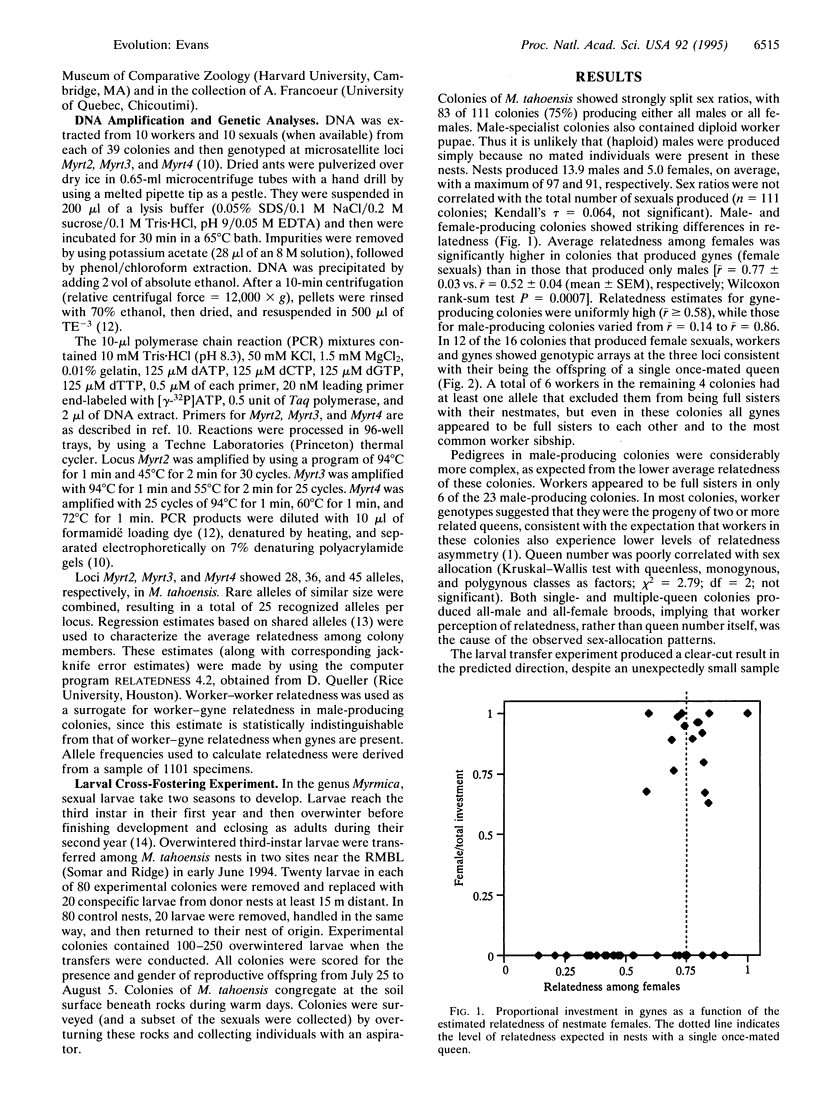
Abstract
The genetic relationships of colony members in the ant Myrmica tahoensis were determined on the basis of highly polymorphic microsatellite DNA loci. These analyses show that colonies fall into one of two classes. In roughly half of the sampled colonies, workers and female offspring appear to be full sisters. The remaining colonies contain offspring produced by two or more queens. Colonies that produce female sexuals are always composed of highly related females, while colonies that produce males often show low levels of nestmate relatedness. These results support theoretical predictions that workers should skew sex allocation in response to relatedness asymmetries found within colonies. The existence of a relatedness threshold below which female sexuals are not produced suggests a possible mechanism for worker perception of relatedness. Two results indicate that workers use genetic cues, not queen number, in making sex-allocation decisions. (i) The number of queens in a colony was not significantly correlated with either the level of relatedness asymmetry or the sex ratio. (ii) Sex-ratio shifts consistent with a genetically based mechanism of relatedness assessment were seen in an experiment involving transfers of larvae among unrelated nests. Thus workers appear to make sex-allocation decisions on the basis of larval cues and appear to be able to adjust sex ratios long after egg laying.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. D., Sherman P. W. Local mate competition and parental investment in social insects. Science. 1977 Apr 29;196(4289):494–500. doi: 10.1126/science.196.4289.494. [DOI] [PubMed] [Google Scholar]
- Evans J. D. Parentage analyses in ant colonies using simple sequence repeat loci. Mol Ecol. 1993 Dec;2(6):393–397. doi: 10.1111/j.1365-294x.1993.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Mueller U. G. Haplodiploidy and the evolution of facultative sex ratios in a primitively eusocial bee. Science. 1991 Oct 18;254(5030):442–444. doi: 10.1126/science.254.5030.442. [DOI] [PubMed] [Google Scholar]
- Nonacs P., Carlin N. F. When can ants discriminate the sex of brood? A new aspect of queen-worker conflict. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9670–9673. doi: 10.1073/pnas.87.24.9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts W. K., Manning C. J., Wakeland E. K. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature. 1991 Aug 15;352(6336):619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- Trivers R. L., Hare H. Haploidploidy and the evolution of the social insect. Science. 1976 Jan 23;191(4224):249–263. doi: 10.1126/science.1108197. [DOI] [PubMed] [Google Scholar]



