the lower panels. Figure 4a shows that TgAb-positive patients rendered disease-free by thyroidectomy may display a transient early rise in TgAb in response to the thyroglobulin released by the surgery and/or RAI treatment, but thereafter TgAb progressively declines and may become undetectable during the early years of follow-up, especially if the initial TgAb concentration was low. Disease-free patients with high TgAb concentrations typically display a slow progressive TgAb decline over years but may not achieve full TgAb-negativity, possibly because of the long-lived memory of plasma cells [108]. The left-hand panels show the TgAb concentrations expressed as percentage of an initial (0–3 month) value. Figure 4b shows that when patients are disease-free, TgAb concentrations typically fall by more than 50% during the first postoperative year [26▪,30▪▪,34,100] and thereafter continue to fall to less than 10% of initial value over subsequent years. These TgAb trends contrast with those of the PTC patients with persistent or recurrent disease shown in the lower panels. Figure 4c shows that when there is active disease, TgAb concentrations may show a decline in the early months following thyroidectomy that is not sustained, or may be followed by a progressive TgAb rise or even a de-novo TgAb appearance sometimes years after TgAb absence, necessitating concurrent TgAb measurement with every thyroglobulin test [30▪▪,44,107]. Figure 4d illustrates that although some patients with active disease have a 50% TgAb decline in the first postoperative year, TgAb rarely declines to less than 10% unless the disease is successfully treated.
FIGURE 4.
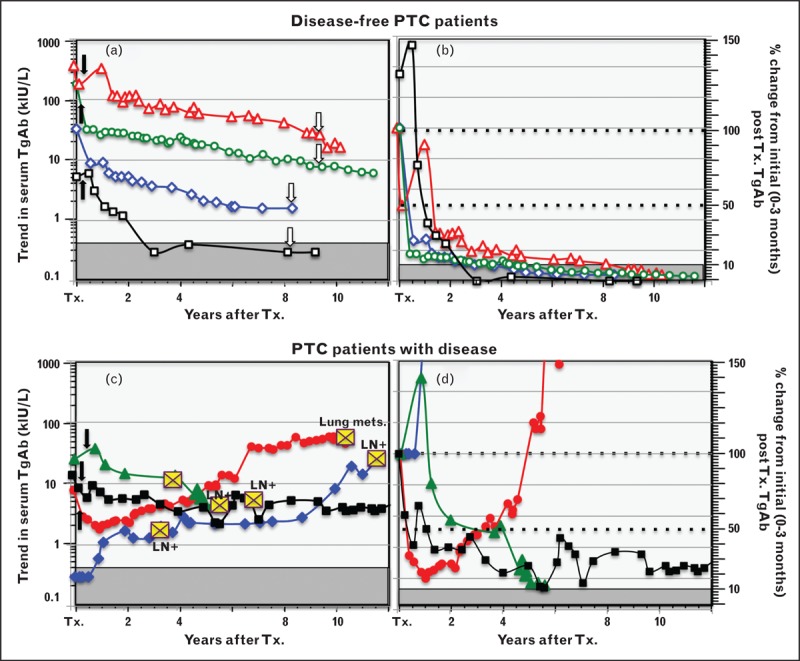
Thyroglobulin autoantibody trend and percentage change used as a surrogate differentiated thyroid cancer tumor-marker. This figure illustrates how the trend in TgAb concentrations (left panels a and c) and the percentage change in TgAb concentrations (Kronus/RSR method) relative to an initial (0–3 month) postoperative TgAb value (panels b and d) can be used as a surrogate DTC tumor-marker. Figure 4a shows TgAb trends for four PTC patients who were judged disease-free by ultrasound (open arrows) in the postoperative period. Figure 4b shows these data converted to percentage of the initial value. Disease-free patients may have a transient early TgAb rise in response to thyroglobulin released by surgical injury or RAI treatment (solid arrows), but thereafter TgAb values typically decline over time (years) to less than 10% of initial level. These disease-free patients were selected to have different initial TgAb concentrations to illustrate that declining TgAb concentrations do not necessarily become undetectable during follow-up, unless the initial TgAb was low. Figures 4c and 4d show comparative data for four PTC patients with persistent or recurrent disease detected during follow-up (indicated by yellow crosses). A de-novo TgAb appearance, a TgAb rise or a stable TgAb concentration that fails to fall below 10% of initial value are indications of active disease. DTC, differentiated thyroid cancer; LN, lymph node metastases; PTC, papillary histotype; RAI, radioiodine; TgAb, thyroglobulin autoantibody; Tx., thyroidectomy.
CONCLUSION
Now that RAI treatment is no longer considered necessary to treat low-risk DTC, postoperative serum thyroglobulin monitoring will primarily be made for disease-free patients who have functioning thyroid remnants that typically give rise to serum basal Tg2GIMA concentrations in the 0.05–0.5 μg/l or less range when TSH is suppressed. When TgAb is absent (∼75% of DTC), the serum basal Tg2GIMA trend and doubling time are important prognostic parameters. However, when TgAb is present, Tg2GIMA measurement is unreliable because of interference causing Tg2GIMA underestimation and the trend in TgAb concentrations (measured by the same method) becomes the primary (surrogate) tumor-marker.
Acknowledgements
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154:787–803 [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 3▪▪.Giovanella L, Clark P, Chiovato L, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol 2014; 171:R33–R46 [DOI] [PMC free article] [PubMed] [Google Scholar]; This review by an expert European panel presents number of questions and recommendations regarding second-generation thyroglobulin measurement.
- 4.Reiners C. Thyroid cancer in 2013: advances in our understanding of differentiated thyroid cancer. Nat Rev Endocrinol 2013; 10:69–70 [DOI] [PubMed] [Google Scholar]
- 5▪.Durante C, Costante G, Filetti S. Differentiated thyroid carcinoma: defining new paradigms for postoperative management. Endocr Relat Cancer 2013; 20:141–154 [DOI] [PubMed] [Google Scholar]; This article discusses the new risk-stratified approach to RAI treatment and postoperative DTC management.
- 6.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140:317–322 [DOI] [PubMed] [Google Scholar]
- 7.Tuttle RM, Leboeuf R. Follow up approaches in thyroid cancer: a risk adapted paradigm. Endocrinol Metab Clin North Am 2008; 37:419–435 [DOI] [PubMed] [Google Scholar]
- 8.Diessl S, Holzberger B, Mäder U, et al. Impact of moderate versus stringent TSH suppression on survival in advanced differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2012; 76:586–592 [DOI] [PubMed] [Google Scholar]
- 9.Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol 2006; 94:692–700 [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, Park HS, Kim SW, et al. Clinical characteristics of papillary thyroid microcarcinoma less than or equal to 5mm on ultrasonography. Eur Arch Otorhinolaryngol 2013; 270:2969–2974 [DOI] [PubMed] [Google Scholar]
- 11.Mehanna H, Al-Maqbili T, Carter B, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab 2014; (in press) [DOI] [PubMed] [Google Scholar]
- 12.Zing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013; 381:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nixon IJ, Ganly I, Patel SG, et al. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid 2013; 23:683–694 [DOI] [PubMed] [Google Scholar]
- 14▪.Durante C, Tognini S, Montesano T, et al. Clinical aggressiveness and long-term outcome in patients with papillary thyroid cancer and circulating antithyroglobulin autoantibodies. Thyroid 2014; 24:1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study emphasizes the importance of reliable TgAb detection because DTC patients with TgAb are at higher risk for recurrence.
- 15.Sabra MM, Grewal RK, Ghossein RA, Tuttle RM. Higher administered activities of radioactive iodine are associated with less structural persistent response in older, but not younger, papillary thyroid cancer patients with lateral neck lymph node metastases. Thyroid 2014; 24:1088–1095 [DOI] [PubMed] [Google Scholar]
- 16▪▪.Giovanella L, Treglia G, Sadeghi R, et al. Unstimulated high-sensitive thyroglobulin in follow-up of differentiated thyroid cancer patients: a meta-analysis. J Clin Endocrinol Metab 2014; 99:440–447 [DOI] [PubMed] [Google Scholar]; This is a comprehensive review of recent studies showing that sensitive (second-generation) basal thyroglobulin measurement overcomes the need for rhTSH stimulation.
- 17.Zophel K, Wunderlich G, Smith BR. Serum thyroglobulin measurements with a high sensitivity enzyme-linked immunosorbent assay: is there a clinical benefit in patients with differentiated thyroid carcinoma? Thyroid 2003; 13:861–865 [DOI] [PubMed] [Google Scholar]
- 18.Smallridge RC, Meek SE, Morgan MA, et al. Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patients. J Clin Endocrinol Metab 2007; 92:82–87 [DOI] [PubMed] [Google Scholar]
- 19.Spencer CA, Fatemi S, Singer P, et al. Serum basal thyroglobulin measured by a 2nd generation assay correlates with the recombinant human TSH-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid 2010; 20:587–595 [DOI] [PubMed] [Google Scholar]
- 20.Chindris AM, Diehl NN, Crook JE, et al. Undetectable sensitive serum thyroglobulin (<0.1 ng/ml) in 163 patients with follicular cell-derived thyroid cancer: results of rhTSH stimulation and neck ultrasonography and long-term biochemical and clinical follow-up. J Clin Endocrinol Metab 2012; 97:2714–2723 [DOI] [PubMed] [Google Scholar]
- 21.Meijer JA, le Cessie S, van den Hout WB, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf) 2010; 72:534–542 [DOI] [PubMed] [Google Scholar]
- 22.Miyauchi A, Kudo T, Miya A, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 2011; 21:707–716 [DOI] [PubMed] [Google Scholar]
- 23.Pacini F, Sabra MM, Tuttle RM. Clinical relevance of thyroglobulin doubling time in the management of patients with differentiated thyroid cancer. Thyroid 2011; 21:691–692 [DOI] [PubMed] [Google Scholar]
- 24.Wong H, Wong KP, Yau T, et al. Is there a role for unstimulated thyroglobulin velocity in predicting recurrence in papillary thyroid carcinoma patients with detectable thyroglobulin after radioiodine ablation? Ann Surg Oncol 2012; 19:3479–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovanella L, Trimboli P, Verburg FA, et al. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging 2013; 40:874–880 [DOI] [PubMed] [Google Scholar]
- 26▪.Tsushima Y, Miyauchi A, Ito Y, et al. Prognostic significance of changes in serum thyroglobulin antibody levels of pre- and post-total thyroidectomy in thyroglobulin antibody-positive papillary thyroid carcinoma patients. Endocr J 2013; 60:871–876 [DOI] [PubMed] [Google Scholar]; This study describes the clinical utility of monitoring percentage TgAb changes and trends.
- 27.Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 1998; 83:1121–1127 [DOI] [PubMed] [Google Scholar]
- 28.Mariotti S, Barbesino G, Caturegli P, et al. Assay of thyroglobulin in serum with thyroglobulin autoantibodies: an unobtainable goal? J Clin Endocrinol Metab 1995; 80:468–472 [DOI] [PubMed] [Google Scholar]
- 29.Spencer CA, Bergoglio LM, Kazarosyan M, et al. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab 2005; 90:5566–5575 [DOI] [PubMed] [Google Scholar]
- 30▪▪.Verburg FA, Luster M, Cupini C, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid 2013; 23:1211–1225 [DOI] [PubMed] [Google Scholar]; This review by an expert European panel presents number of questions and recommendations regarding TgAb measurement.
- 31.Preissner CM, O’Kane DJ, Singh RJ, et al. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab 2003; 88:3069–3074 [DOI] [PubMed] [Google Scholar]
- 32.Giovanella L, Keller F, Ceriani L, Tozzoli R. Heterophile antibodies may falsely increase or decrease thyroglobulin measurement in patients with differentiated thyroid carcinoma. Clin Chem Lab Med 2009; 47:952–954 [DOI] [PubMed] [Google Scholar]
- 33.Verburg FA, Wäschle K, Reiners C, Giovanella L, et al. Heterophile antibodies rarely influence the measurement of thyroglobulin and thyroglobulin antibodies in differentiated thyroid cancer patients. Horm Metab Res 2010; 42:736–739 [DOI] [PubMed] [Google Scholar]
- 34.Spencer C. Commentary on: implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid 2013; 23:1190–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Herle AJ, Uller RP, Matthews NL, Brown J, et al. Radioimmunoassay for measurement of thyroglobulin in human serum. J Clin Invest 1973; 52:1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer C, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab 2011; 96:1283–1291 [DOI] [PubMed] [Google Scholar]
- 37▪▪.Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods: strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab 2013; 27:701–712 [DOI] [PubMed] [Google Scholar]; This review presents the data showing the stability of thyroglobulin and TgAb measurements made in archived sera.
- 38.Mariotti S, Cupini C, Giani C. Evaluation of a solid-phase immunoradiometric assay (IRMA) for serum thyroglobulin: effect of antithyroglobulin autoantibody. Clin Chim Acta 1982; 123:347–355 [DOI] [PubMed] [Google Scholar]
- 39.Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem 2008; 54:1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke NJ, Zhang Y, Reitz RE. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J Investig Med 2012; 60:1157–1163 [DOI] [PubMed] [Google Scholar]
- 41.Kushnir MM, Rockwood AL, Roberts WL. Measurement of thyroglobulin by liquid chromatography/tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin Chem 2013; 59:982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Hoofnagle AN, Roth MY. Improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab 2013; 98:1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reviews the underlying rationale behind thyroglobulin LC-MS/MS and discusses the limitations of this new class of thyroglobulin method.
- 43.Nicoloff JT, Spencer CA. Clinical review 12: The use and misuse of the sensitive thyrotropin assays. J Clin Endocrinol Metab 1990; 71:553–558 [DOI] [PubMed] [Google Scholar]
- 44.Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13:1–126 [DOI] [PubMed] [Google Scholar]
- 45.Schlumberger M, Hitzel A, Toubert ME, et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab 2007; 92:2487–2495 [DOI] [PubMed] [Google Scholar]
- 46.Iervasi A, Iervasi G, Ferdeghini M, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2007; 67:434–441 [DOI] [PubMed] [Google Scholar]
- 47.Rosario PW, Purisch S. Does a highly sensitive thyroglobulin (Tg) assay change the clinical management of low-risk patients with thyroid cancer with Tg on T4 < 1 ng/ml determined by traditional assays? Clin Endocrinol (Oxf) 2008; 68:338–342 [DOI] [PubMed] [Google Scholar]
- 48.Grebe SKG. Diagnosis and management of thyroid carcinoma: a focus on serum thyroglobulin. Expert Rev Endocrinol Metab 2009; 4:25–43 [Google Scholar]
- 49.Malandrino P, Latina A, Marescalco S, et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab 2011; 96:1703–1709 [DOI] [PubMed] [Google Scholar]
- 50.Castagna MG, Tala Jury HP, Cipri C, et al. The use of ultrasensitive thyroglobulin assays reduces but not abolishes the need for TSH stimulation in patients with differentiated thyroid carcinoma. J Endocrinol Invest 2011; 34:219–223 [DOI] [PubMed] [Google Scholar]
- 51.Nakabashi CC, Biscolla RP, Kasamatsu T S, et al. Development, characterization and clinical validation of new sensitive immunofluorometric assay for the measurement of serum thyroglobulin. Arq Bras Endocrinol Metabol 2012; 56:658–665 [DOI] [PubMed] [Google Scholar]
- 52.Trimboli P, La Torre D, Ceriani L, et al. High sensitive thyroglobulin assay on thyroxine therapy: can it avoid stimulation test in low and high risk differentiated thyroid carcinoma patients? Horm Metab Res 2013; 45:664–668 [DOI] [PubMed] [Google Scholar]
- 53▪▪.Angell TE, Spencer CA, Rubino B, et al. In search of an unstimulated thyroglobulin baseline value in low-risk papillary thyroid carcinoma patients not receiving radioactive iodine ablation. Thyroid 2014; 24:1127–1133 [DOI] [PubMed] [Google Scholar]; This is the first study of serum thyroglobulin concentrations arising from postsurgical thyroid remnant tissue of patients without RAI treatment.
- 54.Heilo A, Sigstad E, Fagerlid KH, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab 2011; 96:2750–2755 [DOI] [PubMed] [Google Scholar]
- 55.Hay ID, Lee RA, Davidge-Pitts C, et al. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected ‘recurrent’ neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery 2013; 154:1448–1454 [DOI] [PubMed] [Google Scholar]
- 56.Schneider AB, Pervos R. Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J Clin Endocrinol Metab 1978; 47:126–137 [DOI] [PubMed] [Google Scholar]
- 57.Ross HA, Netea-Maier RT, Schakenraad E, et al. Assay bias may invalidate decision limits and affect comparability of serum thyroglobulin assay methods: an approach to reduce interpretation differences. Clin Chim Acta 2008; 394:104–109 [DOI] [PubMed] [Google Scholar]
- 58.Algeciras-Schimnich A, Bruns DE, et al. Failure of current laboratory protocols to detect lot-to-lot reagent differences: findings and possible solutions. Clin Chem 2013; 59:1187–1194 [DOI] [PubMed] [Google Scholar]
- 59.Feldt-Rasmussen U, Schlumberger M. European interlaboratory comparison of serum thyroglobulin measurement. J Endocrinol Invest 1988; 11:175–181 [DOI] [PubMed] [Google Scholar]
- 60.Feldt-Rasmussen U, Profilis C, Colinet E, et al. Human thyroglobulin reference material (CRM 457) 1st part: Assessment of homogeneity, stability and immunoreactivity. Ann Biol Clin 1996; 54:337–342 [PubMed] [Google Scholar]
- 61.Feldt-Rasmussen U, Profilis C, Colinet E, et al. Human thyroglobulin reference material (CRM 457) 2nd part: Physicochemical characterization and certification. Ann Biol Clin 1996; 54:343–348 [PubMed] [Google Scholar]
- 62.Heilig B, Hufner M, Dorken B, Schmidt-Gayk H. Increased heterogeneity of serum thyroglobulin in thyroid cancer patients as determined by monoclonal antibodies. Klin Wochenschr 1986; 64:776–780 [DOI] [PubMed] [Google Scholar]
- 63.Giovanella L, Ceriani L, Ghelfo A, et al. Preoperative undetectable serum thyroglobulin in differentiated thyroid carcinoma: incidence, causes and management strategy. Clin Endocrinol (Oxf) 2007; 67:547–551 [DOI] [PubMed] [Google Scholar]
- 64.van de Graaf SA, Ris-Stalpers C, Pauws E, et al. Up to date with human thyroglobulin. J Endocrinol 2001; 170:307–321 [DOI] [PubMed] [Google Scholar]
- 65.Prentice L, Kiso Y, Fukuma N, et al. Monoclonal thyroglobulin autoantibodies: variable region analysis and epitope recognition. J Clin Endocrinol Metab 1995; 80:977–986 [DOI] [PubMed] [Google Scholar]
- 66.McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies. Thyroid 2004; 14:510–520 [DOI] [PubMed] [Google Scholar]
- 67.Feldt-Rasmussen U, Petersen PH, Blaabjerg O, Horder M. Long-term variability in serum thyroglobulin and thyroid related hormones in healthy subjects. Acta Endocrinol (Copenh) 1980; 95:328–334 [DOI] [PubMed] [Google Scholar]
- 68.Ozkan E, Soydal C, Araz M. The additive clinical value of 18F-FDG PET/CT in defining the recurrence of disease in patients with differentiated thyroid cancer who have isolated increased antithyroglobulin antibody levels. Clin Nucl Med 2012; 37:755–758 [DOI] [PubMed] [Google Scholar]
- 69.Feldt-Rasmussen U, Rasmussen AK. Serum thyroglobulin (Tg) in presence of thyroglobulin autoantibodies (TgAb). Clinical and methodological relevance of the interaction between Tg and TgAb in vivo and in vitro. J Endocrinol Invest 1985; 8:571–576 [DOI] [PubMed] [Google Scholar]
- 70.Clark P, Franklyn J. Can we interpret serum thyroglobulin results? Ann Clin Biochem 2012; 49:313–322 [DOI] [PubMed] [Google Scholar]
- 71.Crane MS, Strachan MW, Toft AD, Beckett GJ. Discordance in thyroglobulin measurements by radioimmunoassay and immunometric assay: a useful means of identifying thyroglobulin assay interference. Ann Clin Biochem 2013; 50:421–432 [DOI] [PubMed] [Google Scholar]
- 72.Black EG, Sheppard MC, Hoffenberg R. Serial serum thyroglobulin measurements in the management of differentiated thyroid carcinoma. Clin Endocrinol 1987; 27:115–120 [DOI] [PubMed] [Google Scholar]
- 73.Stanojevic M, Savin S, Cvejic D, et al. Comparison of the influence of thyroglobulin antibodies on serum thyroglobulin values from two different immunoassays in post surgical differentiated thyroid carcinoma patients. J Clin Lab Anal 2009; 23:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benvenga S, Burek CL, Talor M, et al. Heterogeneity of the thyroglobulin epitopes associated with circulating thyroid hormone autoantibodies in Hashimoto's thyroiditis and nonautoimmune thyroid diseases. J Endocrinol Invest 2002; 25:977–982 [DOI] [PubMed] [Google Scholar]
- 75.Latrofa F, Ricci D, Montanelli L, et al. Thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: comparison of different assays and evaluation of causes of discrepancies. J Clin Endocrinol Metab 2012; 97:3974–3982 [DOI] [PubMed] [Google Scholar]
- 76.Pickett AJ, Jones M, Evans C. Causes of discordance between thyroglobulin antibody assays. Ann Clin Biochem 2012; 49:463–467 [DOI] [PubMed] [Google Scholar]
- 77.Krahn J, Dembinski T. Thyroglobulin and antithyroglobulin assays in thyroid cancer monitoring. Clin Biochem 2009; 42:416–419 [DOI] [PubMed] [Google Scholar]
- 78.Taylor KP, Parkington D, Bradbury S, et al. Concordance between thyroglobulin antibody assays. Ann Clin Biochem 2011; 48:367–369 [DOI] [PubMed] [Google Scholar]
- 79.Nygaard B, Bentzen J, Laurberg P, et al. Large discrepancy in the results of sensitive measurements of thyroglobulin antibodies in the follow-up on thyroid cancer: a diagnostic dilemma. Eur Thyroid J 2013; 1:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weigle WO, High GJ. The behaviour of autologous thyroglobulin in the circulation of rabbits immunized with either heterologous or altered homologous thyroglobulin. J Immunol 1967; 98:1105–1114 [PubMed] [Google Scholar]
- 81.Feldt-Rasmussen U, Petersen PH, Date J, Madsen CM. Sequential changes in serum thyroglobulin (Tg) and its autoantibodies (TgAb) following subtotal thyroidectomy of patients with preoperatively detectable TgAb. Clin Endocrinol (Oxf) 1980; 12:29–38 [DOI] [PubMed] [Google Scholar]
- 82.Feldt-Rasmussen U. Serum thyroglobulin and thyroglobulin autoantibodies in thyroid disease. Allergy 1983; 38:369–387 [DOI] [PubMed] [Google Scholar]
- 83.van der Laken CJ, Voskuyl AE, Roos JC, et al. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and antiinfliximab in responders and nonresponders to therapy for rheumatoid arthritis. Ann Rheum Dis 2007; 66:253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hrafnkelsson J, Tulinius H, Kjeld M, et al. Serum thyroglobulin as a risk factor for thyroid carcinoma. Acta Oncol 2000; 39:973–977 [DOI] [PubMed] [Google Scholar]
- 85.Petric R, Perhavec A, Gazic B, Besic N. Preoperative serum thyroglobulin concentration is an independent predictive factor of malignancy in follicular neoplasms of the thyroid gland. J Surg Oncol 2012; 105:351–356 [DOI] [PubMed] [Google Scholar]
- 86.Lee EK, Chung KW, Min H S, et al. Preoperative serum thyroglobulin as a useful predictive marker to differentiate follicular thyroid cancer from benign nodules in indeterminate nodules. J Korean Med Sci 2012; 27:1014–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheffler P, Forest VI, Leboeuf R, et al. Serum thyroglobulin improves the sensitivity of the McGill thyroid nodule score for well differentiated thyroid cancer. Thyroid 2014; 24:852–857 [DOI] [PubMed] [Google Scholar]
- 88.Kim ES, Lim DJ, Baek KH, et al. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 2010; 20:885–891 [DOI] [PubMed] [Google Scholar]
- 89.Feldt-Rasmussen U, Rasmussen AK. Autoimmunity in differentiated thyroid cancer: significance and related clinical problems. Hormones (Athens) 2010; 9:109–117 [DOI] [PubMed] [Google Scholar]
- 90.Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers, (DTC). J Clin Endocrinol Metab 2011; 96:3615–3627 [DOI] [PubMed] [Google Scholar]
- 91.Jankovic B, Le KT, Hershman JM. Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 2013; 98:474–482 [DOI] [PubMed] [Google Scholar]
- 92.Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid 2014; 24:488–493 [DOI] [PubMed] [Google Scholar]
- 93.Chung JK, Park YJ, Kim TY, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf) 2002; 57:215–221 [DOI] [PubMed] [Google Scholar]
- 94.Seo JH, Lee SW, Ahn BC, Lee J. Recurrence detection in differentiated thyroid cancer patients with elevated serum level of antithyroglobulin antibody: special emphasis on using (18)F-FDG PET/CT. Clin Endocrinol (Oxf) 2010; 72:558–563 [DOI] [PubMed] [Google Scholar]
- 95.Soyluk O, Boztepe H, Aral F, et al. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid 2011; 21:1301–1308 [DOI] [PubMed] [Google Scholar]
- 96.Giovanella L, Suriano S, Ceriani L, Verburg FA. Undetectable thyroglobulin in patients with differentiated thyroid carcinoma and residual radioiodine uptake on a postablation whole-body scan. Clin Nucl Med 2011; 36:109–112 [DOI] [PubMed] [Google Scholar]
- 97.Latrofa F, Ricci D, Montanelli L. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab 2012; 97:2380–2387 [DOI] [PubMed] [Google Scholar]
- 98.Chiovato L, Latrofa F, Braverman L E, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med 2003; 139:346–351 [DOI] [PubMed] [Google Scholar]
- 99.Gorges R, Maniecki M, Jentzen W, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol 2005; 153:49–55 [DOI] [PubMed] [Google Scholar]
- 100.Kim WG, Yoon JH, Kim WB, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 2008; 93:4683–4689 [DOI] [PubMed] [Google Scholar]
- 101.Brassard M, Borget I, Edet-Sanson A, et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab 2011; 96:1352–1359 [DOI] [PubMed] [Google Scholar]
- 102.Kelders A, Kennes LN, Krohn T, et al. Relationship between positive thyroglobulin doubling time and 18F-FDG PET/CT-positive, 131I-negative lesions. Nucl Med Commun 2014; 34:176–181 [DOI] [PubMed] [Google Scholar]
- 103.Tomoda C, Miyauchi A. Undetectable serum thyroglobulin levels in patients with medullary thyroid carcinoma after total thyroidectomy without radioiodine ablation. Thyroid 2012; 22:680–682 [DOI] [PubMed] [Google Scholar]
- 104.Durante C, Montesano T, Attard M, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab 2012; 97:2748–2753 [DOI] [PubMed] [Google Scholar]
- 105.Nascimento C, Borget I, Troalen F, et al. Ultrasensitive serum thyroglobulin measurement is useful for the follow-up of patients treated with total thyroidectomy without radioactive iodine ablation. Eur J Endocrinol 2013; 169:689–693 [DOI] [PubMed] [Google Scholar]
- 106.Shih ML, Lee JA, Hsieh CB, et al. Thyroidectomy for Hashimoto's thyroiditis: complications and associated cancers. Thyroid 2008; 18:729–734 [DOI] [PubMed] [Google Scholar]
- 107.Tumino S, Belfiore A. Appearance of antithyroglobulin antibodies as the sole sign of metastatic lymph nodes in a patient operated on for papillary thyroid cancer: a case report. Thyroid 2000; 10:431–433 [DOI] [PubMed] [Google Scholar]
- 108.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8:363–372 [DOI] [PubMed] [Google Scholar]


