Abstract
Background
Angiogenic gene therapy is a promising treatment paradigm for patients with ischemic heart disease. In this study, we used micro–positron emission tomography (microPET) to monitor the transgene expression, function, and effects in a whole-body system.
Methods and Results
Adenovirus with cytomegalovirus promoter driving an angiogenic gene (vascular endothelial growth factor [VEGF]) linked to a PET reporter gene (herpes simplex virus type 1 mutant thymidine kinase; Ad-CMV-VEGF121-CMV-HSV1-sr39tk) was used to transfect rat embryonic cardiomyoblasts in vitro. Expression of both genes correlated strongly (r=0.98; P<0.001). Afterward, rats underwent ligation of the left anterior descending artery followed by injection of 1×1010 pfu of Ad-CMV-VEGF121-CMV-HSV1-sr39tk (study; n=35) or Ad-null (control; n=15) at the peri-infarct region. Noninvasive microPET imaging was used to assess the uptake of 9-(4-[18F]-fluoro-hydroxymethylbutyl)guanine ([18F]-FHBG) PET reporter probe by cells expressing the HSV1-sr39tk PET reporter gene. Cardiac transgene expression peaked at day 1 and declined over the next 2 weeks. Repeat adenoviral injections at day 60 yielded no detectable signal. The in vivo reporter gene expression (% injected dose/g of [18F]-FHBG) correlated well with ex vivo gamma counting (r=0.92), myocardial tissue HSV1-sr39TK enzyme activity (r=0.95), and myocardial tissue VEGF level (r=0.94; P<0.001 for all). The VEGF121 isoform induced significant increases in capillaries and small blood vessels. However, the level of neovasculature did not translate into significant improvements in functional parameters such as myocardial contractility by echocardiography, perfusion by nitrogen-13 ammonia imaging, and metabolism by [18F]-fluorodeoxyglucose imaging.
Conclusions
Taken together, these findings establish the feasibility of molecular imaging for monitoring angiogenic gene expression with a PET reporter gene and probe noninvasively, quantitatively, and repetitively. The principles demonstrated here can be used to evaluate other therapeutic genes of interest in animal models before future clinical trials are initiated.
Keywords: gene therapy, angiogenesis, imaging, myocardium, nuclear medicine
Ischemic heart disease is a leading cause of morbidity and mortality in the United States. Despite recent advances in medical therapies, a significant proportion of patients remain symptomatic. Thus, an alternative treatment approach collectively termed “therapeutic angiogenesis” has undergone intense investigation.1 One of the most widely studied angiogenic factors is the vascular endothelial growth factor (VEGF), a homodimeric 34- to 46-kDa heparin-binding glycoprotein. As a result of posttranscriptional mRNA splicing, mature monomeric VEGF transcripts contain 121-, 165-, 189-, or 206-amino acid residues. VEGF121 and VEGF165 are soluble proteins that can diffuse extracellularly, whereas VEGF189 and VEGF206 are bound to heparin-containing proteoglycans on the cell surface or in the basement membrane.2 In animal models and phase 1 clinical trials, VEGF therapy (delivered as protein, plasmid, or adenovirus) significantly improved myocardial perfusion and function.1 In contrast, recent double-blind, randomized clinical trials have shown less consistent if not disappointing results.3–5 Potential reasons for these discrepancies include (1) increased resistance to angiogenic stimulation in the selected patient population,4 (2) short half-life of angiogenic growth factors, resulting in regression of newly formed capillaries,6 and (3) lack of available imaging methods to carefully track cardiac transgene expression, function, and effects.7
Over the past decade, advances in imaging technologies have allowed investigators to visualize targeted cellular processes at a molecular or genetic level in whole-body living subjects.8 Most applications were targeted toward imaging transgene expression in tumor models.9 Similar development of noninvasive surrogate markers for monitoring cardiac transgene expression would be a major advance for cardiology. Recently, we demonstrated the feasibility of imaging the location, magnitude, and duration of a single PET (positron emission tomography) reporter gene (HSV1-sr39tk) expression in the myocardium of living rats using a dedicated small-animal microPET with high spatial resolution (2 mm3 compared with 6 mm3 for clinical PET).10,11 We subsequently showed that the expression of 2 PET reporter genes (HSV1-sr39tk and mutant dopamine 2 receptor) linked together by an internal ribosomal entry site had good correlations in both cell assays (r=0.98, P<0.001) and rat myocardium (r=0.86, P<0.001).12 Therefore, the present study is a logical progression of our previous works on cardiac gene imaging. In a rat model of myocardial infarction (MI), we hypothesize that by substituting one of the PET reporter genes with a therapeutic gene, we can monitor the therapeutic gene of interest (VEGF121) by imaging the linked reporter gene (HSV1-sr39tk).
Methods
Virus Construction and Amplification
A replication-defective recombinant adenovirus carrying dual-expression cassettes separated by a polyA tail with cytomegalovirus (CMV) promoters driving the expression of human VEGF121 gene and HSV1-sr39tk reporter gene (Ad-CMV-VEGF121-CMV-HSV1-sr39tk) was constructed, purified, and amplified as described previously.13,14 The Ad-null and single-cassette Ad-CMV-HSV1-sr39tk served as negative and positive control viruses, respectively.13 Henceforth, “HSV1-sr39tk” refers to reporter gene and “HSV1-sr39TK” refers to reporter enzyme.
Cell-Transfection Studies
Rat embryonic H9c2 cardiomyoblasts (American Type Culture Collection) were cultured as described previously.15 Cells were transfected with Ad-CMV-VEGF121-CMV-HSV1-sr39tk at various plaque forming units (pfu; 0, 1×108, 5×108, 1×109, 5×109, and 1×1010 pfu) for 90 minutes. H9c2 cells transduced with equal titers of Ad-null served as negative controls. After 48 hours, the total amount of VEGF121 in the cell medium was measured quantitatively with a QuantiGlo human ELISA kit (R&D System) according to the manufacturer’s protocol. HSV1-sr39TK level within H9c2 cells was determined by the penciclovir phosphorylation assay. Results are expressed as percent conversion of 8-[3H]PCV in (disintegrations per minute [dpm]/μg protein of cell or tissue extract)/(dpm of control sample) ×100 as described previously.10 All experiments were performed in triplicate.
Western Blot Analysis
Cell lysates were prepared from H9c2 cells transfected with Ad-null, Ad-CMV-HSV1-sr39tk, or Ad-CMV-VEGF121-CMV-HSV1-sr39tk as described previously.13 Mouse monoclonal IgG anti-human VEGF antibody (Oncogene Research) and rabbit polyclonal anti-herpes simplex virus (HSV) type 1 thymidine kinase (HSV1-TK) antibody (kind gift from Dr Margaret Black, Washington State University) were used to qualitatively determine VEGF121 and HSV1-sr39TK levels, respectively.
Induction of MI
Adult female Sprague-Dawley rats (weight 200 to 300 g; Charles River Laboratories, Wilmington, Mass) received isoflurane (2%) for general anesthesia during mechanical ventilation. MI was induced by ligation of the left anterior descending coronary artery 2 to 3 mm from the tip of the left auricle with a 6-0 polypropylene suture. This resulted in myocardial blanching and ST-segment elevation on an ECG monitor (Silogic EC-60 model). Afterward, 3 separate sites around the peri-infarct region were injected with a total of 50 μL of viral volume. The study was performed in accordance with protocols approved by the University Animal Research Committee.
Animal Study Design
Figure 1 depicts the vector construct and research design. Animals were divided into the study group receiving 1×1010 pfu of Ad-CMV-VEGF121-CMV-HSV1-sr39tk (n=35) or the control group receiving Ad-null (n=15) injection. The overall surgical mortality was ≈22% (8 in the VEGF study group and 3 in the control group). To ensure equal randomization, all animals underwent echocardiography, [13N]-NH3, and [18F]-fluorodeoxyglucose ([18F]-FDG) imaging at baseline (day 2). Animals without clear evidence of MI were categorized into the sham surgery group (≈20%; 7 in the VEGF group and 3 in the control group). Subsequently, 20 animals in the VEGF study group and 9 in the Ad-null control group with similar degrees of MI were included for analysis.
Figure 1.
A, Schematic of Ad-CMV-VEGF121-CMV-HSV1-sr39tk–mediated gene expression. Two separate gene cassettes with CMV promoters (PCMV) driving expression of VEGF121 therapeutic gene and HSV1-sr39tk reporter gene separated by polyA tails. Translated product of VEGF121 is soluble and excreted extracellularly, whereas translated product of HSV1-sr39tk (HSV1-sr39TK) traps [18F]-FHBG intracellularly by phosphorylation. B, Outline of experimental design.
Imaging the Kinetics of Cardiac Transgene Expression With MicroPET
The pharmacokinetics of VEGF121 therapeutic gene expression were monitored by imaging the linked HSV1-sr39tk reporter gene expression every 1 to 3 days in 6 study animals with the 9-(4-[18F]-fluoro-3-hydroxymethylbutyl)guanine ([18F]-FHBG; 1.62±0.32 mCi; T1/2 ≈110 minutes) reporter probe. Three of these animals underwent a second intramyocardial injection (1×1010 pfu of Ad-CMV-VEGF121-CMV-HSV1-sr39tk) at day 60 and were scanned on days 62 and 64. Images from 50 to 60 minutes after [18F]-FHBG injection were reconstructed by filtered back-projection (FBP) and the maximum a posteriori probability (MAP) algorithm. Tracer activity (mCi/g) was divided by the injected dose (mCi) to obtain a tissue uptake index, expressed as percentage injected dose per gram of tissue (% ID/g) as described previously.10,11 Image acquisition was performed by 2 investigators blinded to the study conditions (I.Y.C. and M.S.).
Assessment of Myocardial Perfusion and Metabolism With MicroPET
Animals were injected with [13N]-NH3 radiotracers (1.42±0.28 mCi; T1/2 ≈10 minutes) via tail vein and imaged for 15 minutes to assess perfusion deficits after MI. Afterward, [18F]-FDG radiotracers (1.39±0.22 mCi; T1/2 ≈110 minutes) were injected to identify viable and nonviable tissues as described previously.10,16 Axial microPET image data were resliced into 3 cardiac axes with CAPP software (CTI). Regions of interest (ROIs) were drawn around the perfusion and metabolism defects by visual inspection with Image J software (National Institutes of Health). The percentage defect was calculated by dividing the number of pixels in the ROI by the total number of pixels in the polar map. Image analysis was performed by an investigator blinded to the study conditions (J.R.T.).
Assessment of Left Ventricular Contractility With Echocardiography
Rats were sedated with tribromoethanol (0.25 mg/g IP). Echocardiographic images were obtained with an Acuson Sequoia C256 equipped with a 15-MHz probe. Left ventricular ejection fraction and percent fractional shortening were calculated as described previously.17 All measurements were averaged on 3 consecutive cardiac cycles and analyzed by 2 investigators blinded to the study conditions (A.C. and J.-J.M.).
Ex Vivo Validation With Gamma Well Counting and Enzyme Assays
After imaging, explanted hearts (n=29) were counted for [18F] radioactivity in a gamma well counter (Cobra II Auto-Gamma).10 The total myocardial accumulation of [18F]-FHBG was expressed as % ID/g tissue. Afterward, hearts (n=12) were homogenized to determine VEGF121 and HSV1-sr39TK enzyme activities by ELISA and penciclovir phosphorylation assays, respectively, as described previously.10
Immunohistochemical Stainings
Explanted hearts (n=17) were processed for routine light microscopy and immunohistochemistry as described previously.15 The following primary antibodies and dilutions were used: mouse monoclonal IgG anti-human VEGF antibody at a dilution of 1:50; rabbit polyclonal HSV1-TK antibody at 1:500; mouse monoclonal anti-smooth muscle actin (α-SMA; Sigma Chemical) at 1:400; and goat polyclonal anti-CD31 antibody (Santa Cruz Biotechnology) at 1:200. To estimate capillary density, 5 randomly selected fields near the peri-infarct region were evaluated in 10 sections per heart. Computerized images were captured at a magnification of ×200. Capillary density (positive CD31 stain) was defined as the mean number of capillaries per square millimeter. The number of small blood vessels (positive α-SMA stain) was counted in a similar fashion.
Statistical Analysis
Data are expressed as mean±SEM. A probability value of <0.05 was considered statistically significant. Linear regression analysis was performed to assess the linear relationship between 2 variables. The significance of correlation was obtained by performing the 2-tailed Student’s t test against the null hypothesis that the correlation coefficient was zero.
Results
Cell Assays Showed Strong Correlations Between Reporter and Therapeutic Genes
For H9c2 cardiomyoblasts transfected with Ad-CMV-VEGF121-CMV-HSV1-sr39tk, both the VEGF121 and HSV1-sr39TK proteins were recognized at ≈18 and ≈46 kDa by their antibodies on Western blots (Figure 2A). The wider band for HSV1-sr39TK was due to nonspecific binding by the rabbit polyclonal HSV1-TK antibody.13 Precise quantification of the therapeutic and reporter gene activities was analyzed by ELISA and penciclovir phosphorylation enzyme assays, respectively. Over the range of viral titers tested, a high correlation (r=0.98; P<0.001) existed between the expressions of the 2 linked genes (Figure 2B).
Figure 2.
In vitro protein levels of VEGF121 and HSV1-sr39TK. A, Rat embryonic cardiomyoblasts were transduced with Ad-null (column 1; negative control), Ad-CMV-HSV1-sr39tk (column 2; positive control), and Ad-CMV-VEGF121-CMV-HSV1-sr39tk (column 3). Forty-eight hours later, cell media and lysates were assayed qualitatively by Western blot and (B) quantitatively by ELISA and penciclovir phosphorylation assays.
Noninvasive Monitoring of Cardiac Transgene Expression
As determined by myocardial uptake of [18F]-FHBG reporter probe, cardiac transgene expression peaked at day 1 (0.311±0.021% ID/g), progressively declined afterward, and was no longer detected by day 14 (Figure 3A). To address the clinical question of whether repeated injections are effective for increasing therapeutic efficacy, we evaluated transgene expression in a subset of the animals (n=3) that underwent a second intramyocardial injection of Ad-CMV-VEGF121-CMV-HSV1-sr39tk at day 60. Scans performed on days 62 and 64 showed no detectable [18F]-FHBG signal, thus indicating the absence of HSV1-sr39tk gene expression (Figure 3B). As expected, control rats injected with Ad-null showed background cardiac [18F]-FHBG activity (0.033±0.005% ID/g; data not shown).
Figure 3.
Noninvasive imaging of kinetics of cardiac transgene expression. A, Gene expression peaked at day 1 and rapidly decreased thereafter. Second injection (arrow) of Ad-CMV-VEGF121-CMV-HSV-sr39tk at day 60 yielded no detectable signal on day 62 and day 64. Error bars represent mean±SEM. B, Representative rat scanned longitudinally with transaxial [18F]-FHBG microPET images shown at similar slice levels of chest cavity. Gray scale is normalized to individual peak activity of each image. In this rat, myocardial [18F]-FHBG accumulation was visualized at anterolateral wall (arrow) from day 1 to day 14.
In Vivo Gene, Perfusion, and Metabolism Imaging With MicroPET
Figure 4 shows the [13N]-NH3 perfusion, [18F]-FDG metabolism, and overlaid [18F]-FHBG reporter probe images. As expected, control and sham animals injected with Ad-null showed only background [18F]-FHBG activity that faintly outlined the shape of the chest cavity in blue (0.025 to 0.035% ID/g). In contrast, study animals injected with Ad-CMV-VEGF121-CMV-HSV1-sr39tk showed intense [18F]-FHBG signal around the region of perfusion and metabolism defects in red (0.300 to 0.350% ID/g). Importantly, in vivo ROI-derived [18F]-FHBG activity correlated well with ex vivo [18F] gamma counting of the explanted heart (r=0.92, P<0.001), ex vivo tissue HSV1-sr39TK enzyme activity (r=0.95, P<0.001), and ex vivo tissue levels of VEGF121 (r=0.94, P<0.001). Likewise, ex vivo tissue levels of VEGF121 and HSV1-sr39TK showed a strong correlation with each other (r=0.90, P<0.001).
Figure 4.
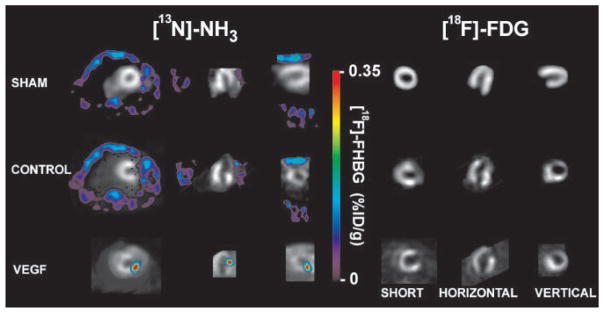
In vivo gene, perfusion, and metabolism imaging with microPET. At day 2, representative images showing normal perfusion ([13N]-NH3) and metabolism ([18F]-FDG) in sham rat (unsuccessful MI), anterolateral infarction in control rat (Ad-null), and anterolateral infarction in study rat (Ad-CMV-VEGF121-CMV-HSV1-sr39tk) in short, vertical, and horizontal axes (gray scale). Color scale is expressed as % ID/g for [18F]-FHBG uptake. Both sham and control animals had background [18F]-FHBG signal only (blue) that outlined shape of chest cavity. In contrast, study rat showed robust HSV1-sr39tk reporter gene activity near site of injection.
VEGF121 Induces Neovascularization in Ischemic Myocardium
The left ventricular infarct areas seen on in vivo perfusion and metabolism images were confirmed by ex vivo hematoxylin-and-eosin and trichrome stains (Figure 5, a through d). Expression of the 2 linked genes was also demonstrated immunohistochemically. The HSV1-TK stained positive around the needle track where the virus was injected in the epicardium (Figure 5, e and f). The VEGF121 stained positive transmurally because it is a soluble protein that can diffuse extracellularly (Figure 5, g and h). As expected, control rats injected with Ad-null had negative HSV1-TK staining and a basal level of VEGF121 staining (data not shown). Around the peri-infarct region, the mean densities of capillaries (747±104 versus 450±101 per mm2; Figure 5, i and j) and small blood vessels (8.1±0.8 versus 5.1±1.2 per mm2; Figure 5, k and l) were significantly higher in the study group than in the control group (P<0.05 for both). We did not notice any evidence of hemangioma-like proliferations by gross or microscopic examination.
Figure 5.
Validation of in vivo imaging with histology and immunohistochemistry. Site of MI in representative study rat shown by hematoxylin and eosin stain (a, b) and trichrome stain (c, d) in low and high power, respectively. HSV1-sr39TK protein stained at site of viral injection (e, f), whereas soluble VEGF121 protein stained vessels diffusely throughout myocardium in low and high power (g, h). CD31 antibody demonstrated capillaries in both study (i) and control (j) rats. Likewise, α-SMA stained small blood vessels in both study (k) and control (l) rats at site of MI.
New Microvessels Do Not Translate Into Significant Functional Improvement
To address whether this level of neovasculature was physiologically relevant, we performed detailed functional imaging studies in sham (failed induction of MI; n=10), control (Ad-null; n=6), and study (Ad-CMV-VEGF121-CMV-HSV1-sr39tk; n=10) animals at baseline (day 2) and at week 10. At baseline, there was no significant difference in left ventricular ejection fraction for study and control rats. After 10 weeks of VEGF121 treatment, left ventricular ejection fraction showed mild improvement in the study group (43.4±8.1% to 47.3±12.5%) compared with the control group (47.5±9.3% to 45.2±8.4%), but this did not reach statistical significance. Similarly, fractional shortening showed no significant changes in the study (18.8±5.4% to 21.4±8.4%) or control (21.5±6.2% to 20.0±5.6%) groups. However, owing to the small size of rat myocardium, we were unable to accurately and consistently determine any significant changes in regional contractility involving the left anterior wall. For comparison of [13N]-NH3 and [18F]-FDG imaging, the VEGF-treated study animals showed an encouraging trend toward lower perfusion defects (15.2±3.1% to 13.8±2.6%) and metabolism deficits (12.7±4.3% to 11.5±4.6%), but the changes were not statistically significant. The control group also did not show any significant changes in perfusion (14.0±4.0% to 15.3±4.1%) or metabolism (13.4±2.3% to 15.1±3.0%) scores (P=NS).
Discussion
This is the first cardiac study to validate the novel approach of linking a therapeutic gene (VEGF121) with a PET reporter gene (HSV1-sr39tk). Our findings are as follows: (1) the dual-cassette adenoviral vector can efficiently transfect rat embryonic cardiomyoblasts (in vitro) and rat myocardium (in vivo), with strong correlations between the 2 genes; (2) noninvasive imaging shows that adenovirus-mediated gene expression peaks at 1 to 3 days, lasts ≈2 weeks, and is not aided by repeated injections; (3) in vivo images of HSV1-sr39tk reporter gene activity correspond well with traditional invasive techniques such as ex vivo gamma counting, enzyme assays, and immunohistochemistry; (4) VEGF121 gene transfer induces formation of new capillaries and small blood vessels as determined by CD31 and α-SMA stains, respectively; and (5) under conditions of the present study, the microscopic level of neovasculature does not translate into significant changes in clinically relevant physiological parameters such as myocardial contractility, perfusion, and metabolism.
Several features of our research deserve further discussion. First, 10 of 50 surgical animals (≈20%) showed no wall-motion abnormality on echocardiography and no defects on [13N]-NH3 and [18F]-FDG imaging. This unsuccessful occlusion of the left anterior descending coronary artery is consistent with 2 large studies that reported 17% to 36% failure rates in rat and mouse models.18,19 We strongly believe that early identification during baseline scans and subsequent exclusion of these sham animals are important. If categorized into the study group, these sham animals can favorably bias the interpretation of therapeutic efficacy by VEGF121 treatment. Second, the noninvasive nature of gene imaging allowed us to perform repetitive assessment of transgene expression, even after second adenoviral injections within the same animals. The significant reduction of [18F]-FHBG activity observed over time is likely due to animal host immune response against adenovirus and is also expected to occur in human studies.7 This short period of adenovirus-mediated gene expression may limit its efficacy, because Dor et al6 have shown that premature cessation of the VEGF stimulus can cause regression of newly acquired vessels. Third, we delivered a single VEGF121 gene at 1×1010 pfu because it was the comparable gene and dosage used in several clinical trials.1,5 However, Whitlock et al14 recently showed that adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF (121, 165, and 189) is more effective at inducing angiogenesis than individual VEGF cDNAs. Finally, we assessed the functional effects of VEGF in a rat MI model. In contrast, VEGF treatment is likely to be more efficacious during chronic myocardial ischemia, as recently demonstrated by Hughes et al20 in porcine myocardium using regional perfusion and segmental wall-motion analysis.
Despite the lack of functional improvement with the VEGF121 gene, our primary goal was to assess the feasibility of monitoring the magnitude, location, and duration of the angiogenic gene expression indirectly by imaging the linked PET reporter gene. We believe similar imaging approaches can and should be applied toward validation of other potential therapeutic genes such as fibroblast growth factor, nitric oxide, and hypoxia-inducible factor-1α before proceeding to clinical studies.1,2 These therapeutic genes can be linked to a variety of reporter genes (HSV1-sr39tk, transferrin, or firefly luciferase), under the control of specific promoters (cardiac tissue specific or tetracycline inducible), delivered by less immunogenic vectors (lentivirus or gutless adenovirus), and monitored by multimodality imaging approaches (PET, MRI, or optical).5,8,9 In doing so, one can expeditiously evaluate the optimal combinations of angiogenic factors, delivery strategy, and duration of gene expression that are needed to induce therapeutic angiogenesis while minimizing potential toxicities.
In theory, microPET imaging of therapeutic gene expression can be achieved by either a direct or an indirect approach.9 The direct approach involves imaging radioactive probes that target therapeutic gene products such as mRNA or enzyme.8,9 However, this approach requires synthesizing a customized probe for every therapeutic gene of interest. In contrast, the indirect approach, which involves a reporter gene and reporter probe, is more generalizable for gene therapy application. The reporter probe itself does not have to be changed if one wishes to study a new biological process, which saves valuable time needed to synthesize, test, and validate new radiotracer agents.9 In addition to quantitative gene imaging, PET imaging offers functional information on physiological variables such as perfusion and metabolism. In post-MI rats, Kudo et al16 showed that microPET imaging of perfusion defects and metabolic deficits as measured by uptake of [13N]-NH3 and [18F]-FDG radiotracers, respectively, correlated well with postmortem histology (r=0.93 and r=0.97; P<0.01).
In summary, gene transfer holds great promise for the treatment of cardiovascular diseases. For cardiac gene therapy to be successful, biological issues related to pharmacokinetics and functional and physiological effects of gene expression will need to be fully evaluated before clinical trials.7 Molecular imaging is an extremely powerful technique that has the potential of analyzing these factors noninvasively, repetitively, and quantitatively in an intact, living whole-body system.8 In addition, it can be used to track cell survival, monitor endogenous transcriptional regulation, screen transgenic animal phenotypes, study protein-protein interactions, and expedite novel drug discovery.8,9,15 Because microPET can be scaled up to a clinical PET scanner, the present study should have direct translational relevance. Eventually, the goal is to use molecular imaging to help develop gene therapy protocols that are safe, beneficial, and quantifiable.
Acknowledgments
This work was supported by funding from the National Institutes of Health (2 RO1 CA82214-05 to Dr Gambhir), the Small Animal Imaging Resource Program (R24 CA92865 to Dr Gambhir), the Department of Energy (DE-FG02-03ER63687 to Dr Gambhir), and GlaxoSmithKline (cardiovascular grant to Dr Wu).
References
- 1.Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 3.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 4.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 5.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 6.Dor Y, Djonov V, Abramovitch R, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pislaru S, Janssens SP, Gersh BJ, et al. Defining gene transfer before expecting gene therapy: putting the horse before the cart. Circulation. 2002;106:631–636. doi: 10.1161/01.cir.0000019621.18368.b7. [DOI] [PubMed] [Google Scholar]
- 8.Blasberg RG, Tjuvajev JG. Molecular-genetic imaging: current and future perspectives. J Clin Invest. 2003;111:1620–1629. doi: 10.1172/JCI18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 10.Wu JC, Inubushi M, Sundaresan G, et al. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation. 2002;106:180–183. doi: 10.1161/01.cir.0000023620.59633.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inubushi M, Wu JC, Gambhir SS, et al. Positron-emission tomography reporter gene expression imaging in rat myocardium. Circulation. 2003;107:326–332. doi: 10.1161/01.cir.0000044385.60972.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen IY, Wu JC, Min JJ, et al. MicroPET imaging of cardiac gene expression in rats using bicistronic adenoviral vector–mediated gene delivery. Circulation. 2004;109:1415–1420. doi: 10.1161/01.CIR.0000121727.59564.5B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambhir SS, Bauer E, Black ME, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci U S A. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitlock PR, Hackett NR, Leopold PL, et al. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004;9:67–75. doi: 10.1016/j.ymthe.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo T, Fukuchi K, Annala AJ, et al. Noninvasive measurement of myocardial activity concentrations and perfusion defect sizes in rats with a new small-animal positron emission tomograph. Circulation. 2002;106:118–123. doi: 10.1161/01.cir.0000020221.28996.78. [DOI] [PubMed] [Google Scholar]
- 17.Watson LE, Sheth M, Denyer RF, et al. Baseline echocardiographic values for adult male rats. J Am Soc Echocardiogr. 2004;17:161–167. doi: 10.1016/j.echo.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Johns TN, Olson BJ. Experimental myocardial infarction, I: a method of coronary occlusion in small animals. Ann Surg. 1954;140:675–682. doi: 10.1097/00000658-195411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper CJ, Pfeffer JM, Finn P, et al. Characteristics of a model of myocardial infarction produced by coronary artery ligation in the rat. Cardiovasc Pathol. 1995;4:189–194. doi: 10.1016/1054-8807(95)00021-v. [DOI] [PubMed] [Google Scholar]
- 20.Hughes GC, Biswas SS, Yin B, et al. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]






